Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach
et al., International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.09.098, Nov 2020
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
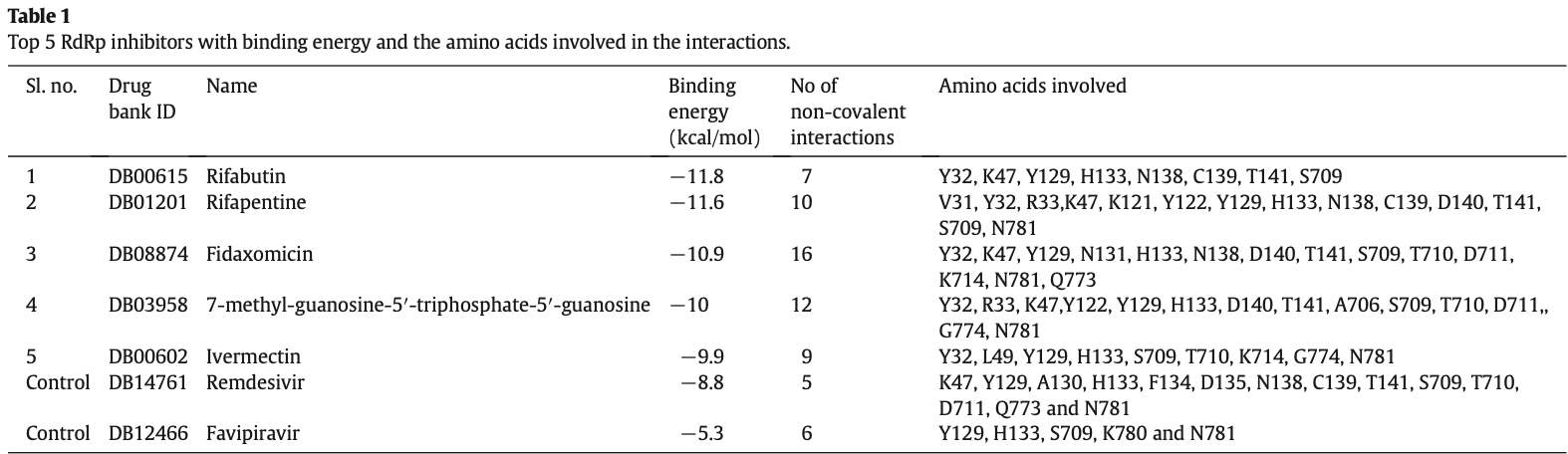
In silico study showing that ivermectin, rifabutin, rifapentine, fidaxomicin, and 7-methyl-guanosine-5′-triphosphate-5′-guanosine could be potential inhibitors of the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp). Authors used molecular docking to screen 44 RNA polymerase inhibitor drug candidates, finding that these five compounds had the highest binding affinity to RdRp, even higher than remdesivir and favipiravir which were used as positive controls. The amino acids Y32, K47, Y122, Y129, H133, N138, D140, T141, S709 and N781 in RdRp were identified as a potential common drug surface hotspot for interaction with the inhibitors.
74 preclinical studies support the efficacy of ivermectin for COVID-19:
Ivermectin, better known for antiparasitic activity, is a broad spectrum antiviral with activity against many viruses including H7N771, Dengue37,72,73 , HIV-173, Simian virus 4074, Zika37,75,76 , West Nile76, Yellow Fever77,78, Japanese encephalitis77, Chikungunya78, Semliki Forest virus78, Human papillomavirus57, Epstein-Barr57, BK Polyomavirus79, and Sindbis virus78.
Ivermectin inhibits importin-α/β-dependent nuclear import of viral proteins71,73,74,80 , shows spike-ACE2 disruption at 1nM with microfluidic diffusional sizing38, binds to glycan sites on the SARS-CoV-2 spike protein preventing interaction with blood and epithelial cells and inhibiting hemagglutination41,81, shows dose-dependent inhibition of wildtype and omicron variants36, exhibits dose-dependent inhibition of lung injury61,66, may inhibit SARS-CoV-2 via IMPase inhibition37, may inhibit SARS-CoV-2 induced formation of fibrin clots resistant to degradation9, inhibits SARS-CoV-2 3CLpro54, may inhibit SARS-CoV-2 RdRp activity28, may minimize viral myocarditis by inhibiting NF-κB/p65-mediated inflammation in macrophages60, may be beneficial for COVID-19 ARDS by blocking GSDMD and NET formation82, may interfere with SARS-CoV-2's immune evasion via ORF8 binding4, may inhibit SARS-CoV-2 by disrupting CD147 interaction83-86, may inhibit SARS-CoV-2 attachment to lipid rafts via spike NTD binding2, shows protection against inflammation, cytokine storm, and mortality in an LPS mouse model sharing key pathological features of severe COVID-1959,87, may be beneficial in severe COVID-19 by binding IGF1 to inhibit the promotion of inflammation, fibrosis, and cell proliferation that leads to lung damage8, may minimize SARS-CoV-2 induced cardiac damage40,48, may counter immune evasion by inhibiting NSP15-TBK1/KPNA1 interaction and restoring IRF3 activation88, may disrupt SARS-CoV-2 N and ORF6 protein nuclear transport and their suppression of host interferon responses1, reduces TAZ/YAP nuclear import, relieving SARS-CoV-2-driven suppression of IRF3 and NF-κB antiviral pathways35, increases Bifidobacteria which play a key role in the immune system89, has immunomodulatory51 and anti-inflammatory70,90 properties, and has an extensive and very positive safety profile91.
1.
Gayozo et al., Binding affinities analysis of ivermectin, nucleocapsid and ORF6 proteins of SARS-CoV-2 to human importins α isoforms: A computational approach, Biotecnia, doi:10.18633/biotecnia.v27.2485.
2.
