Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches
et al., Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1824816, Sep 2020
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
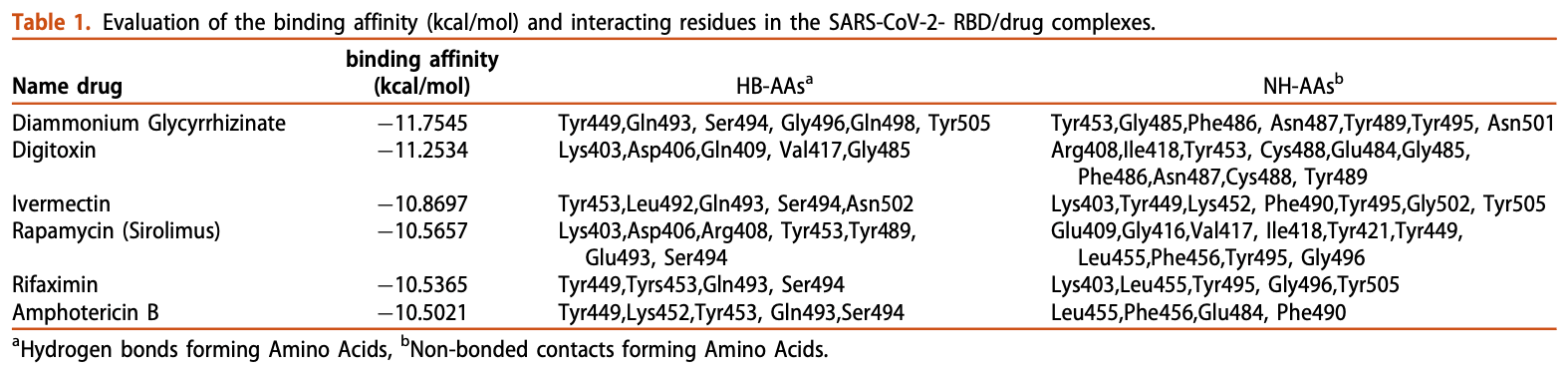
In silico study showing favorable binding of several FDA-approved drugs to the SARS-CoV-2 spike protein receptor binding domain (RBD). Authors performed structure-based virtual screening of FDA drug libraries and identified ivermectin, diammonium glycyrrhizinate, digitoxin, rapamycin, rifaximin, and amphotericin B as lead candidates that bind strongly to the RBD, blocking its interaction with the ACE2 receptor. Further analysis showed these drugs formed stable complexes with the RBD during molecular dynamics simulations. The results suggest these approved drugs may inhibit SARS-CoV-2 infection by disrupting ACE2 binding.
74 preclinical studies support the efficacy of ivermectin for COVID-19:
Ivermectin, better known for antiparasitic activity, is a broad spectrum antiviral with activity against many viruses including H7N771, Dengue37,72,73 , HIV-173, Simian virus 4074, Zika37,75,76 , West Nile76, Yellow Fever77,78, Japanese encephalitis77, Chikungunya78, Semliki Forest virus78, Human papillomavirus57, Epstein-Barr57, BK Polyomavirus79, and Sindbis virus78.
Ivermectin inhibits importin-α/β-dependent nuclear import of viral proteins71,73,74,80 , shows spike-ACE2 disruption at 1nM with microfluidic diffusional sizing38, binds to glycan sites on the SARS-CoV-2 spike protein preventing interaction with blood and epithelial cells and inhibiting hemagglutination41,81, shows dose-dependent inhibition of wildtype and omicron variants36, exhibits dose-dependent inhibition of lung injury61,66, may inhibit SARS-CoV-2 via IMPase inhibition37, may inhibit SARS-CoV-2 induced formation of fibrin clots resistant to degradation9, inhibits SARS-CoV-2 3CLpro54, may inhibit SARS-CoV-2 RdRp activity28, may minimize viral myocarditis by inhibiting NF-κB/p65-mediated inflammation in macrophages60, may be beneficial for COVID-19 ARDS by blocking GSDMD and NET formation82, may interfere with SARS-CoV-2's immune evasion via ORF8 binding4, may inhibit SARS-CoV-2 by disrupting CD147 interaction83-86, may inhibit SARS-CoV-2 attachment to lipid rafts via spike NTD binding2, shows protection against inflammation, cytokine storm, and mortality in an LPS mouse model sharing key pathological features of severe COVID-1959,87, may be beneficial in severe COVID-19 by binding IGF1 to inhibit the promotion of inflammation, fibrosis, and cell proliferation that leads to lung damage8, may minimize SARS-CoV-2 induced cardiac damage40,48, may counter immune evasion by inhibiting NSP15-TBK1/KPNA1 interaction and restoring IRF3 activation88, may disrupt SARS-CoV-2 N and ORF6 protein nuclear transport and their suppression of host interferon responses1, reduces TAZ/YAP nuclear import, relieving SARS-CoV-2-driven suppression of IRF3 and NF-κB antiviral pathways35, increases Bifidobacteria which play a key role in the immune system89, has immunomodulatory51 and anti-inflammatory70,90 properties, and has an extensive and very positive safety profile91.
1.
Gayozo et al., Binding affinities analysis of ivermectin, nucleocapsid and ORF6 proteins of SARS-CoV-2 to human importins α isoforms: A computational approach, Biotecnia, doi:10.18633/biotecnia.v27.2485.
2.
Lefebvre et al., Characterization and Fluctuations of an Ivermectin Binding Site at the Lipid Raft Interface of the N-Terminal Domain (NTD) of the Spike Protein of SARS-CoV-2 Variants, Viruses, doi:10.3390/v16121836.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Bagheri-Far et al., Non-spike protein inhibition of SARS-CoV-2 by natural products through the key mediator protein ORF8, Molecular Biology Research Communications, doi:10.22099/mbrc.2024.50245.2001.
