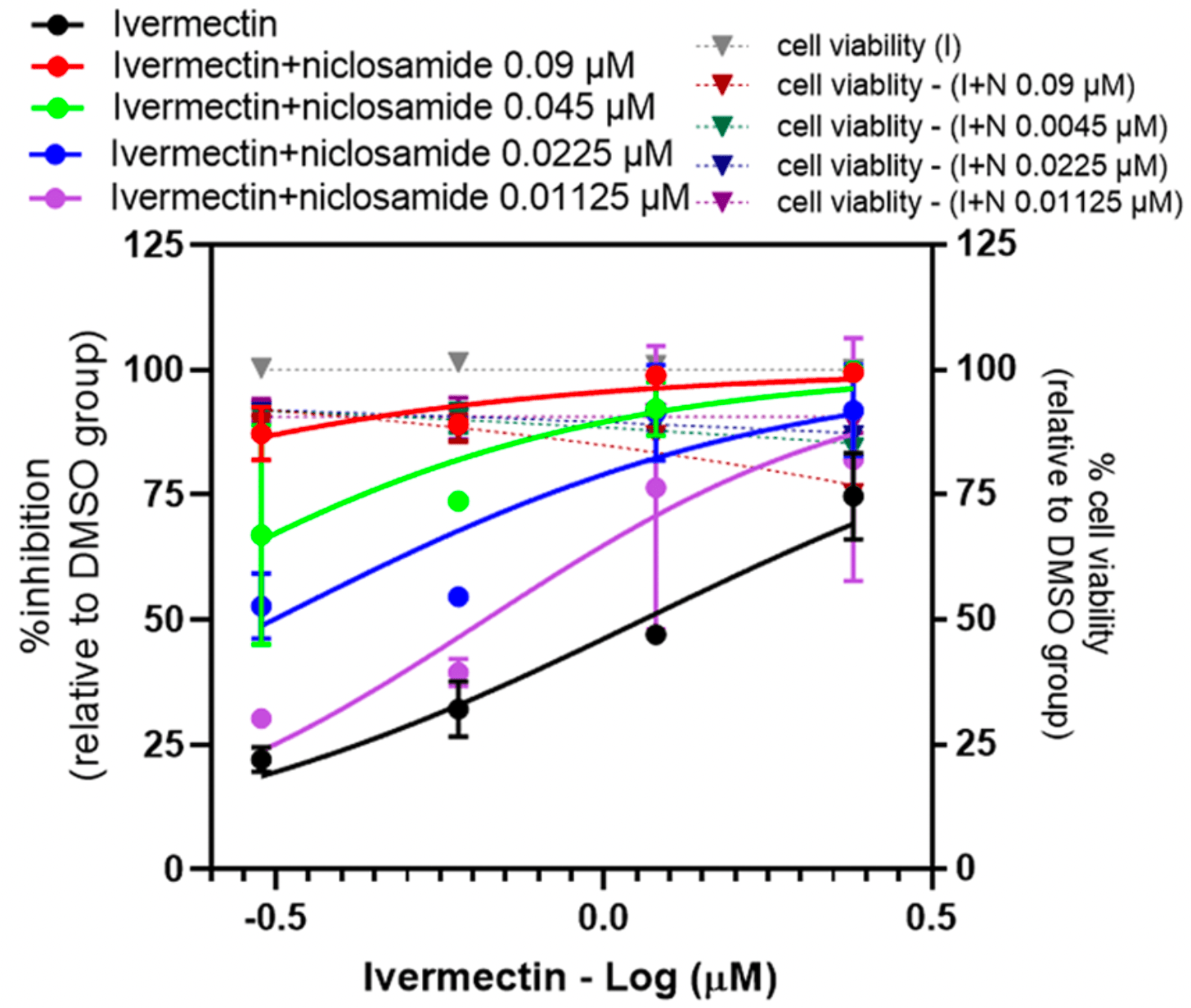
Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations
et al., BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8, Jun 2022 (preprint)
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing a strong synergistic effect of combinations of ivermectin, niclosamide, and chloroquine, with >10x reduction in IC50 compared to individual drugs.
74 preclinical studies support the efficacy of ivermectin for COVID-19:
Ivermectin, better known for antiparasitic activity, is a broad spectrum antiviral with activity against many viruses including H7N771, Dengue37,72,73 , HIV-173, Simian virus 4074, Zika37,75,76 , West Nile76, Yellow Fever77,78, Japanese encephalitis77, Chikungunya78, Semliki Forest virus78, Human papillomavirus57, Epstein-Barr57, BK Polyomavirus79, and Sindbis virus78.
Ivermectin inhibits importin-α/β-dependent nuclear import of viral proteins71,73,74,80 , shows spike-ACE2 disruption at 1nM with microfluidic diffusional sizing38, binds to glycan sites on the SARS-CoV-2 spike protein preventing interaction with blood and epithelial cells and inhibiting hemagglutination41,81, shows dose-dependent inhibition of wildtype and omicron variants36, exhibits dose-dependent inhibition of lung injury61,66, may inhibit SARS-CoV-2 via IMPase inhibition37, may inhibit SARS-CoV-2 induced formation of fibrin clots resistant to degradation9, inhibits SARS-CoV-2 3CLpro54, may inhibit SARS-CoV-2 RdRp activity28, may minimize viral myocarditis by inhibiting NF-κB/p65-mediated inflammation in macrophages60, may be beneficial for COVID-19 ARDS by blocking GSDMD and NET formation82, may interfere with SARS-CoV-2's immune evasion via ORF8 binding4, may inhibit SARS-CoV-2 by disrupting CD147 interaction83-86, may inhibit SARS-CoV-2 attachment to lipid rafts via spike NTD binding2, shows protection against inflammation, cytokine storm, and mortality in an LPS mouse model sharing key pathological features of severe COVID-1959,87, may be beneficial in severe COVID-19 by binding IGF1 to inhibit the promotion of inflammation, fibrosis, and cell proliferation that leads to lung damage8, may minimize SARS-CoV-2 induced cardiac damage40,48, may counter immune evasion by inhibiting NSP15-TBK1/KPNA1 interaction and restoring IRF3 activation88, may disrupt SARS-CoV-2 N and ORF6 protein nuclear transport and their suppression of host interferon responses1, reduces TAZ/YAP nuclear import, relieving SARS-CoV-2-driven suppression of IRF3 and NF-κB antiviral pathways35, increases Bifidobacteria which play a key role in the immune system89, has immunomodulatory51 and anti-inflammatory70,90 properties, and has an extensive and very positive safety profile91.
Study covers ivermectin and niclosamide.
1.
Gayozo et al., Binding affinities analysis of ivermectin, nucleocapsid and ORF6 proteins of SARS-CoV-2 to human importins α isoforms: A computational approach, Biotecnia, doi:10.18633/biotecnia.v27.2485.
2.
