Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder
et al., Pharmaceutics, doi:10.3390/pharmaceutics14071432, Jul 2022
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Development and analysis of an inhalable dry powder formulation of ivermectin. Authors optimized the formulation to have good aerosolization properties for lung delivery. The powder maintained ivermectin's ability to inhibit SARS-CoV-2 replication in cells, demonstrating its potential as an inhaled antiviral therapeutic.
75 preclinical studies support the efficacy of ivermectin for COVID-19:
Ivermectin, better known for antiparasitic activity, is a broad spectrum antiviral with activity against many viruses including H7N772, Dengue38,73,74 , HIV-174, Simian virus 4075, Zika38,76,77 , West Nile77, Yellow Fever78,79, Japanese encephalitis78, Chikungunya79, Semliki Forest virus79, Human papillomavirus58, Epstein-Barr58, BK Polyomavirus80, and Sindbis virus79.
Ivermectin inhibits importin-α/β-dependent nuclear import of viral proteins72,74,75,81 , shows spike-ACE2 disruption at 1nM with microfluidic diffusional sizing39, binds to glycan sites on the SARS-CoV-2 spike protein preventing interaction with blood and epithelial cells and inhibiting hemagglutination42,82, shows dose-dependent inhibition of wildtype and omicron variants37, exhibits dose-dependent inhibition of lung injury62,67, may inhibit SARS-CoV-2 via IMPase inhibition38, may inhibit SARS-CoV-2 induced formation of fibrin clots resistant to degradation10, inhibits SARS-CoV-2 3CLpro55, may inhibit SARS-CoV-2 RdRp activity1,29, may minimize viral myocarditis by inhibiting NF-κB/p65-mediated inflammation in macrophages61, may be beneficial for COVID-19 ARDS by blocking GSDMD and NET formation83, may interfere with SARS-CoV-2's immune evasion via ORF8 binding5, may inhibit SARS-CoV-2 by disrupting CD147 interaction84-87, may inhibit SARS-CoV-2 attachment to lipid rafts via spike NTD binding3, shows protection against inflammation, cytokine storm, and mortality in an LPS mouse model sharing key pathological features of severe COVID-1960,88, may be beneficial in severe COVID-19 by binding IGF1 to inhibit the promotion of inflammation, fibrosis, and cell proliferation that leads to lung damage9, may minimize SARS-CoV-2 induced cardiac damage41,49, may counter immune evasion by inhibiting NSP15-TBK1/KPNA1 interaction and restoring IRF3 activation89, may disrupt SARS-CoV-2 N and ORF6 protein nuclear transport and their suppression of host interferon responses2, reduces TAZ/YAP nuclear import, relieving SARS-CoV-2-driven suppression of IRF3 and NF-κB antiviral pathways36, increases Bifidobacteria which play a key role in the immune system90, has immunomodulatory52 and anti-inflammatory71,91 properties, and has an extensive and very positive safety profile92.
1.
Li et al., Drug–Target Interaction Prediction via Dual-Interaction Fusion, Molecules, doi:10.3390/molecules31030498.
2.
Gayozo et al., Binding affinities analysis of ivermectin, nucleocapsid and ORF6 proteins of SARS-CoV-2 to human importins α isoforms: A computational approach, Biotecnia, doi:10.18633/biotecnia.v27.2485.
3.
Lefebvre et al., Characterization and Fluctuations of an Ivermectin Binding Site at the Lipid Raft Interface of the N-Terminal Domain (NTD) of the Spike Protein of SARS-CoV-2 Variants, Viruses, doi:10.3390/v16121836.
4.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
5.
Bagheri-Far et al., Non-spike protein inhibition of SARS-CoV-2 by natural products through the key mediator protein ORF8, Molecular Biology Research Communications, doi:10.22099/mbrc.2024.50245.2001.
6.
de Oliveira Só et al., In Silico Comparative Analysis of Ivermectin and Nirmatrelvir Inhibitors Interacting with the SARS-CoV-2 Main Protease, Preprints, doi:10.20944/preprints202404.1825.v1.
7.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
8.
Oranu et al., Validation of the binding affinities and stabilities of ivermectin and moxidectin against SARS-CoV-2 receptors using molecular docking and molecular dynamics simulation, GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2024.26.1.0030.
9.
Zhao et al., Identification of the shared gene signatures between pulmonary fibrosis and pulmonary hypertension using bioinformatics analysis, Frontiers in Immunology, doi:10.3389/fimmu.2023.1197752.
10.
Vottero et al., Computational Prediction of the Interaction of Ivermectin with Fibrinogen, Molecular Sciences, doi:10.3390/ijms241411449.
11.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
12.
Umar et al., Inhibitory potentials of ivermectin, nafamostat, and camostat on spike protein and some nonstructural proteins of SARS-CoV-2: Virtual screening approach, Jurnal Teknologi Laboratorium, doi:10.29238/teknolabjournal.v11i1.344.
13.
Alvarado et al., Interaction of the New Inhibitor Paxlovid (PF-07321332) and Ivermectin With the Monomer of the Main Protease SARS-CoV-2: A Volumetric Study Based on Molecular Dynamics, Elastic Networks, Classical Thermodynamics and SPT, Computational Biology and Chemistry, doi:10.1016/j.compbiolchem.2022.107692.
14.
Aminpour et al., In Silico Analysis of the Multi-Targeted Mode of Action of Ivermectin and Related Compounds, Computation, doi:10.3390/computation10040051.
15.
Parvez et al., Insights from a computational analysis of the SARS-CoV-2 Omicron variant: Host–pathogen interaction, pathogenicity, and possible drug therapeutics, Immunity, Inflammation and Disease, doi:10.1002/iid3.639.
16.
Francés-Monerris et al., Microscopic interactions between ivermectin and key human and viral proteins involved in SARS-CoV-2 infection, Physical Chemistry Chemical Physics, doi:10.1039/D1CP02967C.
17.