5.
de Oliveira Só et al., In Silico Comparative Analysis of Ivermectin and Nirmatrelvir Inhibitors Interacting with the SARS-CoV-2 Main Protease, Preprints, doi:10.20944/preprints202404.1825.v1.
6.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
7.
Oranu et al., Validation of the binding affinities and stabilities of ivermectin and moxidectin against SARS-CoV-2 receptors using molecular docking and molecular dynamics simulation, GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2024.26.1.0030.
8.
Zhao et al., Identification of the shared gene signatures between pulmonary fibrosis and pulmonary hypertension using bioinformatics analysis, Frontiers in Immunology, doi:10.3389/fimmu.2023.1197752.
9.
Vottero et al., Computational Prediction of the Interaction of Ivermectin with Fibrinogen, Molecular Sciences, doi:10.3390/ijms241411449.
10.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
11.
Umar et al., Inhibitory potentials of ivermectin, nafamostat, and camostat on spike protein and some nonstructural proteins of SARS-CoV-2: Virtual screening approach, Jurnal Teknologi Laboratorium, doi:10.29238/teknolabjournal.v11i1.344.
12.
Alvarado et al., Interaction of the New Inhibitor Paxlovid (PF-07321332) and Ivermectin With the Monomer of the Main Protease SARS-CoV-2: A Volumetric Study Based on Molecular Dynamics, Elastic Networks, Classical Thermodynamics and SPT, Computational Biology and Chemistry, doi:10.1016/j.compbiolchem.2022.107692.
13.
Aminpour et al., In Silico Analysis of the Multi-Targeted Mode of Action of Ivermectin and Related Compounds, Computation, doi:10.3390/computation10040051.
14.
Parvez et al., Insights from a computational analysis of the SARS-CoV-2 Omicron variant: Host–pathogen interaction, pathogenicity, and possible drug therapeutics, Immunity, Inflammation and Disease, doi:10.1002/iid3.639.
15.
Francés-Monerris et al., Microscopic interactions between ivermectin and key human and viral proteins involved in SARS-CoV-2 infection, Physical Chemistry Chemical Physics, doi:10.1039/D1CP02967C.
16.
González-Paz et al., Comparative study of the interaction of ivermectin with proteins of interest associated with SARS-CoV-2: A computational and biophysical approach, Biophysical Chemistry, doi:10.1016/j.bpc.2021.106677.
17.
González-Paz (B) et al., Structural Deformability Induced in Proteins of Potential Interest Associated with COVID-19 by binding of Homologues present in Ivermectin: Comparative Study Based in Elastic Networks Models, Journal of Molecular Liquids, doi:10.1016/j.molliq.2021.117284.
18.
Rana et al., A Computational Study of Ivermectin and Doxycycline Combination Drug Against SARS-CoV-2 Infection, Research Square, doi:10.21203/rs.3.rs-755838/v1.
19.
Muthusamy et al., Virtual Screening Reveals Potential Anti-Parasitic Drugs Inhibiting the Receptor Binding Domain of SARS-CoV-2 Spike protein, Journal of Virology & Antiviral Research, www.scitechnol.com/abstract/virtual-screening-reveals-potential-antiparasitic-drugs-inhibiting-the-receptor-binding-domain-of-sarscov2-spike-protein-16398.html.
20.
Qureshi et al., Mechanistic insights into the inhibitory activity of FDA approved ivermectin against SARS-CoV-2: old drug with new implications, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1906750.
21.
Schöning et al., Highly-transmissible Variants of SARS-CoV-2 May Be More Susceptible to Drug Therapy Than Wild Type Strains, Research Square, doi:10.21203/rs.3.rs-379291/v1.
22.
Bello et al., Elucidation of the inhibitory activity of ivermectin with host nuclear importin α and several SARS-CoV-2 targets, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1911857.
23.
Udofia et al., In silico studies of selected multi-drug targeting against 3CLpro and nsp12 RNA-dependent RNA-polymerase proteins of SARS-CoV-2 and SARS-CoV, Network Modeling Analysis in Health Informatics and Bioinformatics, doi:10.1007/s13721-021-00299-2.
24.
Choudhury et al., Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach, Future Medicine, doi:10.2217/fvl-2020-0342.
25.
Kern et al., Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing, Frontiers in Pharmacology, doi:10.3389/fphar.2021.625678.
26.
Saha et al., The Binding mechanism of ivermectin and levosalbutamol with spike protein of SARS-CoV-2, Structural Chemistry, doi:10.1007/s11224-021-01776-0.
27.
Eweas et al., Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2, Frontiers in Microbiology, doi:10.3389/fmicb.2020.592908.
28.
Parvez (B) et al., Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.09.098.
29.
Francés-Monerris (B) et al., Has Ivermectin Virus-Directed Effects against SARS-CoV-2? Rationalizing the Action of a Potential Multitarget Antiviral Agent, ChemRxiv, doi:10.26434/chemrxiv.12782258.v1.
30.
Kalhor et al., Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1824816.
31.
Swargiary, A., Ivermectin as a promising RNA-dependent RNA polymerase inhibitor and a therapeutic drug against SARS-CoV2: Evidence from in silico studies, Research Square, doi:10.21203/rs.3.rs-73308/v1.
32.
Maurya, D., A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID-19 Patients, American Chemical Society (ACS), doi:10.26434/chemrxiv.12630539.v1.
33.
Lehrer et al., Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, In Vivo, 34:5, 3023-3026, doi:10.21873/invivo.12134.
34.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
35.