Lefebvre et al., Characterization and Fluctuations of an Ivermectin Binding Site at the Lipid Raft Interface of the N-Terminal Domain (NTD) of the Spike Protein of SARS-CoV-2 Variants, Viruses, doi:10.3390/v16121836.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Bagheri-Far et al., Non-spike protein inhibition of SARS-CoV-2 by natural products through the key mediator protein ORF8, Molecular Biology Research Communications, doi:10.22099/mbrc.2024.50245.2001.
5.
de Oliveira Só et al., In Silico Comparative Analysis of Ivermectin and Nirmatrelvir Inhibitors Interacting with the SARS-CoV-2 Main Protease, Preprints, doi:10.20944/preprints202404.1825.v1.
6.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
7.
Oranu et al., Validation of the binding affinities and stabilities of ivermectin and moxidectin against SARS-CoV-2 receptors using molecular docking and molecular dynamics simulation, GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2024.26.1.0030.
8.
Zhao et al., Identification of the shared gene signatures between pulmonary fibrosis and pulmonary hypertension using bioinformatics analysis, Frontiers in Immunology, doi:10.3389/fimmu.2023.1197752.
9.
Vottero et al., Computational Prediction of the Interaction of Ivermectin with Fibrinogen, Molecular Sciences, doi:10.3390/ijms241411449.
10.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
11.
Umar et al., Inhibitory potentials of ivermectin, nafamostat, and camostat on spike protein and some nonstructural proteins of SARS-CoV-2: Virtual screening approach, Jurnal Teknologi Laboratorium, doi:10.29238/teknolabjournal.v11i1.344.
12.
Alvarado et al., Interaction of the New Inhibitor Paxlovid (PF-07321332) and Ivermectin With the Monomer of the Main Protease SARS-CoV-2: A Volumetric Study Based on Molecular Dynamics, Elastic Networks, Classical Thermodynamics and SPT, Computational Biology and Chemistry, doi:10.1016/j.compbiolchem.2022.107692.
13.
Aminpour et al., In Silico Analysis of the Multi-Targeted Mode of Action of Ivermectin and Related Compounds, Computation, doi:10.3390/computation10040051.
14.
Parvez et al., Insights from a computational analysis of the SARS-CoV-2 Omicron variant: Host–pathogen interaction, pathogenicity, and possible drug therapeutics, Immunity, Inflammation and Disease, doi:10.1002/iid3.639.
15.
Francés-Monerris et al., Microscopic interactions between ivermectin and key human and viral proteins involved in SARS-CoV-2 infection, Physical Chemistry Chemical Physics, doi:10.1039/D1CP02967C.
16.
González-Paz et al., Comparative study of the interaction of ivermectin with proteins of interest associated with SARS-CoV-2: A computational and biophysical approach, Biophysical Chemistry, doi:10.1016/j.bpc.2021.106677.
17.
González-Paz (B) et al., Structural Deformability Induced in Proteins of Potential Interest Associated with COVID-19 by binding of Homologues present in Ivermectin: Comparative Study Based in Elastic Networks Models, Journal of Molecular Liquids, doi:10.1016/j.molliq.2021.117284.
18.
Rana et al., A Computational Study of Ivermectin and Doxycycline Combination Drug Against SARS-CoV-2 Infection, Research Square, doi:10.21203/rs.3.rs-755838/v1.
19.
Muthusamy et al., Virtual Screening Reveals Potential Anti-Parasitic Drugs Inhibiting the Receptor Binding Domain of SARS-CoV-2 Spike protein, Journal of Virology & Antiviral Research, www.scitechnol.com/abstract/virtual-screening-reveals-potential-antiparasitic-drugs-inhibiting-the-receptor-binding-domain-of-sarscov2-spike-protein-16398.html.
20.
Qureshi et al., Mechanistic insights into the inhibitory activity of FDA approved ivermectin against SARS-CoV-2: old drug with new implications, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1906750.
21.
Schöning et al., Highly-transmissible Variants of SARS-CoV-2 May Be More Susceptible to Drug Therapy Than Wild Type Strains, Research Square, doi:10.21203/rs.3.rs-379291/v1.
22.
Bello et al., Elucidation of the inhibitory activity of ivermectin with host nuclear importin α and several SARS-CoV-2 targets, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1911857.
23.
Udofia et al., In silico studies of selected multi-drug targeting against 3CLpro and nsp12 RNA-dependent RNA-polymerase proteins of SARS-CoV-2 and SARS-CoV, Network Modeling Analysis in Health Informatics and Bioinformatics, doi:10.1007/s13721-021-00299-2.
24.
Choudhury et al., Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach, Future Medicine, doi:10.2217/fvl-2020-0342.
25.
Kern et al., Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing, Frontiers in Pharmacology, doi:10.3389/fphar.2021.625678.
26.
Saha et al., The Binding mechanism of ivermectin and levosalbutamol with spike protein of SARS-CoV-2, Structural Chemistry, doi:10.1007/s11224-021-01776-0.
27.
Eweas et al., Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2, Frontiers in Microbiology, doi:10.3389/fmicb.2020.592908.
28.
Parvez (B) et al., Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.09.098.
29.
Francés-Monerris (B) et al., Has Ivermectin Virus-Directed Effects against SARS-CoV-2? Rationalizing the Action of a Potential Multitarget Antiviral Agent, ChemRxiv, doi:10.26434/chemrxiv.12782258.v1.
30.
Kalhor et al., Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1824816.
31.
Swargiary, A., Ivermectin as a promising RNA-dependent RNA polymerase inhibitor and a therapeutic drug against SARS-CoV2: Evidence from in silico studies, Research Square, doi:10.21203/rs.3.rs-73308/v1.
32.
Maurya, D., A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID-19 Patients, American Chemical Society (ACS), doi:10.26434/chemrxiv.12630539.v1.
33.
Lehrer et al., Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, In Vivo, 34:5, 3023-3026, doi:10.21873/invivo.12134.
34.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
35.
Kofler et al., M-Motif, a potential non-conventional NLS in YAP/TAZ and other cellular and viral proteins that inhibits classic protein import, iScience, doi:10.1016/j.isci.2025.112105.
36.
Shahin et al., The selective effect of Ivermectin on different human coronaviruses; in-vitro study, Research Square, doi:10.21203/rs.3.rs-4180797/v1.
37.
Jitobaom et al., Identification of inositol monophosphatase as a broad‐spectrum antiviral target of ivermectin, Journal of Medical Virology, doi:10.1002/jmv.29552.
38.
Fauquet et al., Microfluidic Diffusion Sizing Applied to the Study of Natural Products and Extracts That Modulate the SARS-CoV-2 Spike RBD/ACE2 Interaction, Molecules, doi:10.3390/molecules28248072.
39.
García-Aguilar et al., In Vitro Analysis of SARS-CoV-2 Spike Protein and Ivermectin Interaction, International Journal of Molecular Sciences, doi:10.3390/ijms242216392.
40.
Liu et al., SARS-CoV-2 viral genes Nsp6, Nsp8, and M compromise cellular ATP levels to impair survival and function of human pluripotent stem cell-derived cardiomyocytes, Stem Cell Research & Therapy, doi:10.1186/s13287-023-03485-3.
41.
Boschi et al., SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects, bioRxiv, doi:10.1101/2022.11.24.517882.