González-Paz et al., Comparative study of the interaction of ivermectin with proteins of interest associated with SARS-CoV-2: A computational and biophysical approach, Biophysical Chemistry, doi:10.1016/j.bpc.2021.106677.
18.
González-Paz (B) et al., Structural Deformability Induced in Proteins of Potential Interest Associated with COVID-19 by binding of Homologues present in Ivermectin: Comparative Study Based in Elastic Networks Models, Journal of Molecular Liquids, doi:10.1016/j.molliq.2021.117284.
19.
Rana et al., A Computational Study of Ivermectin and Doxycycline Combination Drug Against SARS-CoV-2 Infection, Research Square, doi:10.21203/rs.3.rs-755838/v1.
20.
Muthusamy et al., Virtual Screening Reveals Potential Anti-Parasitic Drugs Inhibiting the Receptor Binding Domain of SARS-CoV-2 Spike protein, Journal of Virology & Antiviral Research, www.scitechnol.com/abstract/virtual-screening-reveals-potential-antiparasitic-drugs-inhibiting-the-receptor-binding-domain-of-sarscov2-spike-protein-16398.html.
21.
Qureshi et al., Mechanistic insights into the inhibitory activity of FDA approved ivermectin against SARS-CoV-2: old drug with new implications, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1906750.
22.
Schöning et al., Highly-transmissible Variants of SARS-CoV-2 May Be More Susceptible to Drug Therapy Than Wild Type Strains, Research Square, doi:10.21203/rs.3.rs-379291/v1.
23.
Bello et al., Elucidation of the inhibitory activity of ivermectin with host nuclear importin α and several SARS-CoV-2 targets, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1911857.
24.
Udofia et al., In silico studies of selected multi-drug targeting against 3CLpro and nsp12 RNA-dependent RNA-polymerase proteins of SARS-CoV-2 and SARS-CoV, Network Modeling Analysis in Health Informatics and Bioinformatics, doi:10.1007/s13721-021-00299-2.
25.
Choudhury et al., Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach, Future Medicine, doi:10.2217/fvl-2020-0342.
26.
Kern et al., Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing, Frontiers in Pharmacology, doi:10.3389/fphar.2021.625678.
27.
Saha et al., The Binding mechanism of ivermectin and levosalbutamol with spike protein of SARS-CoV-2, Structural Chemistry, doi:10.1007/s11224-021-01776-0.
28.
Eweas et al., Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2, Frontiers in Microbiology, doi:10.3389/fmicb.2020.592908.
29.
Parvez (B) et al., Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.09.098.
30.
Francés-Monerris (B) et al., Has Ivermectin Virus-Directed Effects against SARS-CoV-2? Rationalizing the Action of a Potential Multitarget Antiviral Agent, ChemRxiv, doi:10.26434/chemrxiv.12782258.v1.
31.
Kalhor et al., Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1824816.
32.
Swargiary, A., Ivermectin as a promising RNA-dependent RNA polymerase inhibitor and a therapeutic drug against SARS-CoV2: Evidence from in silico studies, Research Square, doi:10.21203/rs.3.rs-73308/v1.
33.
Maurya, D., A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID-19 Patients, American Chemical Society (ACS), doi:10.26434/chemrxiv.12630539.v1.
34.
Lehrer et al., Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, In Vivo, 34:5, 3023-3026, doi:10.21873/invivo.12134.
35.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
36.
Kofler et al., M-Motif, a potential non-conventional NLS in YAP/TAZ and other cellular and viral proteins that inhibits classic protein import, iScience, doi:10.1016/j.isci.2025.112105.
37.
Shahin et al., The selective effect of Ivermectin on different human coronaviruses; in-vitro study, Research Square, doi:10.21203/rs.3.rs-4180797/v1.
38.
Jitobaom et al., Identification of inositol monophosphatase as a broad‐spectrum antiviral target of ivermectin, Journal of Medical Virology, doi:10.1002/jmv.29552.
39.
Fauquet et al., Microfluidic Diffusion Sizing Applied to the Study of Natural Products and Extracts That Modulate the SARS-CoV-2 Spike RBD/ACE2 Interaction, Molecules, doi:10.3390/molecules28248072.
40.
García-Aguilar et al., In Vitro Analysis of SARS-CoV-2 Spike Protein and Ivermectin Interaction, International Journal of Molecular Sciences, doi:10.3390/ijms242216392.
41.
Liu et al., SARS-CoV-2 viral genes Nsp6, Nsp8, and M compromise cellular ATP levels to impair survival and function of human pluripotent stem cell-derived cardiomyocytes, Stem Cell Research & Therapy, doi:10.1186/s13287-023-03485-3.
42.
Boschi et al., SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects, bioRxiv, doi:10.1101/2022.11.24.517882.
43.
De Forni et al., Synergistic drug combinations designed to fully suppress SARS-CoV-2 in the lung of COVID-19 patients, PLoS ONE, doi:10.1371/journal.pone.0276751.
44.
Saha (B) et al., Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder, Pharmaceutics, doi:10.3390/pharmaceutics14071432.
45.
Jitobaom (B) et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8.
46.
Croci et al., Liposomal Systems as Nanocarriers for the Antiviral Agent Ivermectin, International Journal of Biomaterials, doi:10.1155/2016/8043983.
47.
Zheng et al., Red blood cell-hitchhiking mediated pulmonary delivery of ivermectin: Effects of nanoparticle properties, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121719.
48.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
49.
Liu (B) et al., Genome-wide analyses reveal the detrimental impacts of SARS-CoV-2 viral gene Orf9c on human pluripotent stem cell-derived cardiomyocytes, Stem Cell Reports, doi:10.1016/j.stemcr.2022.01.014.
50.
Segatori et al., Effect of Ivermectin and Atorvastatin on Nuclear Localization of Importin Alpha and Drug Target Expression Profiling in Host Cells from Nasopharyngeal Swabs of SARS-CoV-2- Positive Patients, Viruses, doi:10.3390/v13102084.