Kofler et al., M-Motif, a potential non-conventional NLS in YAP/TAZ and other cellular and viral proteins that inhibits classic protein import, iScience, doi:10.1016/j.isci.2025.112105.
36.
Shahin et al., The selective effect of Ivermectin on different human coronaviruses; in-vitro study, Research Square, doi:10.21203/rs.3.rs-4180797/v1.
37.
Jitobaom et al., Identification of inositol monophosphatase as a broad‐spectrum antiviral target of ivermectin, Journal of Medical Virology, doi:10.1002/jmv.29552.
38.
Fauquet et al., Microfluidic Diffusion Sizing Applied to the Study of Natural Products and Extracts That Modulate the SARS-CoV-2 Spike RBD/ACE2 Interaction, Molecules, doi:10.3390/molecules28248072.
39.
García-Aguilar et al., In Vitro Analysis of SARS-CoV-2 Spike Protein and Ivermectin Interaction, International Journal of Molecular Sciences, doi:10.3390/ijms242216392.
40.
Liu et al., SARS-CoV-2 viral genes Nsp6, Nsp8, and M compromise cellular ATP levels to impair survival and function of human pluripotent stem cell-derived cardiomyocytes, Stem Cell Research & Therapy, doi:10.1186/s13287-023-03485-3.
41.
Boschi et al., SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects, bioRxiv, doi:10.1101/2022.11.24.517882.
42.
De Forni et al., Synergistic drug combinations designed to fully suppress SARS-CoV-2 in the lung of COVID-19 patients, PLoS ONE, doi:10.1371/journal.pone.0276751.
43.
Saha (B) et al., Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder, Pharmaceutics, doi:10.3390/pharmaceutics14071432.
44.
Jitobaom (B) et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8.
45.
Croci et al., Liposomal Systems as Nanocarriers for the Antiviral Agent Ivermectin, International Journal of Biomaterials, doi:10.1155/2016/8043983.
46.
Zheng et al., Red blood cell-hitchhiking mediated pulmonary delivery of ivermectin: Effects of nanoparticle properties, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121719.
47.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
48.
Liu (B) et al., Genome-wide analyses reveal the detrimental impacts of SARS-CoV-2 viral gene Orf9c on human pluripotent stem cell-derived cardiomyocytes, Stem Cell Reports, doi:10.1016/j.stemcr.2022.01.014.
49.
Segatori et al., Effect of Ivermectin and Atorvastatin on Nuclear Localization of Importin Alpha and Drug Target Expression Profiling in Host Cells from Nasopharyngeal Swabs of SARS-CoV-2- Positive Patients, Viruses, doi:10.3390/v13102084.
50.
Jitobaom (C) et al., Favipiravir and Ivermectin Showed in Vitro Synergistic Antiviral Activity against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-941811/v1.
51.
Munson et al., Niclosamide and ivermectin modulate caspase-1 activity and proinflammatory cytokine secretion in a monocytic cell line, British Society For Nanomedicine Early Career Researcher Summer Meeting, 2021, web.archive.org/web/20230401070026/https://michealmunson.github.io/COVID.pdf.
52.
Mountain Valley MD, Mountain Valley MD Receives Successful Results From BSL-4 COVID-19 Clearance Trial on Three Variants Tested With Ivectosol™, 5/18, www.globenewswire.com/en/news-release/2021/05/18/2231755/0/en/Mountain-Valley-MD-Receives-Successful-Results-From-BSL-4-COVID-19-Clearance-Trial-on-Three-Variants-Tested-With-Ivectosol.html.
53.
Yesilbag et al., Ivermectin also inhibits the replication of bovine respiratory viruses (BRSV, BPIV-3, BoHV-1, BCoV and BVDV) in vitro, Virus Research, doi:10.1016/j.virusres.2021.198384.
54.
Mody et al., Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents, Communications Biology, doi:10.1038/s42003-020-01577-x.
55.
Jeffreys et al., Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2022.106542.
56.
Surnar et al., Clinically Approved Antiviral Drug in an Orally Administrable Nanoparticle for COVID-19, ACS Pharmacol. Transl. Sci., doi:10.1021/acsptsci.0c00179.
57.
Li et al., Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment, J. Cellular Physiology, doi:10.1002/jcp.30055.
58.
Caly et al., The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research, doi:10.1016/j.antiviral.2020.104787.
59.
Zhang et al., Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflammation Research, doi:10.1007/s00011-008-8007-8.
60.
Gao et al., Ivermectin ameliorates acute myocarditis via the inhibition of importin-mediated nuclear translocation of NF-κB/p65, International Immunopharmacology, doi:10.1016/j.intimp.2024.112073.
61.
Abd-Elmawla et al., Suppression of NLRP3 inflammasome by ivermectin ameliorates bleomycin-induced pulmonary fibrosis, Journal of Zhejiang University-SCIENCE B, doi:10.1631/jzus.B2200385.
62.
Uematsu et al., Prophylactic administration of ivermectin attenuates SARS-CoV-2 induced disease in a Syrian Hamster Model, The Journal of Antibiotics, doi:10.1038/s41429-023-00623-0.
63.
Albariqi et al., Pharmacokinetics and Safety of Inhaled Ivermectin in Mice as a Potential COVID-19 Treatment, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121688.
64.
Errecalde et al., Safety and Pharmacokinetic Assessments of a Novel Ivermectin Nasal Spray Formulation in a Pig Model, Journal of Pharmaceutical Sciences, doi:10.1016/j.xphs.2021.01.017.
65.
Madrid et al., Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation, Heliyon, doi:10.1016/j.heliyon.2020.e05820.
66.
Ma et al., Ivermectin contributes to attenuating the severity of acute lung injury in mice, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2022.113706.