42.
De Forni et al., Synergistic drug combinations designed to fully suppress SARS-CoV-2 in the lung of COVID-19 patients, PLoS ONE, doi:10.1371/journal.pone.0276751.
43.
Saha (B) et al., Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder, Pharmaceutics, doi:10.3390/pharmaceutics14071432.
44.
Jitobaom (B) et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8.
45.
Croci et al., Liposomal Systems as Nanocarriers for the Antiviral Agent Ivermectin, International Journal of Biomaterials, doi:10.1155/2016/8043983.
46.
Zheng et al., Red blood cell-hitchhiking mediated pulmonary delivery of ivermectin: Effects of nanoparticle properties, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121719.
47.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
48.
Liu (B) et al., Genome-wide analyses reveal the detrimental impacts of SARS-CoV-2 viral gene Orf9c on human pluripotent stem cell-derived cardiomyocytes, Stem Cell Reports, doi:10.1016/j.stemcr.2022.01.014.
49.
Segatori et al., Effect of Ivermectin and Atorvastatin on Nuclear Localization of Importin Alpha and Drug Target Expression Profiling in Host Cells from Nasopharyngeal Swabs of SARS-CoV-2- Positive Patients, Viruses, doi:10.3390/v13102084.
50.
Jitobaom (C) et al., Favipiravir and Ivermectin Showed in Vitro Synergistic Antiviral Activity against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-941811/v1.
51.
Munson et al., Niclosamide and ivermectin modulate caspase-1 activity and proinflammatory cytokine secretion in a monocytic cell line, British Society For Nanomedicine Early Career Researcher Summer Meeting, 2021, web.archive.org/web/20230401070026/https://michealmunson.github.io/COVID.pdf.
52.
Mountain Valley MD, Mountain Valley MD Receives Successful Results From BSL-4 COVID-19 Clearance Trial on Three Variants Tested With Ivectosol™, 5/18, www.globenewswire.com/en/news-release/2021/05/18/2231755/0/en/Mountain-Valley-MD-Receives-Successful-Results-From-BSL-4-COVID-19-Clearance-Trial-on-Three-Variants-Tested-With-Ivectosol.html.
53.
Yesilbag et al., Ivermectin also inhibits the replication of bovine respiratory viruses (BRSV, BPIV-3, BoHV-1, BCoV and BVDV) in vitro, Virus Research, doi:10.1016/j.virusres.2021.198384.
54.
Mody et al., Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents, Communications Biology, doi:10.1038/s42003-020-01577-x.
55.
Jeffreys et al., Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2022.106542.
56.
Surnar et al., Clinically Approved Antiviral Drug in an Orally Administrable Nanoparticle for COVID-19, ACS Pharmacol. Transl. Sci., doi:10.1021/acsptsci.0c00179.
57.
Li et al., Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment, J. Cellular Physiology, doi:10.1002/jcp.30055.
58.
Caly et al., The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research, doi:10.1016/j.antiviral.2020.104787.
59.
Zhang et al., Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflammation Research, doi:10.1007/s00011-008-8007-8.
60.
Gao et al., Ivermectin ameliorates acute myocarditis via the inhibition of importin-mediated nuclear translocation of NF-κB/p65, International Immunopharmacology, doi:10.1016/j.intimp.2024.112073.
61.
Abd-Elmawla et al., Suppression of NLRP3 inflammasome by ivermectin ameliorates bleomycin-induced pulmonary fibrosis, Journal of Zhejiang University-SCIENCE B, doi:10.1631/jzus.B2200385.
62.
Uematsu et al., Prophylactic administration of ivermectin attenuates SARS-CoV-2 induced disease in a Syrian Hamster Model, The Journal of Antibiotics, doi:10.1038/s41429-023-00623-0.
63.
Albariqi et al., Pharmacokinetics and Safety of Inhaled Ivermectin in Mice as a Potential COVID-19 Treatment, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121688.
64.
Errecalde et al., Safety and Pharmacokinetic Assessments of a Novel Ivermectin Nasal Spray Formulation in a Pig Model, Journal of Pharmaceutical Sciences, doi:10.1016/j.xphs.2021.01.017.
65.
Madrid et al., Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation, Heliyon, doi:10.1016/j.heliyon.2020.e05820.
66.
Ma et al., Ivermectin contributes to attenuating the severity of acute lung injury in mice, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2022.113706.
67.
de Melo et al., Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin, EMBO Mol. Med., doi:10.15252/emmm.202114122.
68.
Arévalo et al., Ivermectin reduces in vivo coronavirus infection in a mouse experimental model, Scientific Reports, doi:10.1038/s41598-021-86679-0.
69.
Chaccour et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Scientific Reports, doi:10.1038/s41598-020-74084-y.
70.
Yan et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflammation Research, doi:10.1007/s00011-011-0307-8.
71.
Götz et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Scientific Reports, doi:10.1038/srep23138.
72.
Tay et al., Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Research, doi:10.1016/j.antiviral.2013.06.002.
73.
Wagstaff et al., Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochemical Journal, doi:10.1042/BJ20120150.
74.
Wagstaff (B) et al., An AlphaScreen®-Based Assay for High-Throughput Screening for Specific Inhibitors of Nuclear Import, SLAS Discovery, doi:10.1177/1087057110390360.
75.
Barrows et al., A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection, Cell Host & Microbe, doi:10.1016/j.chom.2016.07.004.
76.
Yang et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Research, doi:10.1016/j.antiviral.2020.104760.
77.
Mastrangelo et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dks147.
78.
Varghese et al., Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses, Antiviral Research, doi:10.1016/j.antiviral.2015.12.012.
79.
Bennett et al., Role of a nuclear localization signal on the minor capsid Proteins VP2 and VP3 in BKPyV nuclear entry, Virology, doi:10.1016/j.virol.2014.10.013.
80.
Kosyna et al., The importin α/β-specific inhibitor Ivermectin affects HIF-dependent hypoxia response pathways, Biological Chemistry, doi:10.1515/hsz-2015-0171.
81.
Scheim et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
82.
Liu (C) et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
83.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
84.
Scheim (B), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
85.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
86.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
87.
DiNicolantonio et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
88.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
89.
Hazan et al., Treatment with Ivermectin Increases the Population of Bifidobacterium in the Gut, ACG 2023, acg2023posters.eventscribe.net/posterspeakers.asp.
Jitobaom et al., 18 Jun 2022, peer-reviewed, 8 authors.
Contact: prasert.aue@mahidol.ac.th (corresponding author).
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations
BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8
Background: COVID-19 pandemic has claimed millions of lives and devastated the health service system, livelihood, and economy in many countries worldwide. Despite the vaccination programs in many countries, the spread of the pandemic continues, and effective treatment is still urgently needed. Although some antiviral drugs have been shown to be effective, they are not widely available. Repurposing of anti-parasitic drugs with in vitro anti-SARS-CoV-2 activity is a promising approach being tested in many clinical trials. Combination of these drugs is a plausible way to enhance their effectiveness.