51.
Jitobaom (C) et al., Favipiravir and Ivermectin Showed in Vitro Synergistic Antiviral Activity against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-941811/v1.
52.
Munson et al., Niclosamide and ivermectin modulate caspase-1 activity and proinflammatory cytokine secretion in a monocytic cell line, British Society For Nanomedicine Early Career Researcher Summer Meeting, 2021, web.archive.org/web/20230401070026/https://michealmunson.github.io/COVID.pdf.
53.
Mountain Valley MD, Mountain Valley MD Receives Successful Results From BSL-4 COVID-19 Clearance Trial on Three Variants Tested With Ivectosol™, 5/18, www.globenewswire.com/en/news-release/2021/05/18/2231755/0/en/Mountain-Valley-MD-Receives-Successful-Results-From-BSL-4-COVID-19-Clearance-Trial-on-Three-Variants-Tested-With-Ivectosol.html.
54.
Yesilbag et al., Ivermectin also inhibits the replication of bovine respiratory viruses (BRSV, BPIV-3, BoHV-1, BCoV and BVDV) in vitro, Virus Research, doi:10.1016/j.virusres.2021.198384.
55.
Mody et al., Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents, Communications Biology, doi:10.1038/s42003-020-01577-x.
56.
Jeffreys et al., Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2022.106542.
57.
Surnar et al., Clinically Approved Antiviral Drug in an Orally Administrable Nanoparticle for COVID-19, ACS Pharmacol. Transl. Sci., doi:10.1021/acsptsci.0c00179.
58.
Li (B) et al., Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment, J. Cellular Physiology, doi:10.1002/jcp.30055.
59.
Caly et al., The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research, doi:10.1016/j.antiviral.2020.104787.
60.
Zhang et al., Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflammation Research, doi:10.1007/s00011-008-8007-8.
61.
Gao et al., Ivermectin ameliorates acute myocarditis via the inhibition of importin-mediated nuclear translocation of NF-κB/p65, International Immunopharmacology, doi:10.1016/j.intimp.2024.112073.
62.
Abd-Elmawla et al., Suppression of NLRP3 inflammasome by ivermectin ameliorates bleomycin-induced pulmonary fibrosis, Journal of Zhejiang University-SCIENCE B, doi:10.1631/jzus.B2200385.
63.
Uematsu et al., Prophylactic administration of ivermectin attenuates SARS-CoV-2 induced disease in a Syrian Hamster Model, The Journal of Antibiotics, doi:10.1038/s41429-023-00623-0.
64.
Albariqi et al., Pharmacokinetics and Safety of Inhaled Ivermectin in Mice as a Potential COVID-19 Treatment, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121688.
65.
Errecalde et al., Safety and Pharmacokinetic Assessments of a Novel Ivermectin Nasal Spray Formulation in a Pig Model, Journal of Pharmaceutical Sciences, doi:10.1016/j.xphs.2021.01.017.
66.
Madrid et al., Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation, Heliyon, doi:10.1016/j.heliyon.2020.e05820.
67.
Ma et al., Ivermectin contributes to attenuating the severity of acute lung injury in mice, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2022.113706.
68.
de Melo et al., Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin, EMBO Mol. Med., doi:10.15252/emmm.202114122.
69.
Arévalo et al., Ivermectin reduces in vivo coronavirus infection in a mouse experimental model, Scientific Reports, doi:10.1038/s41598-021-86679-0.
70.
Chaccour et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Scientific Reports, doi:10.1038/s41598-020-74084-y.
71.
Yan et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflammation Research, doi:10.1007/s00011-011-0307-8.
72.
Götz et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Scientific Reports, doi:10.1038/srep23138.
73.
Tay et al., Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Research, doi:10.1016/j.antiviral.2013.06.002.
74.
Wagstaff et al., Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochemical Journal, doi:10.1042/BJ20120150.
75.
Wagstaff (B) et al., An AlphaScreen®-Based Assay for High-Throughput Screening for Specific Inhibitors of Nuclear Import, SLAS Discovery, doi:10.1177/1087057110390360.
76.
Barrows et al., A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection, Cell Host & Microbe, doi:10.1016/j.chom.2016.07.004.
77.
Yang et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Research, doi:10.1016/j.antiviral.2020.104760.
78.
Mastrangelo et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dks147.
79.
Varghese et al., Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses, Antiviral Research, doi:10.1016/j.antiviral.2015.12.012.
80.
Bennett et al., Role of a nuclear localization signal on the minor capsid Proteins VP2 and VP3 in BKPyV nuclear entry, Virology, doi:10.1016/j.virol.2014.10.013.
81.
Kosyna et al., The importin α/β-specific inhibitor Ivermectin affects HIF-dependent hypoxia response pathways, Biological Chemistry, doi:10.1515/hsz-2015-0171.
82.
Scheim et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
83.
Liu (C) et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
84.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
85.
Scheim (B), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
86.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
87.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
88.
DiNicolantonio et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
89.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
90.
Hazan et al., Treatment with Ivermectin Increases the Population of Bifidobacterium in the Gut, ACG 2023, acg2023posters.eventscribe.net/posterspeakers.asp.
91.
DiNicolantonio (B) et al., Anti-inflammatory activity of ivermectin in late-stage COVID-19 may reflect activation of systemic glycine receptors, Open Heart, doi:10.1136/openhrt-2021-001655.
92.
Descotes, J., Medical Safety of Ivermectin, ImmunoSafe Consultance, web.archive.org/web/20240313025927/https://www.medincell.com/wp-content/uploads/2021/03/Clinical_Safety_of_Ivermectin-March_2021.pdf.
93.
Wissel et al., Tolerability, Safety, and Pharmacokinetics of Ivermectin After Nasal Application in Healthy Adult Subjects, The Journal of Clinical Pharmacology, doi:10.1002/jcph.70137.
94.