67.
de Melo et al., Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin, EMBO Mol. Med., doi:10.15252/emmm.202114122.
68.
Arévalo et al., Ivermectin reduces in vivo coronavirus infection in a mouse experimental model, Scientific Reports, doi:10.1038/s41598-021-86679-0.
69.
Chaccour et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Scientific Reports, doi:10.1038/s41598-020-74084-y.
70.
Yan et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflammation Research, doi:10.1007/s00011-011-0307-8.
71.
Götz et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Scientific Reports, doi:10.1038/srep23138.
72.
Tay et al., Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Research, doi:10.1016/j.antiviral.2013.06.002.
73.
Wagstaff et al., Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochemical Journal, doi:10.1042/BJ20120150.
74.
Wagstaff (B) et al., An AlphaScreen®-Based Assay for High-Throughput Screening for Specific Inhibitors of Nuclear Import, SLAS Discovery, doi:10.1177/1087057110390360.
75.
Barrows et al., A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection, Cell Host & Microbe, doi:10.1016/j.chom.2016.07.004.
76.
Yang et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Research, doi:10.1016/j.antiviral.2020.104760.
77.
Mastrangelo et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dks147.
78.
Varghese et al., Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses, Antiviral Research, doi:10.1016/j.antiviral.2015.12.012.
79.
Bennett et al., Role of a nuclear localization signal on the minor capsid Proteins VP2 and VP3 in BKPyV nuclear entry, Virology, doi:10.1016/j.virol.2014.10.013.
80.
Kosyna et al., The importin α/β-specific inhibitor Ivermectin affects HIF-dependent hypoxia response pathways, Biological Chemistry, doi:10.1515/hsz-2015-0171.
81.
Scheim et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
82.
Liu (C) et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
83.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
84.
Scheim (B), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
85.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
86.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
87.
DiNicolantonio et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
88.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
89.
Hazan et al., Treatment with Ivermectin Increases the Population of Bifidobacterium in the Gut, ACG 2023, acg2023posters.eventscribe.net/posterspeakers.asp.
Kalhor et al., 24 Sep 2020, Iran, peer-reviewed, 5 authors.
Contact: rahimi.h1981@gmail.com.
In silico studies are an important part of preclinical research, however results may be very different in vivo.
Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches
Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1824816
Most recently, the new coronavirus (SARS-CoV-2) has been recognized as a pandemic by the World Health Organization (WHO) while this virus shares substantial similarity with SARS-CoV. So far, no definitive vaccine or drug has been developed to cure Covid-19 disease, since many important aspects about Covid-19 such as pathogenesis and proliferation pathways are still unclear. It was proven that human ACE2 is the main receptor for the entry of Covid-19 into lower respiratory tract epithelial cells through interaction with SARS-CoV-2 S protein. Based on this observation, it is expected that the virus infection can be inhibited if protein-protein interaction is prevented. In this study, using structurebased virtual screening of FDA databases, several lead drugs were discovered based on the ACE2binding pocket of SARS-CoV-2 S protein. Then, binding affinity, binding modes, critical interactions, and pharmaceutical properties of the lead drugs were evaluated. Among the previously approved drugs, Diammonium Glycyrrhizinate, Digitoxin, Ivermectin, Rapamycin, Rifaximin, and Amphotericin B represented the most desirable features, and can be possible candidates for Covid-19 therapies. Furthermore, molecular dynamics (MD) simulation was accomplished for three S protein/drug complexes with the highest binding affinity and best conformation and binding free energies were also computed with the Molecular Mechanics/Poisson-Boltzmann Surface Area (MM/PBSA) method. Results demonstrated the stable binding of these compounds to the S protein; however, in order to confirm the curative effect of these drugs, clinical trials must be done.
References
Abraham, Murtola, Schulz, Smith, Hess et al., GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers, SoftwareX, doi:10.1016/j.softx.2015.06.001
Agostini, Andres, Sims, Graham, Sheahan et al., Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease, mBio, doi:10.1128/mBio.00221-18
Alobid, Bernal, Calvo, Vilaseca, Berenguer et al., Treatment of rhinocerebral mucormycosis by combination of endoscopic sinus debridement and amphotericin B, American Journal of Rhinology, doi:10.1177/1945892401
Amarelle, Lecuona, The antiviral effects of Na, K-ATPase inhibition: A minireview, International Journal of Molecular Sciences, doi:10.3390/ijms19082154