Methods: The in vitro anti-SARS-CoV-2 activity of combinations of niclosamide, ivermectin and chloroquine were evaluated in Vero E6 and lung epithelial cells, Calu-3. Results: All the two-drug combinations showed higher potency resulting in up to 4-fold reduction in the half maximal inhibitory concentration (IC 50 ) values compared to individual drugs. Among these combinations, niclosamideivermectin achieved the highest inhibitory level of over 99%. Combination synergy analysis showed niclosamideivermectin combination to have the best synergy score with a mean Loewe synergy score of 4.28 and a peak synergy score of 24.6 in Vero E6 cells and a mean Loewe synergy score of 3.82 and a peak synergy score of 10.86 in Calu-3 cells.
Conclusions: The present study demonstrated the benefit of drug combinations on anti-SARS-CoV-2 activity. Niclosamide and ivermectin showed the best synergistic profile and should be further tested in clinical trials.
Abbreviations
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s40360-022-00580-8.
Additional file 1. Authors' contributions KJ performed drug treatment experiments, viral quantifications, analysis and was a major contributor in writing and revising the manuscript. CB performed virus infection, viral quantifications, and the optimization of the plaque assay for SARS-CoV-2 and prepared virus stock. SM performed virus isolation and the optimization of the plaque assay for SARS-CoV-2. NP performed analysis and prepared drug stock solutions. SB prepared cell lines and drug stock solutions. AT designed the study and edited the manuscript. PA 3, 4 reviewed and edited the manuscript. PA 1* designed and supervised the study, performed funding acquisitions, writing, and editing the manuscript. All authors read and approved the final manuscript.
Declarations Ethics approval and consent to participate Not applicable. This work does not involve the use of human subjects and animals. All the procedures do not require IRB approval.
Consent for publication Not applicable. This work does not contain data from any individual person.
Competing interests The authors declare that they have no competing interests. • fast, convenient online submission • thorough peer review by experienced researchers in your field • rapid publication on acceptance • support for research data, including large and complex data types •..
References
Ahmed, Karim, Ross, Hossain, Clemens et al., A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis
Andrews, Thyssen, Lorke, The biology and toxicology of molluscicides, Bayluscide, Pharmacol Ther
Arshad, Pertinez, Box, Tatham, Rajoli et al., Prioritization of anti-SARS-CoV-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics, Clin Pharmacol Ther
Baraka, Mahmoud, Marschke, Geary, Homeida et al., Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus, Eur J Clin Pharmacol
Barrows, Campos, Powell, Prasanth, Schott-Lerner et al., A screen of FDA-approved drugs for inhibitors of Zika virus infection, Cell Host Microbe
Behera, Patro, Singh, Chandanshive, Ravikumar et al., Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: a matched case-control study, PLoS One
Bobrowski, Chen, Eastman, Itkin, Shinn et al., Synergistic and antagonistic drug combinations against SARS-CoV-2, Mol Ther
Bryant, Lawrie, Dowswell, Fordham, Mitchell et al., Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines, Am J Ther
Burock, Daum, Keilholz, Neumann, Walther et al., Phase II trial to investigate the safety and efficacy of orally applied niclosamide in patients with metachronous or sychronous metastases of a colorectal cancer progressing after therapy: the NIKOLO trial, BMC Cancer
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antivir Res
Chaccour, Casellas, Blanco-Di Matteo, Pineda, Fernandez-Montero et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial, EClinicalMedicine
Chiu, Green, Baylis, Eline, Rosegay et al., Absorption, tissue distribution, and excretion of tritium-labeled ivermectin in cattle, sheep, and rat, J Agric Food Chem
Dyer, Covid-19: study claims real global deaths are twice official figures, BMJ
Elalfy, Besheer, El-Mesery, El-Gilany, Elazez et al., Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19, J Med Virol
Ganguly, Rottet, Yee, Smith, Khin et al., SYBR green one-step qRT-PCR for the detection of SARS-CoV-2 RNA in saliva, bioRxiv, doi:10.1101/2020.05.29.109702
Gassen, Niemeyer, Muth, Corman, Martinelli et al., SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-coronavirus infection, Nat Commun
Götz, Magar, Dornfeld, Giese, Pohlmann et al., Influenza a viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Sci Rep
Hoffmann, Mösbauer, Hofmann-Winkler, Kaul, Kleine-Weber et al., Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2, Nature
Jans, Wagstaff, The broad spectrum host-directed agent ivermectin as an antiviral for SARS-CoV-2 ?, Biochem Biophys Res Commun
Jeon, Ko, Lee, Choi, Byun et al., Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob, Agents Chemother
Jurgeit, Mcdowell, Moese, Meldrum, Schwendener et al., Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects, PLoS Pathog
Kanjanasirirat, Suksatu, Manopwisedjaroen, Munyoo, Tuchinda et al., High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin a as anti-SARS-CoV-2 agents, Sci Rep
Kongmanas, Punyadee, Wasuworawong, Songjaeng, Prommool et al., Immortalized stem cell-derived hepatocyte-like cells: an alternative model for studying dengue pathogenesis and therapy, PLoS Negl Trop Dis
Kory, Meduri, Varon, Iglesias, Marik, Review of the emerging evidence demonstrating the efficacy of Ivermectin in the prophylaxis and treatment of COVID-19, Am J Ther
Lehrer, Rheinstein, Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2, Vivo
Li, Li, Roberts, Arend, Samant et al., Multitargeted therapy of cancer by niclosamide: a new application for an old drug, Cancer Lett
Lifschitz, Virkel, Sallovitz, Sutra, Galtier et al., Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle, Vet Parasitol
Lim, Hor, Tay, Jelani, Tan et al., Efficacy of Ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial, JAMA Intern Med
Lima-Morales, Méndez-Hernández, Flores, Osorno-Romero, Cuecuecha-Rugerio et al., Effectiveness of a multidrug therapy consisting of ivermectin, azithromycin, montelukast and acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico, Int J Infect Dis
Loewe, The problem of synergism and antagonism of combined drugs, Arzneimittelforschung
Madrid, Panchal, Warren, Shurtleff, Endsley et al., Evaluation of Ebola virus inhibitors for drug repurposing, ACS Infect Dis
Mathieu, Ritchie, Ortiz-Ospina, Roser, Hasell et al., A global database of COVID-19 vaccinations, Nat Hum Behav
Mostafa, Kandeil, Elshaier, Kutkat, Moatasim et al., FDA-approved drugs with potent in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2, Pharm J
Muñoz, Ballester, Antonijoan, Gich, Rodríguez et al., Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers, PLoS Negl Trop Dis
Niyomdecha, Suptawiwat, Boonarkart, Jitobaom, Auewarakul, Inhibition of human immunodeficiency virus type 1 by niclosamide through mTORC1 inhibition, Heliyon
Pizzorno, Padey, Dubois, Julien, Traversier et al., In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2, Antivir Res
Popp, Metzendorf, Gould, Kranke, Meybohm et al., Ivermectin for preventing and treating COVID-19, Cochrane Database Syst Rev
Reed, Muench, A simple method of estimating fifty percent enpoint, Am J Epidemiol
Remme, Sole, Dadzie, Alley, Baker et al., Large scale ivermectin distribution and its epidemiological consequences, Acta Leiden
Savarino, Boelaert, Cassone, Majori, Cauda, Effects of chloroquine on viral infections: an old drug against today's diseases?, Lancet Infect Dis
Schmith, Zhou, Lohmer, The approved dose of Ivermectin alone is not the ideal dose for the treatment of COVID-19, Clin Pharmacol Ther