Lefebvre et al., Characterization and Fluctuations of an Ivermectin Binding Site at the Lipid Raft Interface of the N-Terminal Domain (NTD) of the Spike Protein of SARS-CoV-2 Variants, Viruses, doi:10.3390/v16121836.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Bagheri-Far et al., Non-spike protein inhibition of SARS-CoV-2 by natural products through the key mediator protein ORF8, Molecular Biology Research Communications, doi:10.22099/mbrc.2024.50245.2001.
5.
de Oliveira Só et al., In Silico Comparative Analysis of Ivermectin and Nirmatrelvir Inhibitors Interacting with the SARS-CoV-2 Main Protease, Preprints, doi:10.20944/preprints202404.1825.v1.
6.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
7.
Oranu et al., Validation of the binding affinities and stabilities of ivermectin and moxidectin against SARS-CoV-2 receptors using molecular docking and molecular dynamics simulation, GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2024.26.1.0030.
8.
Zhao et al., Identification of the shared gene signatures between pulmonary fibrosis and pulmonary hypertension using bioinformatics analysis, Frontiers in Immunology, doi:10.3389/fimmu.2023.1197752.
9.
Vottero et al., Computational Prediction of the Interaction of Ivermectin with Fibrinogen, Molecular Sciences, doi:10.3390/ijms241411449.
10.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
11.
Umar et al., Inhibitory potentials of ivermectin, nafamostat, and camostat on spike protein and some nonstructural proteins of SARS-CoV-2: Virtual screening approach, Jurnal Teknologi Laboratorium, doi:10.29238/teknolabjournal.v11i1.344.
12.
Alvarado et al., Interaction of the New Inhibitor Paxlovid (PF-07321332) and Ivermectin With the Monomer of the Main Protease SARS-CoV-2: A Volumetric Study Based on Molecular Dynamics, Elastic Networks, Classical Thermodynamics and SPT, Computational Biology and Chemistry, doi:10.1016/j.compbiolchem.2022.107692.
13.
Aminpour et al., In Silico Analysis of the Multi-Targeted Mode of Action of Ivermectin and Related Compounds, Computation, doi:10.3390/computation10040051.
14.
Parvez et al., Insights from a computational analysis of the SARS-CoV-2 Omicron variant: Host–pathogen interaction, pathogenicity, and possible drug therapeutics, Immunity, Inflammation and Disease, doi:10.1002/iid3.639.
15.
Francés-Monerris et al., Microscopic interactions between ivermectin and key human and viral proteins involved in SARS-CoV-2 infection, Physical Chemistry Chemical Physics, doi:10.1039/D1CP02967C.
16.
González-Paz et al., Comparative study of the interaction of ivermectin with proteins of interest associated with SARS-CoV-2: A computational and biophysical approach, Biophysical Chemistry, doi:10.1016/j.bpc.2021.106677.
17.
González-Paz (B) et al., Structural Deformability Induced in Proteins of Potential Interest Associated with COVID-19 by binding of Homologues present in Ivermectin: Comparative Study Based in Elastic Networks Models, Journal of Molecular Liquids, doi:10.1016/j.molliq.2021.117284.
18.
Rana et al., A Computational Study of Ivermectin and Doxycycline Combination Drug Against SARS-CoV-2 Infection, Research Square, doi:10.21203/rs.3.rs-755838/v1.
19.
Muthusamy et al., Virtual Screening Reveals Potential Anti-Parasitic Drugs Inhibiting the Receptor Binding Domain of SARS-CoV-2 Spike protein, Journal of Virology & Antiviral Research, www.scitechnol.com/abstract/virtual-screening-reveals-potential-antiparasitic-drugs-inhibiting-the-receptor-binding-domain-of-sarscov2-spike-protein-16398.html.
20.
Qureshi et al., Mechanistic insights into the inhibitory activity of FDA approved ivermectin against SARS-CoV-2: old drug with new implications, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1906750.
21.
Schöning et al., Highly-transmissible Variants of SARS-CoV-2 May Be More Susceptible to Drug Therapy Than Wild Type Strains, Research Square, doi:10.21203/rs.3.rs-379291/v1.
22.
Bello et al., Elucidation of the inhibitory activity of ivermectin with host nuclear importin α and several SARS-CoV-2 targets, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1911857.
23.
Udofia et al., In silico studies of selected multi-drug targeting against 3CLpro and nsp12 RNA-dependent RNA-polymerase proteins of SARS-CoV-2 and SARS-CoV, Network Modeling Analysis in Health Informatics and Bioinformatics, doi:10.1007/s13721-021-00299-2.
24.
Choudhury et al., Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach, Future Medicine, doi:10.2217/fvl-2020-0342.
25.
Kern et al., Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing, Frontiers in Pharmacology, doi:10.3389/fphar.2021.625678.
26.
Saha et al., The Binding mechanism of ivermectin and levosalbutamol with spike protein of SARS-CoV-2, Structural Chemistry, doi:10.1007/s11224-021-01776-0.
27.
Eweas et al., Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2, Frontiers in Microbiology, doi:10.3389/fmicb.2020.592908.
28.
Parvez (B) et al., Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.09.098.
29.
Francés-Monerris (B) et al., Has Ivermectin Virus-Directed Effects against SARS-CoV-2? Rationalizing the Action of a Potential Multitarget Antiviral Agent, ChemRxiv, doi:10.26434/chemrxiv.12782258.v1.
30.
Kalhor et al., Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1824816.
31.
Swargiary, A., Ivermectin as a promising RNA-dependent RNA polymerase inhibitor and a therapeutic drug against SARS-CoV2: Evidence from in silico studies, Research Square, doi:10.21203/rs.3.rs-73308/v1.
32.
Maurya, D., A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID-19 Patients, American Chemical Society (ACS), doi:10.26434/chemrxiv.12630539.v1.
33.
Lehrer et al., Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, In Vivo, 34:5, 3023-3026, doi:10.21873/invivo.12134.