Mohammed et al., A remodeled ivermectin polycaprolactone-based nanoparticles for inhalation as a promising treatment of pulmonary inflammatory diseases, European Journal of Pharmaceutical Sciences, doi:10.1016/j.ejps.2024.106714.
Saha et al., 8 Jul 2022, peer-reviewed, 5 authors.
Contact: shyamal.das@otago.ac.nz (corresponding author), tushar.saha@postgrad.otago.ac.nz, shubhra.sinha@otago.ac.nz, rhodri.harfoot@otago.ac.nz, miguel.quinones-mateu@otago.ac.nz.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder
Pharmaceutics, doi:10.3390/pharmaceutics14071432
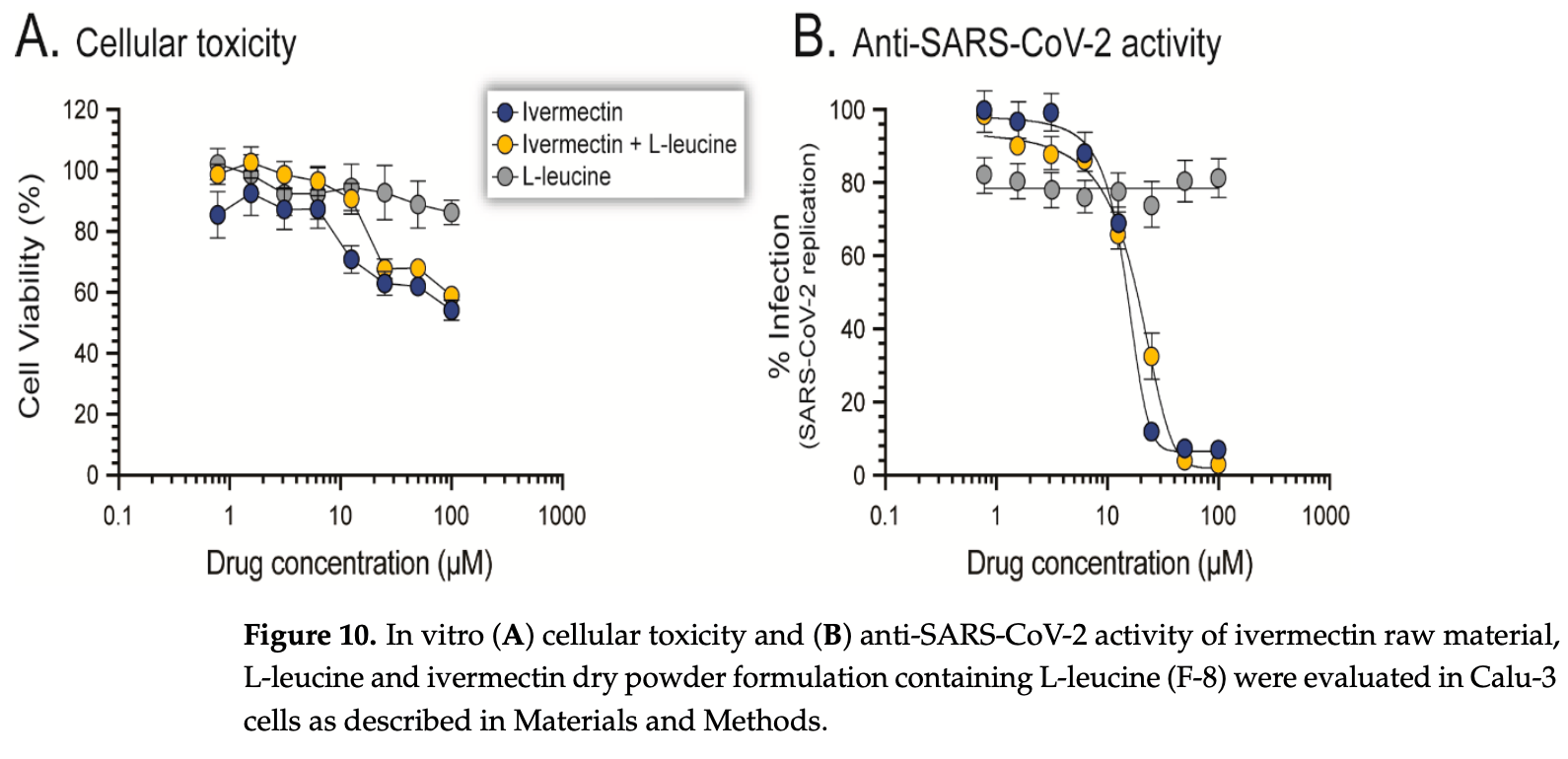
SARS-CoV-2, the causative agent of COVID-19, predominantly affects the respiratory tract. As a consequence, it seems intuitive to develop antiviral agents capable of targeting the virus right on its main anatomical site of replication. Ivermectin, a U.S. FDA-approved anti-parasitic drug, was originally shown to inhibit SARS-CoV-2 replication in vitro, albeit at relatively high concentrations, which is difficult to achieve in the lung. In this study, we tested the spray-drying conditions to develop an inhalable dry powder formulation that could ensure sufficient antiviral drug concentrations, which are difficult to achieve in the lungs based on the oral dosage used in clinical trials. Here, by using ivermectin as a proof-of-concept, we evaluated spray-drying conditions that could lead to the development of antivirals in an inhalable dry powder formulation, which could then be used to ensure sufficient drug concentrations in the lung. Thus, we used ivermectin in proof-of-principle experiments to evaluate our system, including physical characterization and in vitro aerosolization of prepared dry powder. The ivermectin dry powder was prepared with a mini spray-dryer (Buchi B-290), using a 2 3 factorial design and manipulating spray-drying conditions such as feed concentration (0.2% w/v and 0.8% w/v), inlet temperature (80 • C and 100 • C) and presence/absence of L-leucine (0% and 10%). The prepared dry powder was in the size range of 1-5 µm and amorphous in nature with wrinkle morphology. We observed a higher fine particle fraction (82.5 ± 1.4%) in high feed concentration (0.8% w/v), high inlet temperature (100 • C) and the presence of L-leucine (10% w/w). The stability study conducted for 28 days confirmed that the spray-dried powder was stable at 25 ± 2 • C/<15% RH and 25 ± 2 • C/ 53% RH. Interestingly, the ivermectin dry powder formulation inhibited SARS-CoV-2 replication in vitro with a potency similar to ivermectin solution (EC 50 values of 15.8 µM and 14.1 µM, respectively), with a comparable cell toxicity profile in Calu-3 cells. In summary, we were able to manipulate the spray-drying conditions to develop an effective ivermectin inhalable dry powder. Ongoing studies based on this system will allow the development of novel formulations based on single or combinations of drugs that could be used to inhibit SARS-CoV-2 replication in the respiratory tract.