Arase, Ikeda, Murashima, Chayama, Tsubota et al., The long term efficacy of glycyrrhizin in chronic hepatitis C patients, Cancer, doi:10.1002/(sici)1097-0142(19970415)79:81494::aid-cncr83.0.co;2-b
Athanasios, Psychos, Domenikos, Communication: Novel drug combination Doxycycline-Melatonin-Digoxin (D-M-D) for possible Covid-19 treatment, Journal of Modern Medicinal Chemistry, doi:10.12970/2308-8044.2020.08.01
Baba, Shigeta, Antiviral activity of glycyrrhizin against varicella-zoster virus in vitro, Antiviral Research, doi:10.1016/0166-3542(87)90025-8
Bussi, Donadio, Parrinello, Canonical sampling through velocity rescaling, The Journal of Chemical Physics, doi:10.1063/1.2408420
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research, doi:10.1016/j.antiviral.2020.104787
Chang, Tung, Lee, Chen, Hsiao et al., Potential Therapeutic Agents for COVID-19 Based on the Analysis of Protease and RNA Polymerase Docking, Preprints, doi:10.20944/preprints202002.0242.v2
Cinatl, Morgenstern, Bauer, Chandra, Rabenau et al., Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus, The Lancet, doi:10.1016/S0140-6736(03)13615-X
Darden, York, Pedersen, Particle mesh Ewald: An NÁ log (N) method for Ewald sums in large systems, The Journal of Chemical Physics, doi:10.1063/1.464397
De Oliveira, Rocha, Paluch, Costa, Repurposing approved drugs as inhibitors of SARS-CoV-2 S-protein from molecular modeling and virtual screening, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1772885
Delano, Pymol: An open-source molecular graphics tool, CCP4 Newsletter on Protein Crystallography
Elfiky, Anti-HCV, nucleotide inhibitors, repurposing against COVID-19, Life Sciences, doi:10.1016/j.lfs.2020.117477
Elfiky, Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study, Life Sciences, doi:10.1016/j.lfs.2020.117592
Feng, Wang, Yao, Zhang, Tian, Diammonium glycyrrhizinate, a component of traditional Chinese medicine Gan-Cao, prevents murine T-cell-mediated fulminant hepatitis in IL-10-and IL-6dependent manners, International Immunopharmacology, doi:10.1016/j.intimp.2007.05.011
Gupta, Ammonium glycyrrhizinate: a compherensive review of its traditionaluse, phytochemistry, pharmacology & safety, Asian Journal of Pharmaceutical Education and Research
G€ Otz, Magar, Dornfeld, Giese, Pohlmann et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Scientific Reports, doi:10.1038/srep23138
Hackett, Sylvester, Joss, Calvin, Synergistic effect of rifamycin derivatives and amphotericin B on viral transformation of a murine cell line, Proceedings of the National Academy of Sciences, doi:10.1073/pnas.69.12.3653
Hess, Bekker, Berendsen, Fraaije, Ito et al., Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV), Journal of Computational Chemistry, doi:10.1016/0166-3542(88)90047-2
Jin, Du, Xu, Deng, Liu et al., Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors, Nature, doi:10.1038/s41586-020-2223-y
Kalhor, Rahimi, Akbari Eidgahi, Teimoori-Toolabi, Novel small molecules against two binding sites of Wnt2 protein as potential drug candidates for colorectal cancer: A structure based virtual screening approach, Iranian Journal of Pharmaceutical Research, doi:10.22037/IJPR.2019.15297.13037
Kalhor, Sadeghi, Marashiyan, Kalhor, Aghaei Gharehbolagh et al., Identification of new DNA gyrase inhibitors based on bioactive compounds from streptomyces: Structure-based virtual screening and molecular dynamics simulations approaches, Journal of Biomolecular Structure & Dynamics, doi:10.1080/07391102.2019.1588784
Kandeel, Al-Nazawi, Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease, Life Sciences, doi:10.1016/j.lfs.2020.117627
Koes, Baumgartner, Camacho, Lessons learned in empirical scoring with smina from the CSAR 2011 benchmarking exercise, Journal of Chemical Information and Modeling, doi:10.1021/ci300604z
Kumari, Kumar, Consortium, Lynn, g_mmpbsaA A GROMACS tool for high-throughput MM-PBSA calculations, Journal of Chemical Information and Modeling, doi:10.1021/ci500020m
Laskowski, PDBsum new things, Nucleic Acids Research, doi:10.1093/nar/gkn860
Laskowski, Swindells, LigPlotþ: Multiple ligand-protein interaction diagrams for drug discovery, ACS Publications, doi:10.1021/ci200227u
Li, Kim, Blenis, Rapamycin: One drug, many effects, Cell Metabolism, doi:10.1016/j.cmet.2014.01.001
Li, Li, Farzan, Harrison, Structure of SARS coronavirus spike receptor-binding domain complexed with receptor, Science, doi:10.1126/science.1116480
Lin, Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro, Antiviral Research, doi:10.1016/S0166-3542(03)00030-5
Lipinski, Lombardo, Dominy, Feeney, Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Advanced Drug Delivery Reviews, doi:10.1016/S0169-409X(00)00129-0
Lundberg, Pinkham, Baer, Amaya, Narayanan et al., Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication, Antiviral Research, doi:10.1016/j.antiviral.2013.10.004
Mohammadi, Kometiani, Xie, Askari, Role of protein kinase C in the signal pathways that link Naþ/Kþ-ATPase to ERK1/2, Journal of Biological Chemistry, doi:10.1074/jbc.M107892200
Morgenstern, Michaelis, Baer, Doerr, Cinatl, Ribavirin and interferon-b synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines, Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2004.11.128