34.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
35.
Kofler et al., M-Motif, a potential non-conventional NLS in YAP/TAZ and other cellular and viral proteins that inhibits classic protein import, iScience, doi:10.1016/j.isci.2025.112105.
36.
Shahin et al., The selective effect of Ivermectin on different human coronaviruses; in-vitro study, Research Square, doi:10.21203/rs.3.rs-4180797/v1.
37.
Jitobaom et al., Identification of inositol monophosphatase as a broad‐spectrum antiviral target of ivermectin, Journal of Medical Virology, doi:10.1002/jmv.29552.
38.
Fauquet et al., Microfluidic Diffusion Sizing Applied to the Study of Natural Products and Extracts That Modulate the SARS-CoV-2 Spike RBD/ACE2 Interaction, Molecules, doi:10.3390/molecules28248072.
39.
García-Aguilar et al., In Vitro Analysis of SARS-CoV-2 Spike Protein and Ivermectin Interaction, International Journal of Molecular Sciences, doi:10.3390/ijms242216392.
40.
Liu et al., SARS-CoV-2 viral genes Nsp6, Nsp8, and M compromise cellular ATP levels to impair survival and function of human pluripotent stem cell-derived cardiomyocytes, Stem Cell Research & Therapy, doi:10.1186/s13287-023-03485-3.
41.
Boschi et al., SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects, bioRxiv, doi:10.1101/2022.11.24.517882.
42.
De Forni et al., Synergistic drug combinations designed to fully suppress SARS-CoV-2 in the lung of COVID-19 patients, PLoS ONE, doi:10.1371/journal.pone.0276751.
43.
Saha (B) et al., Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder, Pharmaceutics, doi:10.3390/pharmaceutics14071432.
44.
Jitobaom (B) et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8.
45.
Croci et al., Liposomal Systems as Nanocarriers for the Antiviral Agent Ivermectin, International Journal of Biomaterials, doi:10.1155/2016/8043983.
46.
Zheng et al., Red blood cell-hitchhiking mediated pulmonary delivery of ivermectin: Effects of nanoparticle properties, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121719.
47.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
48.
Liu (B) et al., Genome-wide analyses reveal the detrimental impacts of SARS-CoV-2 viral gene Orf9c on human pluripotent stem cell-derived cardiomyocytes, Stem Cell Reports, doi:10.1016/j.stemcr.2022.01.014.
49.
Segatori et al., Effect of Ivermectin and Atorvastatin on Nuclear Localization of Importin Alpha and Drug Target Expression Profiling in Host Cells from Nasopharyngeal Swabs of SARS-CoV-2- Positive Patients, Viruses, doi:10.3390/v13102084.
50.
Jitobaom (C) et al., Favipiravir and Ivermectin Showed in Vitro Synergistic Antiviral Activity against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-941811/v1.
51.
Munson et al., Niclosamide and ivermectin modulate caspase-1 activity and proinflammatory cytokine secretion in a monocytic cell line, British Society For Nanomedicine Early Career Researcher Summer Meeting, 2021, web.archive.org/web/20230401070026/https://michealmunson.github.io/COVID.pdf.
52.
Mountain Valley MD, Mountain Valley MD Receives Successful Results From BSL-4 COVID-19 Clearance Trial on Three Variants Tested With Ivectosol™, 5/18, www.globenewswire.com/en/news-release/2021/05/18/2231755/0/en/Mountain-Valley-MD-Receives-Successful-Results-From-BSL-4-COVID-19-Clearance-Trial-on-Three-Variants-Tested-With-Ivectosol.html.
53.
Yesilbag et al., Ivermectin also inhibits the replication of bovine respiratory viruses (BRSV, BPIV-3, BoHV-1, BCoV and BVDV) in vitro, Virus Research, doi:10.1016/j.virusres.2021.198384.
54.
Mody et al., Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents, Communications Biology, doi:10.1038/s42003-020-01577-x.
55.
Jeffreys et al., Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2022.106542.
56.
Surnar et al., Clinically Approved Antiviral Drug in an Orally Administrable Nanoparticle for COVID-19, ACS Pharmacol. Transl. Sci., doi:10.1021/acsptsci.0c00179.
57.
Li et al., Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment, J. Cellular Physiology, doi:10.1002/jcp.30055.
58.
Caly et al., The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research, doi:10.1016/j.antiviral.2020.104787.
59.
Zhang et al., Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflammation Research, doi:10.1007/s00011-008-8007-8.
60.
Gao et al., Ivermectin ameliorates acute myocarditis via the inhibition of importin-mediated nuclear translocation of NF-κB/p65, International Immunopharmacology, doi:10.1016/j.intimp.2024.112073.
61.
Abd-Elmawla et al., Suppression of NLRP3 inflammasome by ivermectin ameliorates bleomycin-induced pulmonary fibrosis, Journal of Zhejiang University-SCIENCE B, doi:10.1631/jzus.B2200385.
62.
Uematsu et al., Prophylactic administration of ivermectin attenuates SARS-CoV-2 induced disease in a Syrian Hamster Model, The Journal of Antibiotics, doi:10.1038/s41429-023-00623-0.
63.
Albariqi et al., Pharmacokinetics and Safety of Inhaled Ivermectin in Mice as a Potential COVID-19 Treatment, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121688.
64.
Errecalde et al., Safety and Pharmacokinetic Assessments of a Novel Ivermectin Nasal Spray Formulation in a Pig Model, Journal of Pharmaceutical Sciences, doi:10.1016/j.xphs.2021.01.017.
65.
Madrid et al., Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation, Heliyon, doi:10.1016/j.heliyon.2020.e05820.
66.
Ma et al., Ivermectin contributes to attenuating the severity of acute lung injury in mice, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2022.113706.