Schweizer, Haugk, Mckiernan, Gulati, Cheng et al., A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer, PLoS One
Singh, Ryan, Kredo, Chaplin, Fletcher, Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19, Cochrane Database Syst Rev, doi:10.1002/14651858.CD013587
Stachulski, Pidathala, Row, Sharma, Berry et al., Thiazolides as novel antiviral agents. 2. Inhibition of hepatitis C virus replication, J Med Chem
Suputtamongkol, Avirutnan, Mairiang, Angkasekwinai, Niwattayakul et al., Ivermectin accelerates circulating nonstructural protein 1 (NS1) clearance in adult dengue patients: a combined phase 2/3 randomized double-blinded placebo controlled trial, Clin Infect Dis
Tay, Fraser, Chan, Moreland, Rathore et al., Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antivir Res
Thomson, COVID-19: social distancing, ACE 2 receptors, protease inhibitors and beyond?, Int J Clin Pract
Vallejos, Zoni, Bangher, Villamandos, Bobadilla et al., Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial, BMC Infect Dis
Wagstaff, Rawlinson, Hearps, Jans, An AlphaScreen ® -based assay for high-throughput screening for specific inhibitors of nuclear import, J Biomol Screen
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J
Wang, Chen, Tissue distributions of antiviral drugs affect their capabilities of reducing viral loads in COVID-19 treatment, Eur J Pharmacol
Wen, Kuo, Liang, Wang, Liu, Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus, J Med Chem
Wu, Chen, Hsieh, Hwang, Liu, Inhibition of severe acute respiratory syndrome coronavirus replication by Niclosamide, Antimicrob Agents Chemother
Xu, Han, Liu, Pang, Zheng et al., Antivirus effectiveness of ivermectin on dengue virus type 2 in Aedes albopictus, PLoS Negl Trop Dis
Xu, Lee, Wen, Cheng, Huang et al., Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen, Nat Med
Xu, Shi, Li, Zhou, Broad Spectrum antiviral agent Niclosamide and its therapeutic potential, ACS Infect Dis
Yang, Atkinson, Wang, Lee, Bogoyevitch et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antivir Res
Zaidi, Dehgani-Mobaraki, The mechanisms of action of ivermectin against SARS-CoV-2-an extensive review, J Antibiot
Zheng, Wang, Aldahdooh, Malyutina, Shadbahr et al., SynergyFinder plus: toward better interpretation and annotation of drug combination screening datasets, Genomics Proteomics Bioinformatics
DOI record:
{
"DOI": "10.1186/s40360-022-00580-8",
"ISSN": [
"2050-6511"
],
"URL": "http://dx.doi.org/10.1186/s40360-022-00580-8",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>COVID-19 pandemic has claimed millions of lives and devastated the health service system, livelihood, and economy in many countries worldwide. Despite the vaccination programs in many countries, the spread of the pandemic continues, and effective treatment is still urgently needed. Although some antiviral drugs have been shown to be effective, they are not widely available. Repurposing of anti-parasitic drugs with in vitro anti-SARS-CoV-2 activity is a promising approach being tested in many clinical trials. Combination of these drugs is a plausible way to enhance their effectiveness.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>The in vitro anti-SARS-CoV-2 activity of combinations of niclosamide, ivermectin and chloroquine were evaluated in Vero E6 and lung epithelial cells, Calu-3.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>All the two-drug combinations showed higher potency resulting in up to 4-fold reduction in the half maximal inhibitory concentration (IC<jats:sub>50</jats:sub>) values compared to individual drugs. Among these combinations, niclosamide-ivermectin achieved the highest inhibitory level of over 99%. Combination synergy analysis showed niclosamide-ivermectin combination to have the best synergy score with a mean Loewe synergy score of 4.28 and a peak synergy score of 24.6 in Vero E6 cells and a mean Loewe synergy score of 3.82 and a peak synergy score of 10.86 in Calu-3 cells.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>The present study demonstrated the benefit of drug combinations on anti-SARS-CoV-2 activity. Niclosamide and ivermectin showed the best synergistic profile and should be further tested in clinical trials.</jats:p>\n </jats:sec>",
"alternative-id": [
"580"
],
"article-number": "41",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "5 January 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "9 June 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "18 June 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable. This work does not involve the use of human subjects and animals. All the procedures do not require IRB approval."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable. This work does not contain data from any individual person."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Jitobaom",
"given": "Kunlakanya",
"sequence": "first"
},
{
"affiliation": [],
"family": "Boonarkart",
"given": "Chompunuch",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manopwisedjaroen",
"given": "Suwimon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Punyadee",
"given": "Nuntaya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Borwornpinyo",
"given": "Suparerk",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thitithanyanont",
"given": "Arunee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Avirutnan",
"given": "Panisadee",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4745-4291",
"affiliation": [],
"authenticated-orcid": false,
"family": "Auewarakul",
"given": "Prasert",
"sequence": "additional"
}
],
"container-title": "BMC Pharmacology and Toxicology",
"container-title-short": "BMC Pharmacol Toxicol",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
6,
18
]
],
"date-time": "2022-06-18T09:02:54Z",
"timestamp": 1655542974000
},
"deposited": {
"date-parts": [
[
2022,
6,
18
]
],
"date-time": "2022-06-18T09:12:48Z",
"timestamp": 1655543568000
},
"funder": [
{
"DOI": "10.13039/501100004192",
"award": [
"P-20-52262"
],
"doi-asserted-by": "publisher",
"name": "National Science and Technology Development Agency"
},
{
"DOI": "10.13039/501100013238",
"doi-asserted-by": "publisher",
"name": "Faculty of Medicine Siriraj Hospital, Mahidol University"
}
],
"indexed": {
"date-parts": [
[
2022,
6,
18
]
],
"date-time": "2022-06-18T09:43:01Z",
"timestamp": 1655545381613
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
6,
18
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
18
]
],
"date-time": "2022-06-18T00:00:00Z",
"timestamp": 1655510400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
18
]
],
"date-time": "2022-06-18T00:00:00Z",
"timestamp": 1655510400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40360-022-00580-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s40360-022-00580-8/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40360-022-00580-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
6,
18
]
]
},
"published-online": {
"date-parts": [
[
2022,
6,
18
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1136/bmj.n1188",
"author": "O Dyer",
"doi-asserted-by": "publisher",
"first-page": "n1188",
"journal-title": "BMJ",
"key": "580_CR1",
"unstructured": "Dyer O. Covid-19: study claims real global deaths are twice official figures. BMJ. 2021;373:n1188.",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1038/s41562-021-01122-8",
"author": "E Mathieu",
"doi-asserted-by": "publisher",
"first-page": "947",
"journal-title": "Nat Hum Behav",
"key": "580_CR2",
"unstructured": "Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947–53.",
"volume": "5",
"year": "2021"
},
{
"key": "580_CR3",
"unstructured": "FDA Approves First Treatment for COVID-19. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19. Accessed 15 Dec 2021."