Morris, Huey, Lindstrom, Sanner, Belew et al., AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility, Journal of Computational Chemistry, doi:10.1002/jcc.21256
Mukhtar, Mukhtar, Coronavirus (COVID-19): Let's prevent not panic, Journal of Ayub Medical College Abbottabad
Numazaki, Nagata, Sato, Chiba, Effect of glycyrrhizin, cyclosporin A, and tumor necrosis factor a on infection of U-937 and MRC-5 cells by human cytomegalovirus, Journal of Leukocyte Biology, doi:10.1002/jlb.55.1.24
O'boyle, Banck, James, Morley, Vandermeersch et al., Open Babel: An open chemical toolbox, Journal of Cheminformatics, doi:10.1021/ci200227u
Parrinello, Rahman, Polymorphic transitions in single crystals: A new molecular dynamics method Polymorphic transitions in single crystals: A new molecular dynamics method, Journal of Applied Physics, doi:10.1063/1.328693
Pollard, Pollard, Pollard, Classical drug digitoxin inhibits influenza cytokine storm, with implications for COVID-19 therapy, bioRxiv, doi:10.1101/2020.04.09.034983
Rismanbaf, Potential treatments for COVID-19; a narrative literature review, Archives of Academic Emergency Medicine
Roggo, Natural products in drug discovery, CHIMIA International Journal for Chemistry, doi:10.2533/chimia.2007.312
Sander, Freyss, Von Korff, Rufener, DataWarrior: An open-source program for chemistry aware data visualization and analysis, Journal of Chemical Information and Modeling, doi:10.1021/ci500588j
Sato, Goto, Yamamura, Kurokawa, Kageyama et al., Therapeutic basis of glycyrrhizin on chronic hepatitis B, Antiviral Research, doi:10.1016/0166-3542(96)00942-4
Sch€ Uttelkopf, Van Aalten, PRODRG: A tool for highthroughput crystallography of protein-ligand complexes, Acta Crystallographica Section D Biological Crystallography, doi:10.1107/S0907444904011679
Sekhar, Virtual screening based prediction of potential drugs for COVID-19, Preprints, doi:10.20944/preprints202002.0418.v2
Shang, Ye, Shi, Wan, Luo et al., Structural basis of receptor recognition by SARS-CoV-2, Nature, doi:10.1038/s41586-020-2179-y
Shanmugaraj, Malla, Phoolcharoen, Emergence of novel coronavirus 2019-nCoV: Need for rapid vaccine and biologics development, Pathogens, doi:10.3390/pathogens9020148
Shayto, Mrad, Sharara, Use of rifaximin in gastrointestinal and liver diseases, World Journal of Gastroenterology, doi:10.1038/s41421-020-0153-3
Shiri, Pirhadi, Ghasemi, Dynamic structure based pharmacophore modeling of the Acetylcholinesterase reveals several potential inhibitors, Journal of Biomolecular Structure & Dynamics, doi:10.1080/07391102.2018.1468281
Shiri, Pirhadi, Rahmani, Identification of new potential HIV-1 reverse transcriptase inhibitors by QSAR modeling and structure-based virtual screening, Journal of Receptor and Signal Transduction Research, doi:10.1080/10799893.2017.1414844
Siddell, Anderson, Cavanagh, Fujiwara, Klenk et al., Coronaviridae, Intervirology, doi:10.1159/000149390
Song, Gui, Wang, Xiang, Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2, PLOS Pathogens, doi:10.1371/journal.ppat.1007236
St€ Ohr, Costa, Sandmann, Westhaus, Pfaender et al., Host cell mTORC1 is required for HCV RNA replication, Gut, doi:10.1136/gutjnl-2014-308971
Tay, Fraser, Chan, Moreland, Rathore et al., Nuclear ocalization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENVserotypes by the inhibitor Ivermectin, Antiviral Research, doi:10.1016/j.antiviral.2013.06.002
Thakur, Raj, Pharmacological perspective of Glycyrrhiza glabra Linn: A mini-review, Journal of Analytical & Pharmaceutical Research, doi:10.15406/japlr.2017.05.00156
Utsunomiya, Kobayashi, Herndon, Pollard, Suzuki et al., Glycyrrhizin(20b-carboxy-11-oxo-30-norolean-12-en-3b-yl-2-Ob-d-glucopyranuronosyl-a-d-glucopyranosiduronic acid) improves the resistance of thermally injured mice to opportunistic infection of herpes simplex virus type 1, Antimicrobial Agents and Chemotherapy, doi:10.1128/AAC.41.3.551
Van Tilbeurgh, Bezzine, Cambillau, Verger, Carriere, Colipase: Structure and interaction with pancreatic lipase, Biochimica et Biophysica Acta (Bba) -Molecular and Cell Biology of Lipids, doi:10.1016/S1388-1981(99)00149-3
Visualizer, Version 4.5 (software
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin a/b-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, The Biochemical Journal, doi:10.1042/BJ20120150
Wrapp, Wang, Corbett, Goldsmith, Hsieh et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, doi:10.1126/science.abb2507
Wu, Zhao, Yu, Chen, Wang et al., A new coronavirus associated with human respiratory disease in China, Nature, doi:10.1038/s41586-020-2008-3
Xu, Chen, Wang, Feng, Zhou et al., Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission, Science China Life Sciences, doi:10.1007/s11427-020-1637-5
Yu, Sun, Macrocyclic drugs and synthetic methodologies toward macrocycles, Molecules, doi:10.3390/molecules18066230
Zhou, Hou, Shen, Huang, Martin et al., Network-based drug repurposing for novel coronavirus 2019-nCoV/ SARS-CoV-2, Cell Discovery, doi:10.1038/s41421-020-0153-3
Zhu, Zhang, Wang, Li, Yang et al., A novel coronavirus from patients with pneumonia in China, 2019, The New England Journal of Medicine, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.1080/07391102.2020.1824816",