67.
de Melo et al., Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin, EMBO Mol. Med., doi:10.15252/emmm.202114122.
68.
Arévalo et al., Ivermectin reduces in vivo coronavirus infection in a mouse experimental model, Scientific Reports, doi:10.1038/s41598-021-86679-0.
69.
Chaccour et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Scientific Reports, doi:10.1038/s41598-020-74084-y.
70.
Yan et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflammation Research, doi:10.1007/s00011-011-0307-8.
71.
Götz et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Scientific Reports, doi:10.1038/srep23138.
72.
Tay et al., Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Research, doi:10.1016/j.antiviral.2013.06.002.
73.
Wagstaff et al., Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochemical Journal, doi:10.1042/BJ20120150.
74.
Wagstaff (B) et al., An AlphaScreen®-Based Assay for High-Throughput Screening for Specific Inhibitors of Nuclear Import, SLAS Discovery, doi:10.1177/1087057110390360.
75.
Barrows et al., A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection, Cell Host & Microbe, doi:10.1016/j.chom.2016.07.004.
76.
Yang et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Research, doi:10.1016/j.antiviral.2020.104760.
77.
Mastrangelo et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dks147.
78.
Varghese et al., Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses, Antiviral Research, doi:10.1016/j.antiviral.2015.12.012.
79.
Bennett et al., Role of a nuclear localization signal on the minor capsid Proteins VP2 and VP3 in BKPyV nuclear entry, Virology, doi:10.1016/j.virol.2014.10.013.
80.
Kosyna et al., The importin α/β-specific inhibitor Ivermectin affects HIF-dependent hypoxia response pathways, Biological Chemistry, doi:10.1515/hsz-2015-0171.
81.
Scheim et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
82.
Liu (C) et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
83.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
84.
Scheim (B), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
85.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
86.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
87.
DiNicolantonio et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
88.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
89.
Hazan et al., Treatment with Ivermectin Increases the Population of Bifidobacterium in the Gut, ACG 2023, acg2023posters.eventscribe.net/posterspeakers.asp.
Parvez et al., 30 Nov 2020, peer-reviewed, 8 authors.
Contact: sorwersust@yahoo.com, jakir-gen@sust.edu.
In silico studies are an important part of preclinical research, however results may be very different in vivo.
Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach
International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.09.098
The pandemic prevalence of COVID-19 has become a very serious global health issue. Scientists all over the world have been seriously attempting in the discovery of a drug to combat SARS-CoV-2. It has been found that RNAdependent RNA polymerase (RdRp) plays a crucial role in SARS-CoV-2 replication, and thus could be a potential drug target. Here, comprehensive computational approaches including drug repurposing and molecular docking were employed to predict an effective drug candidate targeting RdRp of SARS-CoV-2. This study revealed that Rifabutin, Rifapentine, Fidaxomicin, 7-methyl-guanosine-5′-triphosphate-5′-guanosine and Ivermectin have a potential inhibitory interaction with RdRp of SARS-CoV-2 and could be effective drugs for COVID-19. In addition, virtual screening of the compounds from ZINC database also allowed the prediction of two compounds (ZINC09128258 and ZINC09883305) with pharmacophore features that interact effectively with RdRp of SARS-CoV-2, indicating their potentiality as effective inhibitors of the enzyme. Furthermore, ADME analysis along with analysis of toxicity was also undertaken to check the pharmacokinetics and drug-likeness properties of the two compounds. Comparative structural analysis of protein-inhibitor complexes revealed that the amino acids Y32, K47, Y122, Y129, H133, N138, D140, T141, S709 and N781 are crucial for drug surface hotspot in the RdRp of SARS-CoV-2.
Fig. 10 . PCA results trejectories for the protein-inhibitor complexes. Here, RdRp in complex with (A) Rifabutin, (B) ZINC09128258, and (C) ZINC098833. In this graph, the colour blue to red frames over time.
Declaration of competing interest The authors declare that they have no competing interests.
References
Agostini, Andres, Sims, Graham, Sheahan et al., Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease, MBio, doi:10.1128/mBio.00221-18
Agrawal, Singh, Srivastava, Singh, Kishore et al., Benchmarking of Different Molecular Docking Methods for Protein-Peptide Docking
Biovia, Discovery Studio Modeling Environment, Release 2017
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antivir. Res, doi:10.1016/j.antiviral.2020.104787
Case, Cheatham, Darden, Gohlke, Luo et al., The Amber Biomolecular Simulation Programs, doi:10.1002/jcc.20290
Chan, Yuan, Kok, To, Chu et al., A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster, Lancet, doi:10.1016/S0140-6736(20)30154-9
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet, doi:10.1016/S0140-6736(20)30211-7
Clercq, The design of drugs for HIV and HCV, Nat. Rev. Drug Discov, doi:10.1038/nrd2424
Daina, Michielin, Zoete, SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules, Sci. Rep, doi:10.1038/srep42717
Daina, Zoete, A BOILED-egg to predict gastrointestinal absorption and brain penetration of small molecules, ChemMedChem, doi:10.1002/cmdc.201600182
Delano, Pymol: an open-source molecular graphics tool, CCP4, Newsl Prot. Crystallogr
Dong, Hu, Gao, Discovering drugs to treat coronavirus disease 2019 (COVID-19), Drug Discov. Ther, doi:10.5582/ddt.2020.01012
Ekins, Mestres, Testa, In silico pharmacology for drug discovery: methods for virtual ligand screening and profiling, Br. J. Pharmacol, doi:10.1038/sj.bjp.0707305
Elfiky, Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study, Life Sci, doi:10.1016/j.lfs.2020.117592