Conflicts of Interest: The authors declare no conflict of interest.
References
Ahmed, Karim, Ross, Hossain, Clemens et al., A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.11.191
Ari, Practical strategies for a safe and effective delivery of aerosolized medications to patients with COVID-19, Respir. Med, doi:10.1016/j.rmed.2020.105987
Benke, Farkas, Szabó-Révész, Ambrus, Development of an Innovative, Carrier-Based Dry Powder Inhalation Formulation Containing Spray-Dried Meloxicam Potassium to Improve the In Vitro and In Silico Aerodynamic Properties, Pharmaceutics, doi:10.3390/pharmaceutics12060535
Bp, British Pharmacopoeia; Stationery Office: London, UK
Brunaugh, Seo, Warnken, Ding, Seo et al., Development and evaluation of inhalable composite niclosamide-lysozyme particles: A broad-spectrum, patient-adaptable treatment for coronavirus infections and sequalae, PLoS ONE, doi:10.1371/journal.pone.0246803
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
Carter, Rowley, Fletcher, Stylianopoulos, Measurement of Electrostatic Charge Decay in Pharmaceutical Powders and Polymer Materials Used in Dry Powder Inhaler Device, Drug Dev. Ind. Pharm, doi:10.3109/03639049809089953
Chable-Bessia, Boullé, Neyret, Swain, Hénaut et al., Low Selectivity Indices of Ivermectin and Macrocyclic Lactones on SARS-CoV-2 Replication In Vitro, Covid
Chaccour, Abizanda, Irigoyen-Barrio, Casellas, Aldaz et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Sci. Rep
Chaccour, Hammann, Rabinovich, Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety, Malar. J, doi:10.1186/s12936-017-1801-4
Chang, Yang, Pan, Chang, Liao, Anti-hygroscopic effect of leucine on spray-dried herbal extract powders, Powder Technol, doi:10.1016/j.powtec.2014.06.058
Charmet, Schaeffer, Grant, Galmiche, Cheny et al., Impact of original, B.1.1.7, and B.1.351/P.1 SARS-CoV-2 lineages on vaccine effectiveness of two doses of COVID-19 mRNA vaccines: Results from a nationwide case-control study in France, doi:10.1016/j.lanepe.2021.100171
Chaurasiya, Zhao, Dry Powder for Pulmonary Delivery: A Comprehensive Review, Pharmaceutics, doi:10.3390/pharmaceutics13010031
Chen, Fei, Chen, Sargsyan, Chang et al., Synergistic Inhibition of SARS-CoV-2 Replication Using Disulfiram/Ebselen and Remdesivir, ACS Pharmacol. Transl. Sci, doi:10.1021/acsptsci.1c00022
Chen, Guo, Pan, Zhao, Structure analysis of the receptor binding of 2019-nCoV, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2020.02.071
Chew, Shekunov, Tong, Chow, Savage et al., Effect of amino acids on the dispersion of disodium cromoglycate powders, J. Pharm. Sci, doi:10.1002/jps.20426
Costela-Ruiz, Illescas-Montes, Puerta-Puerta, Ruiz, Melguizo-Rodriguez, SARS-CoV-2 infection: The role of cytokines in COVID-19 disease, Cytokine Growth Factor Rev, doi:10.1016/j.cytogfr.2020.06.001
Dal Negro, Dry powder inhalers and the right things to remember: A concept review, Multidiscip. Respir. Med, doi:10.1186/s40248-015-0012-5
Delandre, Gendrot, Jardot, Le Bideau, Boxberger et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445
Dhama, Sharun, Tiwari, Dadar, Malik et al., COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics, Hum. Vaccin. Immunother, doi:10.1080/21645515.2020.1735227
Dong, Song, Lian, Fu, Gong, Subcutaneously injected ivermectin-loaded mixed micelles: Formulation, pharmacokinetics and local irritation study, Drug Dev, doi:10.3109/10717544.2014.956849
Eedara, Alabsi, Encinas-Basurto, Polt, Ledford et al., Inhalation Delivery for the Treatment and Prevention of COVID-19 Infection, Pharmaceutics, doi:10.3390/pharmaceutics13071077
Eedara, Tucker, Das, In vitro dissolution testing of respirable size anti-tubercular drug particles using a small volume dissolution apparatus, Int. J. Pharm, doi:10.1016/j.ijpharm.2019.01.035
Eedara, Tucker, Zujovic, Rades, Price et al., Crystalline adduct of moxifloxacin with trans-cinnamic acid to reduce the aqueous solubility and dissolution rate for improved residence time in the lungs, Eur. J. Pharm. Sci, doi:10.1016/j.ejps.2019.104961
Elalfy, Besheer, El-Mesery, El-Gilany, Soliman et al., Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19, J. Med. Virol, doi:10.1002/jmv.26880
Emary, Golubchik, Aley, Ariani, Angus et al., Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial, Lancet, doi:10.1016/S0140-6736(21)00628-0
Ferdynand, Nokhodchi, Co-spraying of carriers (mannitol-lactose) as a method to improve aerosolization performance of salbutamol sulfate dry powder inhaler, Drug Deliv. Transl. Res, doi:10.1007/s13346-020-00707-6
Ghasemian, Vatanara, Rouini, Rouholamini Najafabadi, Gilani et al., Inhaled sildenafil nanocomposites: Lung accumulation and pulmonary pharmacokinetics, Pharm. Dev. Technol, doi:10.3109/10837450.2015.1086369
Harfoot, Lawley, Hernandez, Kuang, Grant et al., Characterization of the First SARS-CoV-2 Isolates from Aotearoa New Zealand as Part of a Rapid Response to the COVID-19 Pandemic, doi:10.3390/v14020366
Hassoun, Ho, Muddle, Buttini, Parry et al., Formulating powder-device combinations for salmeterol xinafoate dry powder inhalers, Int. J. Pharm, doi:10.1016/j.ijpharm.2015.05.028
Hassoun, Malmlof, Scheibelhofer, Kumar, Bansal et al., Use of PBPK Modeling To Evaluate the Performance of DissolvIt, a Biorelevant Dissolution Assay for Orally Inhaled Drug Products, Mol. Pharm, doi:10.1021/acs.molpharmaceut.8b01200
Heidary, Gharebaghi, Ivermectin: A systematic review from antiviral effects to COVID-19 complementary regimen, J. Antibiot, doi:10.1038/s41429-020-0336-z
Heimfarth, Serafini, Martins-Filho, Quintans, Quintans-Junior, Drug repurposing and cytokine management in response to COVID-19: A review, Int. Immunopharmacol, doi:10.1016/j.intimp.2020.106947