},
{
"key": "580_CR4",
"unstructured": "Coronavirus (COVID-19) Update: FDA Authorizes Drug Combination for Treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-combination-treatment-covid-19. Accessed 1 Dec 2021."
},
{
"DOI": "10.1016/j.ejphar.2020.173634",
"author": "Y Wang",
"doi-asserted-by": "publisher",
"first-page": "173634",
"journal-title": "Eur J Pharmacol",
"key": "580_CR5",
"unstructured": "Wang Y, Chen L. Tissue distributions of antiviral drugs affect their capabilities of reducing viral loads in COVID-19 treatment. Eur J Pharmacol. 2020;889:173634.",
"volume": "889",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1909",
"author": "U Arshad",
"doi-asserted-by": "publisher",
"first-page": "775",
"journal-title": "Clin Pharmacol Ther",
"key": "580_CR6",
"unstructured": "Arshad U, Pertinez H, Box H, Tatham L, Rajoli RKR, Curley P, et al. Prioritization of anti-SARS-CoV-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics. Clin Pharmacol Ther. 2020;108:775–90.",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00819-20",
"author": "S Jeon",
"doi-asserted-by": "publisher",
"first-page": "e00819",
"issue": "7",
"journal-title": "Antimicrob Agents Chemother",
"key": "580_CR7",
"unstructured": "Jeon S, Ko M, Lee J, Choi I, Byun SY, Park S, et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother. 2020;64(7):e00819–20.",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1002/14651858.CD013587",
"author": "B Singh",
"doi-asserted-by": "publisher",
"first-page": "CD013587",
"issue": "2",
"journal-title": "Cochrane Database Syst Rev",
"key": "580_CR8",
"unstructured": "Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst Rev. 2021;2(2):CD013587. https://doi.org/10.1002/14651858.CD013587.",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"author": "S Ahmed",
"doi-asserted-by": "publisher",
"first-page": "214",
"journal-title": "Int J Infect Dis",
"key": "580_CR9",
"unstructured": "Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–6.",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0247163",
"author": "P Behera",
"doi-asserted-by": "publisher",
"first-page": "e0247163",
"journal-title": "PLoS One",
"key": "580_CR10",
"unstructured": "Behera P, Patro BK, Singh AK, Chandanshive PD, Ravikumar SR, Pradhan SK, et al. Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: a matched case-control study. PLoS One. 2021;16:e0247163.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2020.100720",
"author": "C Chaccour",
"doi-asserted-by": "publisher",
"first-page": "100720",
"journal-title": "EClinicalMedicine",
"key": "580_CR11",
"unstructured": "Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, Ruiz-Castillo P, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine. 2021;32:100720.",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2021.02.014",
"author": "R Lima-Morales",
"doi-asserted-by": "publisher",
"first-page": "598",
"journal-title": "Int J Infect Dis",
"key": "580_CR12",
"unstructured": "Lima-Morales R, Méndez-Hernández P, Flores YN, Osorno-Romero P, Cuecuecha-Rugerio E, Nava-Zamora A, et al. Effectiveness of a multidrug therapy consisting of ivermectin, azithromycin, montelukast and acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico. Int J Infect Dis. 2021;105:598–605.",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.1097/MJT.0000000000001377",
"author": "P Kory",
"doi-asserted-by": "publisher",
"first-page": "e299",
"journal-title": "Am J Ther",
"key": "580_CR13",
"unstructured": "Kory P, Meduri GU, Varon J, Iglesias J, Marik PE. Review of the emerging evidence demonstrating the efficacy of Ivermectin in the prophylaxis and treatment of COVID-19. Am J Ther. 2021;28:e299–318.",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"author": "L Caly",
"doi-asserted-by": "publisher",
"first-page": "104787",
"journal-title": "Antivir Res",
"key": "580_CR14",
"unstructured": "Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;178:104787.",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1016/j.bbrc.2020.10.042",
"author": "DA Jans",
"doi-asserted-by": "publisher",
"first-page": "163",
"journal-title": "Biochem Biophys Res Commun",
"key": "580_CR15",
"unstructured": "Jans DA, Wagstaff KM. The broad spectrum host-directed agent ivermectin as an antiviral for SARS-CoV-2 ? Biochem Biophys Res Commun. 2021;538:163–72.",
"volume": "538",
"year": "2021"
},
{
"DOI": "10.1002/jmv.26880",
"doi-asserted-by": "crossref",
"key": "580_CR16",
"unstructured": "Elalfy H, Besheer T, El-Mesery A, El-Gilany A-H, Abd Elazez MS, Alhawarey A, et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J Med Virol. 2021;93(5):3176–83."
},
{
"author": "J Remme",
"first-page": "177",
"journal-title": "Acta Leiden",
"key": "580_CR17",
"unstructured": "Remme J, De Sole G, Dadzie KY, Alley ES, Baker RH, Habbema JD, et al. Large scale ivermectin distribution and its epidemiological consequences. Acta Leiden. 1990;59:177–91.",
"volume": "59",
"year": "1990"
},
{
"author": "A Mostafa",
"first-page": "443",
"issue": "12",
"journal-title": "Pharm J",
"key": "580_CR18",
"unstructured": "Mostafa A, Kandeil A, AMME Elshaier Y, Kutkat O, Moatasim Y, Rashad AA, et al. FDA-approved drugs with potent in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2. Pharm J. 2020;13(12):443.",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1021/acsinfecdis.0c00052",
"author": "J Xu",
"doi-asserted-by": "publisher",
"first-page": "909",
"journal-title": "ACS Infect Dis",
"key": "580_CR19",
"unstructured": "Xu J, Shi P-Y, Li H, Zhou J. Broad Spectrum antiviral agent Niclosamide and its therapeutic potential. ACS Infect Dis. 2020;6:909–15.",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1038/s41598-020-77003-3",
"author": "P Kanjanasirirat",
"doi-asserted-by": "publisher",
"first-page": "19963",
"journal-title": "Sci Rep",
"key": "580_CR20",
"unstructured": "Kanjanasirirat P, Suksatu A, Manopwisedjaroen S, Munyoo B, Tuchinda P, Jearawuttanakul K, et al. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin a as anti-SARS-CoV-2 agents. Sci Rep. 2020;10:19963.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1093/oxfordjournals.aje.a118408",
"author": "LJ Reed",
"doi-asserted-by": "publisher",
"first-page": "493",
"journal-title": "Am J Epidemiol",
"key": "580_CR21",
"unstructured": "Reed LJ, Muench H. A simple method of estimating fifty percent enpoint. Am J Epidemiol. 1938;27:493–7.",
"volume": "27",
"year": "1938"
},
{
"DOI": "10.1101/2020.05.29.109702",
"doi-asserted-by": "publisher",
"key": "580_CR22",
"unstructured": "Ganguly D, Rottet S, Yee S, Hee W, Smith A, Khin N, Millar A, Fahrer A. SYBR green one-step qRT-PCR for the detection of SARS-CoV-2 RNA in saliva. bioRxiv. 2020:05.29.109702. https://doi.org/10.1101/2020.05.29.109702."