Fang, Zhang, Xie, Lin, Ying et al., Sensitivity of chest CT for COVID-19: comparison to RT-PCR, Radiology, doi:10.1148/radiol.2020200432
Furuta, Gowen, Takahashi, Shiraki, Smee et al., Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antivir. Res, doi:10.1016/j.antiviral.2013.09.015
Ganeshpurkar, Gutti, Singh, RNA-dependent RNA polymerases and their emerging roles in antiviral therapy, Viral Polym, doi:10.1016/b978-0-12-815422-9.00001-2
Harrison, Coronavirus puts drug repurposing on the fast track, Nat. Biotechnol, doi:10.1038/d41587-020-00003-1
Hsu, Chen, Lin, Yang, iGEMDOCK: A Graphical Environment of Enhancing GEMDOCK Using Pharmacological Interactions and Post-screening Analysis
Hubbard, Chen, Davis, Informatics and modeling challenges in fragmentbased drug discovery, Curr. Opin. Drug Discov. Dev
Irwin, Shoichet, ZINCa free database of commercially available compounds for virtual screening, J. Chem. Inf. Model, doi:10.1021/ci049714+
Koes, Camacho, ZINCPharmer: pharmacophore search of the ZINC database, Nucleic Acids Res, doi:10.1093/nar/gks378
Lake, What we know so far: COVID-19 current clinical knowledge and research, Clin. Med, doi:10.7861/clinmed.2019-coron
Laskowski, Swindells, LigPlot+: multiple ligand-protein interaction diagrams for drug discovery, J. Chem. Inf. Model, doi:10.1021/ci200227u
Leach, Molecular Modelling: Principles and Applications
Li, Clercq, Therapeutic options for the 2019 novel coronavirus (2019-nCoV), Nat. Rev. Drug Discov, doi:10.1038/d41573-020-00016-0
Li, Du, Wang, Wu, Li et al., In silico estimation of chemical carcinogenicity with binary and ternary classification methods, Mol. Inform, doi:10.1002/minf.201400127
Li, Yu, Zhang, Ren, Peluffo et al., Network Bioinformatics Analysis Provides Insight Into Drug Repurposing for COVID-2019, doi:10.20944/preprints202003.0286.v1
Lung, Lin, Yang, Chou, Shu et al., The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase, J. Med. Virol, doi:10.1002/jmv.25761
Mercorelli, Palù, Loregian, Drug repurposing for viral infectious diseases: how far are we?, Trends Microbiol, doi:10.1016/j.tim.2018.04.004
Mishra, Dahima, In vitro ADME studies of TUG-891, a GPR-120 inhibitor using SWISS ADME predictor, J. Drug Deliv. Ther, doi:10.22270/jddt.v9i2-s.2710
Pal, Berhanu, Desalegn, Kandi, Severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2): an update, Cureus, doi:10.7759/cureus.7423
Pizzorno, Padey, Terrier, Rosa-Calatrava, Drug repurposing approaches for the treatment of influenza viral infection: reviving old drugs to fight against a longlived enemy, Front. Immunol, doi:10.3389/fimmu.2019.00531
Polsky, Antiviral chemotherapy for infection with human immunodeficiency virus, Rev. Infect. Dis, doi:10.1093/CLINIDS/11.SUPPLEMENT_7.S1648
Porcheddu, Serra, Kelvin, Kelvin, Rubino, Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China, J. Infect. Dev. Count, doi:10.3855/jidc.12600
Prichard, Kern, Orthopoxvirus targets for the development of antiviral therapies, Curr. Drug Targets. Infect. Disord, doi:10.2174/1568005053174627
Rose, Prlić, Altunkaya, Bi, Bradley et al., The RCSB protein data bank: integrative view of protein, gene and 3D structural information, Nucleic Acids Res
Sakano, Takako, Iqbal Mahamood, Yamashita, Molecular dynamics analysis to evaluate docking pose prediction, Biophys. Physicobiol, doi:10.2142/biophysico.13.0
Salentin, Schreiber, Haupt, Adasme, Schroeder, PLIPfully automated protein-ligand interaction profiler, Nucleic Acids Res, doi:10.7490/F1000RESEARCH.1098304.1
Schneidman-Duhovny, Dror, Inbar, Nussinov, Wolfson, Deterministic pharmacophore detection via multiple flexible alignment of drug-like molecules, J. Comput. Biol, doi:10.1089/cmb.2007.0130
Schneidman-Duhovny, Dror, Inbar, Nussinov, Wolfson, PharmaGist: a webserver for ligand-based pharmacophore detection, Nucleic Acids Res, doi:10.1093/nar/gkn187
Schneidman-Duhovny, Inbar, Nussinov, Wolfson, PatchDock and SymmDock : Servers for Rigid and Symmetric Docking, doi:10.1093/nar/gki481
Shen, Cheng, Xu, Li, Tang, Estimation of ADME properties with substructure pattern recognition, J. Chem. Inf. Model, doi:10.1021/ci100104j
Tripathi, Ghosh, Talapatra, Bioavailability prediction of phytochemicals present in Calotropis procera (Aiton) R. Br. by using Swiss-ADME tool, World Sci. News
Trott, Olson, AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J. Comput. Chem, doi:10.1002/jcc.21334
Volunteers, Li, Wu, Guo, Yao et al., Caution on kidney dysfunctions of 2019-nCoV patients, MedRxiv, doi:10.1101/2020.02.08.20021212
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Who, Coronavirus Disease (COVID-19) Situation Dashboard
Wishart, Feunang, Guo, Lo, Marcu et al., DrugBank 5.0: a major update to the DrugBank database for 2018, Nucleic Acids Res, doi:10.1093/nar/gkx1037
Worldometer, Coronavirus Cases, Worldometer, doi:10.1101/2020.01.23.20018549V2
Xu, Cheng, Chen, Du, Li et al., In silico prediction of chemical ames mutagenicity, J. Chem. Inf. Model, doi:10.1021/ci300400a
Xu, Hassounah, Colby-Germinario, Oliveira, Fogarty et al., Purification of Zika virus RNA-dependent RNA polymerase and its use to identify smallmolecule Zika inhibitors, J. Antimicrob. Chemother, doi:10.1093/jac/dkw514
Yang, Lu, Liu, Wang, Zhang et al., Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China, MedRxiv, doi:10.1101/2020.02.10.20021675
Yang, Wang, Chen, Hao, Yang, LARMD : Integration of Bioinformatic Resources to Profile Ligand-driven Protein Dynamics With a Case on the Activation of Estrogen Receptor, doi:10.1093/bib/bbz141
Yuen, Ye, Fung, Chan, Jin, SARS-CoV-2 and COVID-19: the most important research questions, Cell Biosci, doi:10.1186/s13578-020-00404-4
Zhang, Zhou, Binding Mechanism of Remdesivir to SARS-CoV-2 RNA Dependent RNA Polymerase, doi:10.20944/preprints202003.0267.v1
Zhou, Dai, Tong, COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression, J. Antimicrob. Chemother, doi:10.1093/jac/dkaa114
Zumla, Chan, Azhar, Hui, Yuen, Coronaviruses-drug discovery and therapeutic options, Nat. Rev. Drug Discov, doi:10.1038/nrd.2015.37
DOI record:
{
"DOI": "10.1016/j.ijbiomac.2020.09.098",
"ISSN": [
"0141-8130"
],
"URL": "http://dx.doi.org/10.1016/j.ijbiomac.2020.09.098",
"alternative-id": [