Heyder, Gebhart, Rudolf, Schiller, Stahlhofen, Deposition of particles in the human respiratory tract in the size range 0.005-15 µm, J. Aerosol. Sci, doi:10.1016/0021-8502(86)90035-2
Hickey, Da Rocha, Pharmaceutical Inhalation Aerosol Technology
Hoppentocht, Hagedoorn, Frijlink, De Boer, Technological and practical challenges of dry powder inhalers and formulations, Adv. Drug Deliv. Rev, doi:10.1016/j.addr.2014.04.004
Kissler, Tedijanto, Goldstein, Grad, Lipsitch, Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period, Science, doi:10.1126/science.abb5793
Kumar, Ter Ellen, Bouma, Troost, Van De Pol et al., Moxidectin and Ivermectin Inhibit SARS-CoV-2 Replication in Vero E6 Cells but Not in Human Primary Bronchial Epithelial Cells, Antimicrob. Agents Chemother, doi:10.1128/AAC.01543-21
Letko, Marzi, Munster, Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses, Nat. Microbiol, doi:10.1038/s41564-020-0688-y
Li, Moore, Vasilieva, Sui, Wong et al., Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus, Nature, doi:10.1038/nature02145
Li, Sun, Parumasivam, Denman, Gengenbach et al., L-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders, Eur. J. Pharm. Biopharm, doi:10.1016/j.ejpb.2016.02.010
Lim, Hor, Tay, Mat, Jelani et al., Efficacy of Ivermectin Treatment on Disease Progression Among Adults with Mild to Moderate COVID-19 and Comorbidities: The I-TECH Randomized Clinical Trial, JAMA Intern. Med, doi:10.1001/jamainternmed.2022.0189
Lopez-Medina, Lopez, Hurtado, Davalos, Ramirez et al., Effect of Ivermectin on Time to Resolution of Symptoms among Adults with Mild COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2021.3071
Mangal, Nie, Xu, Guo, Cavallaro et al., Physico-Chemical Properties, Aerosolization and Dissolution of Co-Spray Dried Azithromycin Particles with L-Leucine for Inhalation, Pharm. Res, doi:10.1007/s11095-017-2334-9
Mansour, Shamma, Ahmed, Sabry, Esmat et al., Safety of inhaled ivermectin as a repurposed direct drug for treatment of COVID-19: A preclinical tolerance study, Int. Immunopharmacol
Mehta, Dry Powder Inhalers: A Focus on Advancements in Novel Drug Delivery Systems, J. Drug Deliv, doi:10.1155/2016/8290963
Momin, Adhikari, Sinha, Larson, Das, Roflumilast Powders for Chronic Obstructive Pulmonary Disease: Formulation Design and the Influence of Device, Inhalation Flow Rate, and Storage Relative Humidity on Aerosolization, Pharmaceutics, doi:10.3390/pharmaceutics13081254
Momin, Sinha, Tucker, Doyle, Das, Dry powder formulation of kanamycin with enhanced aerosolization efficiency for drug-resistant tuberculosis, Int. J. Pharm, doi:10.1016/j.ijpharm.2017.06.004
Momin, Tucker, Das, High dose dry powder inhalers to overcome the challenges of tuberculosis treatment, Int. J. Pharm, doi:10.1016/j.ijpharm.2018.08.061
Nasreen, Chung, He, Brown, Gubbay et al., Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario, Nat. Microbiol, doi:10.1038/s41564-021-01053-0
Patel, Patel, Chakraborty, Shukla, Revealing facts behind spray dried solid dispersion technology used for solubility enhancement, Saudi Pharm. J, doi:10.1016/j.jsps.2013.12.013
Peña-Silva, Duffull, Steer, Jaramillo-Rincon, Gwee et al., Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID-19, Br. J. Clin. Pharmacol, doi:10.1111/bcp.14476
Pignatello, Leonardi, Fuochi, Petronio Petronio, Greco et al., A Method for Efficient Loading of Ciprofloxacin Hydrochloride in Cationic Solid Lipid Nanoparticles: Formulation and Microbiological Evaluation, Nanomaterials, doi:10.3390/nano8050304
Rabi, Al Zoubi, Kasasbeh, Salameh, Al-Nasser, SARS-CoV-2 and Coronavirus Disease, What We Know So Far. Pathogens, doi:10.3390/pathogens9030231
Reis, Silva, Silva, Thabane, Milagres et al., Effect of Early Treatment with Ivermectin among Patients with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2115869
Rolim, Santos, Chaves, Gonçalves, Freitas-Neto et al., Preformulation study of ivermectin raw material, J. Therm. Anal. Calorim, doi:10.1007/s10973-014-3691-9
Rothan, Byrareddy, The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak, J. Autoimmun, doi:10.1016/j.jaut.2020.102433
Schenker, Jacobs, Respiratory effects of organic solvent exposure, Tuber. Lung Dis, doi:10.1016/S0962-8479(96)90069-6
Schmith, Zhou, Lohmer, The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19, Clin. Pharmacol. Ther, doi:10.1002/cpt.1889
Seville, Learoyd, Li, Williamson, Birchall, Amino acid-modified spray-dried powders with enhanced aerosolisation properties for pulmonary drug delivery, Powder Technol, doi:10.1016/j.powtec.2007.03.046
Simon, Amaro, Cabral, Healy, De Sousa, Development of a novel dry powder inhalation formulation for the delivery of rivastigmine hydrogen tartrate, Int. J. Pharm, doi:10.1016/j.ijpharm.2016.01.066
Sohrabi, Alsafi, O'neill, Khan, Kerwan et al., World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19), Int. J. Surg, doi:10.1016/j.ijsu.2020.02.034
Son, Worth Longest, Hindle, Aerosolization characteristics of dry powder inhaler formulations for the excipient enhanced growth (EEG) application: Effect of spray drying process conditions on aerosol performance, Int. J. Pharm, doi:10.1016/j.ijpharm.2013.01.003
Sou, Kaminskas, Nguyen, Carlberg, Mcintosh et al., The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules, Eur. J. Pharm. Biopharm, doi:10.1016/j.ejpb.2012.10.015
Thomas, Moreira, Kitchin, Absalon, Gurtman et al., Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 months, N. Engl. J. Med, doi:10.1056/NEJMoa2110345
Walls, Park, Tortorici, Wall, Mcguire et al., Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein, doi:10.1016/j.cell.2020.02.058
Watkins, Preventing a COVID-19 pandemic, BMJ, doi:10.1136/bmj.m810