"ISSN": [
"0739-1102",
"1538-0254"
],
"URL": "http://dx.doi.org/10.1080/07391102.2020.1824816",
"alternative-id": [
"10.1080/07391102.2020.1824816"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=tbsd20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=tbsd20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-08-01"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2020-09-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2020-09-24"
}
],
"author": [
{
"affiliation": [
{
"name": "Cellular and Molecular Research Center, Qom University of Medical Sciences, Qom, Iran"
},
{
"name": "Molecular Medicine Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran"
}
],
"family": "Kalhor",
"given": "Hourieh",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-5861-2606",
"affiliation": [
{
"name": "Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran"
}
],
"authenticated-orcid": false,
"family": "Sadeghi",
"given": "Solmaz",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9174-5304",
"affiliation": [
{
"name": "Cellular and Molecular Research Center, Qom University of Medical Sciences, Qom, Iran"
},
{
"name": "Spiritual Health Research Center, Qom University of Medical Sciences, Qom, Iran"
},
{
"name": "Department of Pharmacology, School of Medicine, Qom University of Medical Sciences, Qom, Iran"
}
],
"authenticated-orcid": false,
"family": "Abolhasani",
"given": "Hoda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cellular and Molecular Research Center, Qom University of Medical Sciences, Qom, Iran"
},
{
"name": "Department of Genetics, Colleague of Sciences, Kazerun branch, Islamic Azad University, Kazerun, Iran"
}
],
"family": "Kalhor",
"given": "Reyhaneh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Molecular Medicine Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran"
}
],
"family": "Rahimi",
"given": "Hamzeh",
"sequence": "additional"
}
],
"container-title": "Journal of Biomolecular Structure and Dynamics",
"container-title-short": "Journal of Biomolecular Structure and Dynamics",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2020,
9,
24
]
],
"date-time": "2020-09-24T12:10:25Z",
"timestamp": 1600949425000
},
"deposited": {
"date-parts": [
[
2022,
1,
31
]
],
"date-time": "2022-01-31T13:12:23Z",
"timestamp": 1643634743000
},
"indexed": {
"date-parts": [
[
2024,
2,
20
]
],
"date-time": "2024-02-20T10:18:49Z",
"timestamp": 1708424329552
},
"is-referenced-by-count": 30,
"issue": "3",
"issued": {
"date-parts": [
[
2020,
9,
24
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2022,
2,
11
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/07391102.2020.1824816",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "1299-1315",
"prefix": "10.1080",
"published": {
"date-parts": [
[
2020,
9,
24
]
]
},
"published-online": {
"date-parts": [
[
2020,
9,
24
]
]
},
"published-print": {
"date-parts": [
[
2022,
2,
11
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1016/j.softx.2015.06.001",
"doi-asserted-by": "publisher",
"key": "CIT0001"
},
{
"DOI": "10.1128/mBio.00221-18",
"doi-asserted-by": "publisher",
"key": "CIT0002"
},
{
"DOI": "10.1177/194589240101500508",
"doi-asserted-by": "publisher",
"key": "CIT0003"
},
{
"DOI": "10.3390/ijms19082154",
"doi-asserted-by": "publisher",
"key": "CIT0004"
},
{
"DOI": "10.1002/(SICI)1097-0142(19970415)79:8<1494::AID-CNCR8>3.0.CO;2-B",
"doi-asserted-by": "publisher",
"key": "CIT0005"
},
{
"DOI": "10.12970/2308-8044.2020.08.01",
"doi-asserted-by": "publisher",
"key": "CIT0006"
},
{
"DOI": "10.1016/0166-3542(87)90025-8",
"doi-asserted-by": "publisher",
"key": "CIT0007"
},
{
"DOI": "10.1063/1.2408420",
"doi-asserted-by": "publisher",
"key": "CIT0008"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"doi-asserted-by": "publisher",
"key": "CIT0009"
},
{
"DOI": "10.20944/preprints202002.0242.v2",
"doi-asserted-by": "publisher",
"key": "CIT0010"
},
{
"DOI": "10.1016/S0140-6736(03)13615-X",
"doi-asserted-by": "publisher",
"key": "CIT0011"
},
{
"DOI": "10.1063/1.464397",
"doi-asserted-by": "publisher",
"key": "CIT0012"
},
{
"DOI": "10.1080/07391102.2020.1772885",
"doi-asserted-by": "publisher",
"key": "CIT0013"
},
{
"author": "DeLano W. L.",
"first-page": "82",
"journal-title": "CCP4 Newsletter on Protein Crystallography",
"key": "CIT0014",
"volume": "40",
"year": "2002"
},
{
"DOI": "10.1016/j.lfs.2020.117477",
"doi-asserted-by": "publisher",
"key": "CIT0015"
},
{
"DOI": "10.1016/j.lfs.2020.117592",
"doi-asserted-by": "publisher",
"key": "CIT0016"
},
{
"DOI": "10.1016/j.intimp.2007.05.011",
"doi-asserted-by": "publisher",
"key": "CIT0017"
},
{
"DOI": "10.1038/srep23138",
"doi-asserted-by": "publisher",
"key": "CIT0018"
},
{
"DOI": "10.1073/pnas.69.12.3653",
"doi-asserted-by": "publisher",
"key": "CIT0019"
},
{
"DOI": "10.1002/(SICI)1096-987X(199709)18:12<1463::AID-JCC4>3.0.CO;2-H",
"doi-asserted-by": "publisher",
"key": "CIT0020"
},
{
"DOI": "10.1016/0166-3542(88)90047-2",
"doi-asserted-by": "publisher",
"key": "CIT0021"
},
{
"DOI": "10.1038/s41586-020-2223-y",
"doi-asserted-by": "publisher",
"key": "CIT0022"
},
{
"author": "Kalhor H.",