},
{
"DOI": "10.1016/j.heliyon.2020.e04050",
"author": "N Niyomdecha",
"doi-asserted-by": "publisher",
"first-page": "e04050",
"journal-title": "Heliyon",
"key": "580_CR23",
"unstructured": "Niyomdecha N, Suptawiwat O, Boonarkart C, Jitobaom K, Auewarakul P. Inhibition of human immunodeficiency virus type 1 by niclosamide through mTORC1 inhibition. Heliyon. 2020;6:e04050.",
"volume": "6",
"year": "2020"
},
{
"author": "S Zheng",
"first-page": "00008",
"issue": "22",
"journal-title": "Genomics Proteomics Bioinformatics",
"key": "580_CR24",
"unstructured": "Zheng S, Wang W, Aldahdooh J, Malyutina A, Shadbahr T, Tanoli Z, et al. SynergyFinder plus: toward better interpretation and annotation of drug combination screening datasets. Genomics Proteomics Bioinformatics. 2022;S1672-0229(22):00008–0.",
"volume": "S1672-0229",
"year": "2022"
},
{
"author": "S Loewe",
"first-page": "285",
"issue": "6",
"journal-title": "Arzneimittelforschung",
"key": "580_CR25",
"unstructured": "Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3(6):285–90 PMID: 13081480.",
"volume": "3",
"year": "1953"
},
{
"DOI": "10.1016/j.antiviral.2020.104878",
"author": "A Pizzorno",
"doi-asserted-by": "publisher",
"first-page": "104878",
"journal-title": "Antivir Res",
"key": "580_CR26",
"unstructured": "Pizzorno A, Padey B, Dubois J, Julien T, Traversier A, Dulière V, et al. In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2. Antivir Res. 2020;181:104878.",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1021/jm070295s",
"author": "C-C Wen",
"doi-asserted-by": "publisher",
"first-page": "4087",
"journal-title": "J Med Chem",
"key": "580_CR27",
"unstructured": "Wen C-C, Kuo Y-H, Jan J-T, Liang P-H, Wang S-Y, Liu H-G, et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. 2007;50:4087–95.",
"volume": "50",
"year": "2007"
},
{
"DOI": "10.1128/AAC.48.7.2693-2696.2004",
"author": "C-J Wu",
"doi-asserted-by": "publisher",
"first-page": "2693",
"journal-title": "Antimicrob Agents Chemother",
"key": "580_CR28",
"unstructured": "Wu C-J, Jan J-T, Chen C-M, Hsieh H-P, Hwang D-R, Liu H-W, et al. Inhibition of severe acute respiratory syndrome coronavirus replication by Niclosamide. Antimicrob Agents Chemother. 2004;48:2693–6.",
"volume": "48",
"year": "2004"
},
{
"DOI": "10.1038/s41467-019-13659-4",
"author": "NC Gassen",
"doi-asserted-by": "publisher",
"first-page": "5770",
"journal-title": "Nat Commun",
"key": "580_CR29",
"unstructured": "Gassen NC, Niemeyer D, Muth D, Corman VM, Martinelli S, Gassen A, et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-coronavirus infection. Nat Commun. 2019;10:5770.",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1038/nm.4184",
"author": "M Xu",
"doi-asserted-by": "publisher",
"first-page": "1101",
"journal-title": "Nat Med",
"key": "580_CR30",
"unstructured": "Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, Qian X, et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22:1101–7.",
"volume": "22",
"year": "2016"
},
{
"DOI": "10.1021/jm201264t",
"author": "AV Stachulski",
"doi-asserted-by": "publisher",
"first-page": "8670",
"journal-title": "J Med Chem",
"key": "580_CR31",
"unstructured": "Stachulski AV, Pidathala C, Row EC, Sharma R, Berry NG, Lawrenson AS, et al. Thiazolides as novel antiviral agents. 2. Inhibition of hepatitis C virus replication. J Med Chem. 2011;54:8670–80.",
"volume": "54",
"year": "2011"
},
{
"DOI": "10.1021/acsinfecdis.5b00030",
"author": "PB Madrid",
"doi-asserted-by": "publisher",
"first-page": "317",
"journal-title": "ACS Infect Dis",
"key": "580_CR32",
"unstructured": "Madrid PB, Panchal RG, Warren TK, Shurtleff AC, Endsley AN, Green CE, et al. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect Dis. 2015;1:317–26.",
"volume": "1",
"year": "2015"
},
{
"author": "G Thomson",
"first-page": "e13503",
"journal-title": "Int J Clin Pract",
"key": "580_CR33",
"unstructured": "Thomson G. COVID-19: social distancing, ACE 2 receptors, protease inhibitors and beyond? Int J Clin Pract. 2020;74:e13503.",
"volume": "74",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1002976",
"author": "A Jurgeit",
"doi-asserted-by": "publisher",
"first-page": "e1002976",
"journal-title": "PLoS Pathog",
"key": "580_CR34",
"unstructured": "Jurgeit A, McDowell R, Moese S, Meldrum E, Schwendener R, Greber UF. Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects. PLoS Pathog. 2012;8:e1002976.",
"volume": "8",
"year": "2012"
},
{
"DOI": "10.1371/journal.pone.0198389",
"author": "MT Schweizer",
"doi-asserted-by": "publisher",
"first-page": "e0198389",
"journal-title": "PLoS One",
"key": "580_CR35",
"unstructured": "Schweizer MT, Haugk K, McKiernan JS, Gulati R, Cheng HH, Maes JL, et al. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLoS One. 2018;13:e0198389–9.",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1016/0163-7258(82)90064-X",
"author": "P Andrews",
"doi-asserted-by": "publisher",
"first-page": "245",
"journal-title": "Pharmacol Ther",
"key": "580_CR36",
"unstructured": "Andrews P, Thyssen J, Lorke D. The biology and toxicology of molluscicides, Bayluscide. Pharmacol Ther. 1982;19:245–95.",
"volume": "19",
"year": "1982"
},
{
"DOI": "10.1016/j.canlet.2014.04.003",
"author": "Y Li",
"doi-asserted-by": "publisher",
"first-page": "8",
"journal-title": "Cancer Lett",
"key": "580_CR37",
"unstructured": "Li Y, Li PK, Roberts MJ, Arend RC, Samant RS, Buchsbaum DJ. Multi-targeted therapy of cancer by niclosamide: a new application for an old drug. Cancer Lett. 2014;349:8–14.",
"volume": "349",
"year": "2014"
},
{
"DOI": "10.1186/s12885-018-4197-9",
"author": "S Burock",
"doi-asserted-by": "publisher",
"first-page": "297",
"journal-title": "BMC Cancer",
"key": "580_CR38",
"unstructured": "Burock S, Daum S, Keilholz U, Neumann K, Walther W, Stein U. Phase II trial to investigate the safety and efficacy of orally applied niclosamide in patients with metachronous or sychronous metastases of a colorectal cancer progressing after therapy: the NIKOLO trial. BMC Cancer. 2018;18:297.",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.1016/S1473-3099(03)00806-5",
"author": "A Savarino",