"S0141813020344585"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Biological Macromolecules"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijbiomac.2020.09.098"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Elsevier B.V. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Parvez",
"given": "Md. Sorwer Alam",
"sequence": "first"
},
{
"affiliation": [],
"family": "Karim",
"given": "Md. Adnan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hasan",
"given": "Mahmudul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jaman",
"given": "Jomana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karim",
"given": "Ziaul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tahsin",
"given": "Tohura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hasan",
"given": "Md. Nazmul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hosen",
"given": "Mohammad Jakir",
"sequence": "additional"
}
],
"container-title": "International Journal of Biological Macromolecules",
"container-title-short": "International Journal of Biological Macromolecules",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
9,
17
]
],
"date-time": "2020-09-17T10:18:58Z",
"timestamp": 1600337938000
},
"deposited": {
"date-parts": [
[
2022,
6,
25
]
],
"date-time": "2022-06-25T20:56:02Z",
"timestamp": 1656190562000
},
"funder": [
{
"DOI": "10.13039/501100007944",
"doi-asserted-by": "publisher",
"name": "Shahjalal University of Science and Technology"
},
{
"name": "Bangladesh Bureau of Educational Information and Statistics"
},
{
"DOI": "10.13039/501100002701",
"doi-asserted-by": "publisher",
"name": "Ministry of Education"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
16
]
],
"date-time": "2024-03-16T18:58:43Z",
"timestamp": 1710615523569
},
"is-referenced-by-count": 51,
"issued": {
"date-parts": [
[
2020,
11
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
11,
1
]
],
"date-time": "2020-11-01T00:00:00Z",
"timestamp": 1604188800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0141813020344585?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0141813020344585?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "1787-1797",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
11
]
]
},
"published-print": {
"date-parts": [
[
2020,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.3855/jidc.12600",
"article-title": "Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China",
"author": "Porcheddu",
"doi-asserted-by": "crossref",
"first-page": "125",
"journal-title": "J. Infect. Dev. Count.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0005",
"volume": "14",
"year": "2020"
},
{
"author": "Worldometer",
"first-page": "1",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0010",
"series-title": "Coronavirus Cases",
"year": "2020"
},
{
"author": "WHO",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0015",
"series-title": "Coronavirus Disease (COVID-19) Situation Dashboard",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30154-9",
"article-title": "A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster",
"author": "Chan",
"doi-asserted-by": "crossref",
"journal-title": "Lancet",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0020",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"article-title": "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study",
"author": "Chen",
"doi-asserted-by": "crossref",
"journal-title": "Lancet",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0025",
"year": "2020"
},
{
"article-title": "Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China",
"author": "Yang",
"journal-title": "MedRxiv",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0030",
"year": "2020"
},
{
"article-title": "Caution on kidney dysfunctions of 2019-nCoV patients",
"author": "A.-2019-nCoV Volunteers",
"journal-title": "MedRxiv",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0035",
"year": "2020"
},
{
"article-title": "Severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2): an update",
"author": "Pal",
"journal-title": "Cureus",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0040",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1038/nrd.2015.37",
"article-title": "Coronaviruses—drug discovery and therapeutic options",
"author": "Zumla",
"doi-asserted-by": "crossref",
"first-page": "327",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0045",
"volume": "15",
"year": "2016"
},
{
"DOI": "10.1016/j.lfs.2020.117592",
"article-title": "Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study",
"author": "Elfiky",
"doi-asserted-by": "crossref",
"first-page": "117592",
"journal-title": "Life Sci.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0050",
"year": "2020"
},
{
"article-title": "The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase",
"author": "Lung",
"first-page": "jmv.25761",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0055",
"year": "2020"
},
{
"DOI": "10.1038/d41573-020-00016-0",
"article-title": "Therapeutic options for the 2019 novel coronavirus (2019-nCoV)",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "149",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0060",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"article-title": "Favipiravir (T-705), a novel viral RNA polymerase inhibitor",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "446",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0065",
"volume": "100",
"year": "2013"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0070",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1128/mBio.00221-18",
"article-title": "Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease",
"author": "Agostini",
"doi-asserted-by": "crossref",
"journal-title": "MBio",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0075",
"volume": "9",
"year": "2018"
},
{
"author": "Zhang",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0080",
"series-title": "Binding Mechanism of Remdesivir to SARS-CoV-2 RNA Dependent RNA Polymerase",
"year": "2020"
},
{
"DOI": "10.1038/d41587-020-00003-1",
"article-title": "Coronavirus puts drug repurposing on the fast track",
"author": "Harrison",
"doi-asserted-by": "crossref",
"journal-title": "Nat. Biotechnol.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0085",