Williams, Adams, Poochikian, Hauck, Content uniformity and dose uniformity: Current approaches, statistical analyses, and presentation of an alternative approach, with special reference to oral inhalation and nasal drug products, Pharm. Res, doi:10.1023/A:1015114821387
Yang, Yang, Ren, Mei, Effects of formulation and operating variables on zanamivir dry powder inhalation characteristics and aerosolization performance, Drug Deliv, doi:10.3109/10717544.2014.883113
Young, Sung, Traini, Kwok, Chiou et al., Influence of humidity on the electrostatic charge and aerosol performance of dry powder inhaler carrier based systems, Pharm. Res, doi:10.1007/s11095-006-9218-8
Zhu, Tan, Ng, Shen, Zhou et al., Analysis of the influence of relative humidity on the moisture sorption of particles and the aerosolization process in a dry powder inhaler, J. Aerosol. Sci, doi:10.1016/j.jaerosci.2008.02.003
Zijlstra, Hinrichs, De Boer, Frijlink, The role of particle engineering in relation to formulation and deagglomeration principle in the development of a dry powder formulation for inhalation of cetrorelix, Eur. J. Pharm. Sci, doi:10.1016/j.ejps.2004.06.005
DOI record:
{
"DOI": "10.3390/pharmaceutics14071432",
"ISSN": [
"1999-4923"
],
"URL": "http://dx.doi.org/10.3390/pharmaceutics14071432",
"abstract": "<jats:p>SARS-CoV-2, the causative agent of COVID-19, predominantly affects the respiratory tract. As a consequence, it seems intuitive to develop antiviral agents capable of targeting the virus right on its main anatomical site of replication. Ivermectin, a U.S. FDA-approved anti-parasitic drug, was originally shown to inhibit SARS-CoV-2 replication in vitro, albeit at relatively high concentrations, which is difficult to achieve in the lung. In this study, we tested the spray-drying conditions to develop an inhalable dry powder formulation that could ensure sufficient antiviral drug concentrations, which are difficult to achieve in the lungs based on the oral dosage used in clinical trials. Here, by using ivermectin as a proof-of-concept, we evaluated spray-drying conditions that could lead to the development of antivirals in an inhalable dry powder formulation, which could then be used to ensure sufficient drug concentrations in the lung. Thus, we used ivermectin in proof-of-principle experiments to evaluate our system, including physical characterization and in vitro aerosolization of prepared dry powder. The ivermectin dry powder was prepared with a mini spray-dryer (Buchi B-290), using a 23 factorial design and manipulating spray-drying conditions such as feed concentration (0.2% w/v and 0.8% w/v), inlet temperature (80 °C and 100 °C) and presence/absence of L-leucine (0% and 10%). The prepared dry powder was in the size range of 1–5 μm and amorphous in nature with wrinkle morphology. We observed a higher fine particle fraction (82.5 ± 1.4%) in high feed concentration (0.8% w/v), high inlet temperature (100 °C) and the presence of L-leucine (10% w/w). The stability study conducted for 28 days confirmed that the spray-dried powder was stable at 25 ± 2 °C/<15% RH and 25 ± 2 °C/ 53% RH. Interestingly, the ivermectin dry powder formulation inhibited SARS-CoV-2 replication in vitro with a potency similar to ivermectin solution (EC50 values of 15.8 µM and 14.1 µM, respectively), with a comparable cell toxicity profile in Calu-3 cells. In summary, we were able to manipulate the spray-drying conditions to develop an effective ivermectin inhalable dry powder. Ongoing studies based on this system will allow the development of novel formulations based on single or combinations of drugs that could be used to inhibit SARS-CoV-2 replication in the respiratory tract.</jats:p>",
"alternative-id": [
"pharmaceutics14071432"
],
"author": [
{
"affiliation": [],
"family": "Saha",
"given": "Tushar",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sinha",
"given": "Shubhra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harfoot",
"given": "Rhodri",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9170-5601",
"affiliation": [],
"authenticated-orcid": false,
"family": "Quiñones-Mateu",
"given": "Miguel E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Das",
"given": "Shyamal C.",
"sequence": "additional"
}
],
"container-title": "Pharmaceutics",
"container-title-short": "Pharmaceutics",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
11
]
],
"date-time": "2022-07-11T04:06:21Z",
"timestamp": 1657512381000
},
"deposited": {
"date-parts": [
[
2022,
7,
11
]
],
"date-time": "2022-07-11T04:29:51Z",
"timestamp": 1657513791000
},
"funder": [
{
"award": [
"LA394",
"Nil"
],
"name": "Laurenson Bequest Award"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
29
]
],
"date-time": "2023-08-29T13:21:54Z",
"timestamp": 1693315314967
},
"is-referenced-by-count": 5,
"issue": "7",
"issued": {
"date-parts": [
[
2022,
7,
8
]
]
},
"journal-issue": {
"issue": "7",
"published-online": {
"date-parts": [
[
2022,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
8
]
],
"date-time": "2022-07-08T00:00:00Z",
"timestamp": 1657238400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4923/14/7/1432/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1432",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
7,
8
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
8
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref1",
"unstructured": "World Health Organization Coronavirus Disease (COVID-19) Pandemic\nhttps://www.who.int/emergencies/diseases/novel-coronavirus-2019"
},
{