"issue": "2",
"journal-title": "Iranian Journal of Pharmaceutical Research",
"key": "CIT0023",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1080/07391102.2019.1588784",
"doi-asserted-by": "publisher",
"key": "CIT0024"
},
{
"DOI": "10.1016/j.lfs.2020.117627",
"doi-asserted-by": "publisher",
"key": "CIT0025"
},
{
"DOI": "10.1021/ci300604z",
"doi-asserted-by": "publisher",
"key": "CIT0026"
},
{
"DOI": "10.1021/ci500020m",
"doi-asserted-by": "publisher",
"key": "CIT0027"
},
{
"DOI": "10.1093/nar/gkn860",
"doi-asserted-by": "publisher",
"key": "CIT0028"
},
{
"author": "Laskowski R. A.",
"key": "CIT0029",
"volume-title": "LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery",
"year": "2011"
},
{
"DOI": "10.1016/j.cmet.2014.01.001",
"doi-asserted-by": "publisher",
"key": "CIT0030"
},
{
"DOI": "10.1126/science.1116480",
"doi-asserted-by": "publisher",
"key": "CIT0031"
},
{
"DOI": "10.1016/S0166-3542(03)00030-5",
"doi-asserted-by": "publisher",
"key": "CIT0032"
},
{
"DOI": "10.1016/j.addr.2012.09.019",
"doi-asserted-by": "publisher",
"key": "CIT0033"
},
{
"DOI": "10.1016/j.antiviral.2013.10.004",
"doi-asserted-by": "publisher",
"key": "CIT0034"
},
{
"DOI": "10.1074/jbc.M107892200",
"doi-asserted-by": "publisher",
"key": "CIT0035"
},
{
"DOI": "10.1016/j.bbrc.2004.11.128",
"doi-asserted-by": "publisher",
"key": "CIT0036"
},
{
"DOI": "10.1002/jcc.21256",
"doi-asserted-by": "publisher",
"key": "CIT0037"
},
{
"author": "Mukhtar F.",
"first-page": "141",
"issue": "1",
"journal-title": "Journal of Ayub Medical College Abbottabad",
"key": "CIT0038",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.1002/jlb.55.1.24",
"doi-asserted-by": "publisher",
"key": "CIT0039"
},
{
"DOI": "10.1186/1758-2946-3-33",
"doi-asserted-by": "publisher",
"key": "CIT0040"
},
{
"DOI": "10.1063/1.328693",
"doi-asserted-by": "publisher",
"key": "CIT0041"
},
{
"author": "Pollard H. B.",
"journal-title": "bioRxiv",
"key": "CIT0042",
"year": "2020"
},
{
"author": "Rismanbaf A.",
"issue": "1",
"journal-title": "Archives of Academic Emergency Medicine",
"key": "CIT0043",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.2533/chimia.2007.312",
"doi-asserted-by": "publisher",
"key": "CIT0044"
},
{
"DOI": "10.1021/ci500588j",
"doi-asserted-by": "publisher",
"key": "CIT0045"
},
{
"DOI": "10.1016/0166-3542(96)00942-4",
"doi-asserted-by": "publisher",
"key": "CIT0046"
},
{
"DOI": "10.1107/S0907444904011679",
"doi-asserted-by": "publisher",
"key": "CIT0047"
},
{
"DOI": "10.20944/preprints202002.0418.v2",
"doi-asserted-by": "crossref",
"key": "CIT0048",
"unstructured": "Sekhar, T. (2020). Virtual screening based prediction of potential drugs for COVID-19. Preprints.org. https://doi.org/10.20944/preprints202002.0418.v2"
},
{
"DOI": "10.1038/s41586-020-2179-y",
"doi-asserted-by": "publisher",
"key": "CIT0049"
},
{
"DOI": "10.3390/pathogens9020148",
"doi-asserted-by": "publisher",
"key": "CIT0050"
},
{
"DOI": "10.3748/wjg.v22.i29.6638",
"doi-asserted-by": "publisher",
"key": "CIT0051"
},
{
"DOI": "10.1080/07391102.2018.1468281",
"doi-asserted-by": "publisher",
"key": "CIT0052"
},
{
"DOI": "10.1080/10799893.2017.1414844",
"doi-asserted-by": "publisher",
"key": "CIT0053"
},
{
"DOI": "10.1159/000149390",
"doi-asserted-by": "publisher",
"key": "CIT0054"
},
{
"DOI": "10.1371/journal.ppat.1007236",
"doi-asserted-by": "publisher",
"key": "CIT0055"
},
{
"DOI": "10.1136/gutjnl-2014-308971",
"doi-asserted-by": "publisher",
"key": "CIT0056"
},
{
"DOI": "10.1016/j.antiviral.2013.06.002",
"doi-asserted-by": "publisher",
"key": "CIT0057"
},
{
"DOI": "10.15406/japlr.2017.05.00156",
"doi-asserted-by": "publisher",
"key": "CIT0058"
},
{
"author": "Tinku Gupta M. M.",
"first-page": "58",
"issue": "1",
"journal-title": "Asian Journal of Pharmaceutical Education and Research",
"key": "CIT0059",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1016/0165-2478(94)00183-R",
"doi-asserted-by": "publisher",
"key": "CIT0060"
},
{
"DOI": "10.1128/AAC.41.3.551",
"doi-asserted-by": "publisher",
"key": "CIT0061"
},
{
"DOI": "10.1016/S1388-1981(99)00149-3",
"doi-asserted-by": "publisher",
"key": "CIT0062"
},
{
"key": "CIT0063",
"unstructured": "Visualizer, B. D. S. (2017). Version 4.5 (software). http://mayavi.sourceforge.net"
},
{
"DOI": "10.1042/BJ20120150",
"doi-asserted-by": "publisher",
"key": "CIT0064"
},
{
"DOI": "10.1126/science.abb2507",
"doi-asserted-by": "publisher",
"key": "CIT0065"
},
{
"DOI": "10.1038/s41586-020-2008-3",
"doi-asserted-by": "publisher",
"key": "CIT0066"
},
{
"DOI": "10.1007/s11427-020-1637-5",
"doi-asserted-by": "publisher",
"key": "CIT0067"
},
{
"DOI": "10.3390/molecules18066230",
"doi-asserted-by": "publisher",
"key": "CIT0068"
},
{
"DOI": "10.1038/s41421-020-0153-3",
"doi-asserted-by": "publisher",
"key": "CIT0069"
},
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "CIT0070"
}
],
"reference-count": 70,
"references-count": 70,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/07391102.2020.1824816"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Molecular Biology",
"General Medicine",
"Structural Biology"
],
"subtitle": [],
"title": "Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01",
"volume": "40"
}