"year": "2020"
},
{
"DOI": "10.1038/sj.bjp.0707305",
"article-title": "In silico pharmacology for drug discovery: methods for virtual ligand screening and profiling",
"author": "Ekins",
"doi-asserted-by": "crossref",
"first-page": "9",
"journal-title": "Br. J. Pharmacol.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0090",
"volume": "152",
"year": "2007"
},
{
"article-title": "Informatics and modeling challenges in fragment-based drug discovery",
"author": "Hubbard",
"first-page": "289",
"journal-title": "Curr. Opin. Drug Discov. Dev.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0095",
"volume": "10",
"year": "2007"
},
{
"DOI": "10.3389/fimmu.2019.00531",
"article-title": "Drug repurposing approaches for the treatment of influenza viral infection: reviving old drugs to fight against a long-lived enemy",
"author": "Pizzorno",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0100",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/j.tim.2018.04.004",
"article-title": "Drug repurposing for viral infectious diseases: how far are we?",
"author": "Mercorelli",
"doi-asserted-by": "crossref",
"first-page": "865",
"journal-title": "Trends Microbiol.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0105",
"volume": "26",
"year": "2018"
},
{
"author": "Li",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0110",
"series-title": "Network Bioinformatics Analysis Provides Insight Into Drug Repurposing for COVID-2019",
"year": "2020"
},
{
"DOI": "10.1093/nar/gkv1290",
"article-title": "Database resources of the National Center for Biotechnology Information",
"author": "Resource Coordinators",
"doi-asserted-by": "crossref",
"first-page": "D7",
"journal-title": "Nucleic Acids Res.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0115",
"volume": "44",
"year": "2016"
},
{
"article-title": "The RCSB protein data bank: integrative view of protein, gene and 3D structural information",
"author": "Rose",
"first-page": "D271",
"journal-title": "Nucleic Acids Res.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0120",
"volume": "45",
"year": "2017"
},
{
"DOI": "10.1093/nar/gkx1037",
"article-title": "DrugBank 5.0: a major update to the DrugBank database for 2018",
"author": "Wishart",
"doi-asserted-by": "crossref",
"first-page": "D1074",
"journal-title": "Nucleic Acids Res.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0125",
"volume": "46",
"year": "2018"
},
{
"article-title": "AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading",
"author": "Trott",
"journal-title": "J. Comput. Chem.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0130",
"volume": "31",
"year": "2009"
},
{
"article-title": "Pymol: an open-source molecular graphics tool",
"author": "DeLano",
"first-page": "82",
"issue": "1",
"journal-title": "CCP4 Newsl Prot. Crystallogr",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0135",
"volume": "40",
"year": "2002"
},
{
"DOI": "10.1021/ci200227u",
"article-title": "LigPlot+: multiple ligand-protein interaction diagrams for drug discovery",
"author": "Laskowski",
"doi-asserted-by": "crossref",
"first-page": "2778",
"journal-title": "J. Chem. Inf. Model.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0140",
"volume": "51",
"year": "2011"
},
{
"author": "Biovia",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0145",
"series-title": "Discovery Studio Modeling Environment, Release 2017",
"year": "2016"
},
{
"DOI": "10.1093/nar/gkv315",
"article-title": "PLIP — fully automated protein-ligand interaction profiler",
"author": "Salentin",
"doi-asserted-by": "crossref",
"first-page": "W443",
"journal-title": "Nucleic Acids Res.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0150",
"volume": "43",
"year": "2015"
},
{
"DOI": "10.1089/cmb.2007.0130",
"article-title": "Deterministic pharmacophore detection via multiple flexible alignment of drug-like molecules",
"author": "Schneidman-Duhovny",
"doi-asserted-by": "crossref",
"first-page": "737",
"journal-title": "J. Comput. Biol.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0155",
"volume": "15",
"year": "2008"
},
{
"DOI": "10.1093/nar/gkn187",
"article-title": "PharmaGist: a webserver for ligand-based pharmacophore detection",
"author": "Schneidman-Duhovny",
"doi-asserted-by": "crossref",
"first-page": "W223",
"journal-title": "Nucleic Acids Res.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0160",
"volume": "36",
"year": "2008"
},
{
"DOI": "10.1093/nar/gks378",
"article-title": "ZINCPharmer: pharmacophore search of the ZINC database",
"author": "Koes",
"doi-asserted-by": "crossref",
"first-page": "W409",
"journal-title": "Nucleic Acids Res.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0165",
"volume": "40",
"year": "2012"
},
{
"DOI": "10.1021/ci049714+",
"article-title": "ZINC — a free database of commercially available compounds for virtual screening",
"author": "Irwin",
"doi-asserted-by": "crossref",
"first-page": "177",
"journal-title": "J. Chem. Inf. Model.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0170",
"volume": "45",
"year": "2005"
},
{
"DOI": "10.1038/srep42717",
"article-title": "SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules",
"author": "Daina",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0175",
"volume": "7",
"year": "2017"
},
{
"article-title": "Bioavailability prediction of phytochemicals present in Calotropis procera (Aiton) R. Br. by using Swiss-ADME tool",
"author": "Tripathi",
"first-page": "147",
"journal-title": "World Sci. News",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0180",
"volume": "131",
"year": "2019"
},
{
"article-title": "In vitro ADME studies of TUG-891, a GPR-120 inhibitor using SWISS ADME predictor",
"author": "Mishra",
"first-page": "366",
"journal-title": "J. Drug Deliv. Ther.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0185",
"volume": "9",
"year": "2019"
},
{
"key": "10.1016/j.ijbiomac.2020.09.098_bb0190",
"series-title": "Molecular Properties Prediction — Osiris Property Explorer",
"year": "2020"
},
{
"DOI": "10.1021/ci100104j",
"article-title": "Estimation of ADME properties with substructure pattern recognition",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "1034",