"DOI": "10.1021/acsptsci.1c00022",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1016/S0140-6736(21)00628-0",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1056/NEJMoa2110345",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1038/s41564-021-01053-0",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/j.lanepe.2021.100171",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1126/science.abb5793",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1080/21645515.2020.1735227",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1136/bmj.m810",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.3390/pathogens9030231",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1038/nature02145",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1016/j.cytogfr.2020.06.001",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1038/s41564-020-0688-y",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1016/j.jaut.2020.102433",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.ijsu.2020.02.034",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1016/j.bbrc.2020.02.071",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/j.cell.2020.02.058",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1038/s41429-020-0336-z",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1016/j.intimp.2020.106947",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1001/jama.2021.3071",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1056/NEJMoa2115869",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1001/jamainternmed.2022.0189",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1002/cpt.1889",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1111/bcp.14476",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.3390/pharmaceutics13071077",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1016/j.intimp.2021.108004",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1038/s41598-020-74084-y",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1016/j.rmed.2020.105987",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1016/S0962-8479(96)90069-6",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1016/j.ijpharm.2018.08.061",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1186/s40248-015-0012-5",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1016/j.ijpharm.2015.05.028",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1016/0021-8502(86)90035-2",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.3390/pharmaceutics13081254",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1016/j.ejps.2019.104961",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1016/j.ijpharm.2019.01.035",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.3390/v14020366",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1016/j.jsps.2013.12.013",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1002/jps.20426",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1016/j.ejpb.2012.10.015",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1016/j.powtec.2007.03.046",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1371/journal.pone.0246803",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1016/j.powtec.2014.06.058",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1016/j.ejpb.2016.02.010",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1007/s10973-014-3691-9",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.3390/nano8050304",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.3390/pharmaceutics13010031",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.3109/10717544.2014.883113",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"author": "BP",
"key": "ref51",
"series-title": "British Pharmacopoeia",
"year": "2015"
},
{
"DOI": "10.1016/j.ijpharm.2017.06.004",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.3390/pharmaceutics12060535",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.1023/A:1015114821387",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"author": "Hickey",
"key": "ref55",
"series-title": "Pharmaceutical Inhalation Aerosol Technology",
"year": "2019"
},
{
"DOI": "10.1016/j.jaerosci.2008.02.003",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.1016/j.ejps.2004.06.005",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.1016/j.ijpharm.2016.01.066",
"doi-asserted-by": "publisher",
"key": "ref58"
},
{
"DOI": "10.1007/s11095-017-2334-9",
"doi-asserted-by": "publisher",
"key": "ref59"
},
{
"DOI": "10.1016/j.ijpharm.2013.01.003",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.1016/j.addr.2014.04.004",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"DOI": "10.1155/2016/8290963",
"doi-asserted-by": "publisher",
"key": "ref62"
},
{
"DOI": "10.1007/s13346-020-00707-6",
"doi-asserted-by": "publisher",
"key": "ref63"
},
{
"DOI": "10.3109/10837450.2015.1086369",
"doi-asserted-by": "publisher",
"key": "ref64"
},
{
"DOI": "10.1007/s11095-006-9218-8",
"doi-asserted-by": "publisher",
"key": "ref65"
},
{
"DOI": "10.3109/03639049809089953",
"doi-asserted-by": "publisher",
"key": "ref66"
},
{
"DOI": "10.3109/10717544.2014.956849",
"doi-asserted-by": "publisher",
"key": "ref67"
},
{
"DOI": "10.1186/s12936-017-1801-4",
"doi-asserted-by": "publisher",
"key": "ref68"
},
{
"DOI": "10.1021/acs.molpharmaceut.8b01200",
"doi-asserted-by": "publisher",
"key": "ref69"
},
{
"DOI": "10.1002/jmv.26880",
"doi-asserted-by": "publisher",
"key": "ref70"
},
{
"DOI": "10.3390/covid2010005",
"doi-asserted-by": "publisher",
"key": "ref71"
},
{
"DOI": "10.3390/ph15040445",
"doi-asserted-by": "publisher",
"key": "ref72"
},
{
"DOI": "10.1128/AAC.01543-21",
"doi-asserted-by": "publisher",
"key": "ref73"
}
],
"reference-count": 73,
"references-count": 73,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4923/14/7/1432"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmaceutical Science"
],
"subtitle": [],
"title": "Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder",
"type": "journal-article",
"volume": "14"
}