"journal-title": "J. Chem. Inf. Model.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0195",
"volume": "50",
"year": "2010"
},
{
"DOI": "10.1021/ci300400a",
"article-title": "In silico prediction of chemical ames mutagenicity",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "2840",
"journal-title": "J. Chem. Inf. Model.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0200",
"volume": "52",
"year": "2012"
},
{
"DOI": "10.1002/minf.201400127",
"article-title": "In silico estimation of chemical carcinogenicity with binary and ternary classification methods",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "228",
"journal-title": "Mol. Inform.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0205",
"volume": "34",
"year": "2015"
},
{
"author": "Schneidman-duhovny",
"first-page": "363",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0210",
"series-title": "PatchDock and SymmDock : Servers for Rigid and Symmetric Docking",
"volume": "vol. 33",
"year": "2005"
},
{
"author": "Hsu",
"first-page": "1",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0215",
"series-title": "iGEMDOCK: A Graphical Environment of Enhancing GEMDOCK Using Pharmacological Interactions and Post-screening Analysis",
"volume": "vol. 12",
"year": "2011"
},
{
"author": "Leach",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0220",
"series-title": "Molecular Modelling: Principles and Applications",
"year": "2001"
},
{
"author": "Yang",
"first-page": "1",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0225",
"series-title": "LARMD : Integration of Bioinformatic Resources to Profile Ligand-driven Protein Dynamics With a Case on the Activation of Estrogen Receptor",
"volume": "vol. 00",
"year": "2019"
},
{
"author": "Case",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0230",
"series-title": "The Amber Biomolecular Simulation Programs",
"volume": "12255",
"year": "2005"
},
{
"DOI": "10.1002/cmdc.201600182",
"article-title": "A BOILED-egg to predict gastrointestinal absorption and brain penetration of small molecules",
"author": "Daina",
"doi-asserted-by": "crossref",
"first-page": "1117",
"journal-title": "ChemMedChem",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0235",
"volume": "11",
"year": "2016"
},
{
"DOI": "10.7861/clinmed.2019-coron",
"article-title": "What we know so far: COVID-19 current clinical knowledge and research",
"author": "Lake",
"doi-asserted-by": "crossref",
"first-page": "124",
"journal-title": "Clin. Med.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0240",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1186/s13578-020-00404-4",
"article-title": "SARS-CoV-2 and COVID-19: the most important research questions",
"author": "Yuen",
"doi-asserted-by": "crossref",
"first-page": "40",
"journal-title": "Cell Biosci",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0245",
"volume": "10",
"year": "2020"
},
{
"article-title": "Sensitivity of chest CT for COVID-19: comparison to RT-PCR",
"author": "Fang",
"first-page": "200432",
"journal-title": "Radiology.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0250",
"year": "2020"
},
{
"DOI": "10.5582/ddt.2020.01012",
"article-title": "Discovering drugs to treat coronavirus disease 2019 (COVID-19)",
"author": "Dong",
"doi-asserted-by": "crossref",
"first-page": "58",
"journal-title": "Drug Discov. Ther.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0255",
"volume": "14",
"year": "2020"
},
{
"article-title": "COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression",
"author": "Zhou",
"journal-title": "J. Antimicrob. Chemother.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0260",
"year": "2020"
},
{
"DOI": "10.1038/nrd2424",
"article-title": "The design of drugs for HIV and HCV",
"author": "De Clercq",
"doi-asserted-by": "crossref",
"first-page": "1001",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0265",
"volume": "6",
"year": "2007"
},
{
"article-title": "Purification of Zika virus RNA-dependent RNA polymerase and its use to identify small-molecule Zika inhibitors",
"author": "Xu",
"first-page": "727",
"journal-title": "J. Antimicrob. Chemother.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0270",
"volume": "72",
"year": "2017"
},
{
"article-title": "RNA-dependent RNA polymerases and their emerging roles in antiviral therapy",
"author": "Ganeshpurkar",
"first-page": "1",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0275",
"series-title": "Viral Polym.",
"year": "2019"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"first-page": "104787",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0280",
"year": "2020"
},
{
"DOI": "10.1093/clinids/11.Supplement_7.S1648",
"article-title": "Antiviral chemotherapy for infection with human immunodeficiency virus",
"author": "Polsky",
"doi-asserted-by": "crossref",
"first-page": "S1648",
"journal-title": "Rev. Infect. Dis.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0285",
"volume": "11",
"year": "1989"
},
{
"DOI": "10.2174/1568005053174627",
"article-title": "Orthopoxvirus targets for the development of antiviral therapies",
"author": "Prichard",
"doi-asserted-by": "crossref",
"first-page": "17",
"journal-title": "Curr. Drug Targets. Infect. Disord.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0290",
"volume": "5",
"year": "2005"
},
{
"author": "Agrawal",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0295",
"series-title": "Benchmarking of Different Molecular Docking Methods for Protein-Peptide Docking",
"volume": "vol. 19",
"year": "2019"
},
{
"DOI": "10.2142/biophysico.13.0_181",
"article-title": "Molecular dynamics analysis to evaluate docking pose prediction",
"author": "Sakano",
"doi-asserted-by": "crossref",
"first-page": "181",
"journal-title": "Biophys. Physicobiol.",
"key": "10.1016/j.ijbiomac.2020.09.098_bb0300",
"volume": "13",
"year": "2016"
}
],
"reference-count": 60,
"references-count": 60,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0141813020344585"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Molecular Biology",
"General Medicine",
"Biochemistry",
"Structural Biology"
],
"subtitle": [],
"title": "Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "163"
}