"doi-asserted-by": "publisher",
"first-page": "722",
"journal-title": "Lancet Infect Dis",
"key": "580_CR39",
"unstructured": "Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3:722–7.",
"volume": "3",
"year": "2003"
},
{
"DOI": "10.1038/s41586-020-2575-3",
"author": "M Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "588",
"journal-title": "Nature",
"key": "580_CR40",
"unstructured": "Hoffmann M, Mösbauer K, Hofmann-Winkler H, Kaul A, Kleine-Weber H, Krüger N, et al. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020;585:588–90.",
"volume": "585",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104760",
"author": "SNY Yang",
"doi-asserted-by": "publisher",
"first-page": "104760",
"journal-title": "Antivir Res",
"key": "580_CR41",
"unstructured": "Yang SNY, Atkinson SC, Wang C, Lee A, Bogoyevitch MA, Borg NA, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir Res. 2020;177:104760.",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2016.07.004",
"author": "NJ Barrows",
"doi-asserted-by": "publisher",
"first-page": "259",
"journal-title": "Cell Host Microbe",
"key": "580_CR42",
"unstructured": "Barrows NJ, Campos RK, Powell ST, Prasanth KR, Schott-Lerner G, Soto-Acosta R, et al. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe. 2016;20:259–70.",
"volume": "20",
"year": "2016"
},
{
"DOI": "10.1042/BJ20120150",
"author": "KM Wagstaff",
"doi-asserted-by": "publisher",
"first-page": "851",
"journal-title": "Biochem J",
"key": "580_CR43",
"unstructured": "Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443:851–6.",
"volume": "443",
"year": "2012"
},
{
"DOI": "10.1016/j.antiviral.2013.06.002",
"author": "MY Tay",
"doi-asserted-by": "publisher",
"first-page": "301",
"journal-title": "Antivir Res",
"key": "580_CR44",
"unstructured": "Tay MY, Fraser JE, Chan WK, Moreland NJ, Rathore AP, Wang C, et al. Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir Res. 2013;99:301–6.",
"volume": "99",
"year": "2013"
},
{
"DOI": "10.1371/journal.pntd.0006934",
"author": "T-L Xu",
"doi-asserted-by": "publisher",
"first-page": "e0006934",
"journal-title": "PLoS Negl Trop Dis",
"key": "580_CR45",
"unstructured": "Xu T-L, Han Y, Liu W, Pang X-Y, Zheng B, Zhang Y, et al. Antivirus effectiveness of ivermectin on dengue virus type 2 in Aedes albopictus. PLoS Negl Trop Dis. 2018;12:e0006934.",
"volume": "12",
"year": "2018"
},
{
"DOI": "10.1177/1087057110390360",
"author": "KM Wagstaff",
"doi-asserted-by": "publisher",
"first-page": "192",
"journal-title": "J Biomol Screen",
"key": "580_CR46",
"unstructured": "Wagstaff KM, Rawlinson SM, Hearps AC, Jans DA. An AlphaScreen®-based assay for high-throughput screening for specific inhibitors of nuclear import. J Biomol Screen. 2011;16:192–200.",
"volume": "16",
"year": "2011"
},
{
"DOI": "10.1038/srep23138",
"author": "V Götz",
"doi-asserted-by": "publisher",
"first-page": "23138",
"journal-title": "Sci Rep",
"key": "580_CR47",
"unstructured": "Götz V, Magar L, Dornfeld D, Giese S, Pohlmann A, Höper D, et al. Influenza a viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci Rep. 2016;6:23138–8.",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.21873/invivo.12134",
"author": "S Lehrer",
"doi-asserted-by": "publisher",
"first-page": "3023",
"journal-title": "In Vivo",
"key": "580_CR48",
"unstructured": "Lehrer S, Rheinstein PH. Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2. In Vivo. 2020;34:3023–6.",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1038/s41429-021-00491-6",
"author": "AK Zaidi",
"doi-asserted-by": "publisher",
"first-page": "60",
"journal-title": "J Antibiot",
"key": "580_CR49",
"unstructured": "Zaidi AK, Dehgani-Mobaraki P. The mechanisms of action of ivermectin against SARS-CoV-2—an extensive review. J Antibiot. 2022;75:60–71.",
"volume": "75",
"year": "2022"
},
{
"author": "MSM Popp",
"first-page": "CD015017",
"journal-title": "Cochrane Database Syst Rev",
"key": "580_CR50",
"unstructured": "Popp MSM, Metzendorf MI, Gould S, Kranke P, Meybohm P, Skoetz N, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev. 2021;28:CD015017.",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06348-5",
"author": "J Vallejos",
"doi-asserted-by": "publisher",
"first-page": "635",
"journal-title": "BMC Infect Dis",
"key": "580_CR51",
"unstructured": "Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 2021;21:635.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2022.0189",
"author": "SCL Lim",
"doi-asserted-by": "publisher",
"first-page": "426",
"issue": "4",
"journal-title": "JAMA Intern Med",
"key": "580_CR52",
"unstructured": "Lim SCL, Hor CP, Tay KH, Mat Jelani A, Tan WH, Ker HB, et al. Efficacy of Ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial. JAMA Intern Med. 2022;182(4):426–35.",
"volume": "182",
"year": "2022"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"author": "A Bryant",
"doi-asserted-by": "publisher",
"first-page": "e434",
"journal-title": "Am J Ther",
"key": "580_CR53",
"unstructured": "Bryant A, Lawrie TA, Dowswell T, Fordham EJ, Mitchell S, Hill SR, et al. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. 2021;28:e434–60.",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1371/journal.pntd.0006020",
"author": "J Muñoz",
"doi-asserted-by": "publisher",
"first-page": "e0006020",
"journal-title": "PLoS Negl Trop Dis",
"key": "580_CR54",
"unstructured": "Muñoz J, Ballester MR, Antonijoan RM, Gich I, Rodríguez M, Colli E, et al. Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers. PLoS Negl Trop Dis. 2018;12:e0006020.",
"volume": "12",
"year": "2018"
},
{
"DOI": "10.1007/s002280050131",
"author": "OZ Baraka",
"doi-asserted-by": "publisher",
"first-page": "407",
"journal-title": "Eur J Clin Pharmacol",
"key": "580_CR55",
"unstructured": "Baraka OZ, Mahmoud BM, Marschke CK, Geary TG, Homeida MM, Williams JF. Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus. Eur J Clin Pharmacol. 1996;50:407–10.",
"volume": "50",
"year": "1996"
},
{
"DOI": "10.1002/cpt.1889",
"author": "VD Schmith",
"doi-asserted-by": "publisher",
"first-page": "762",
"journal-title": "Clin Pharmacol Ther",
"key": "580_CR56",
"unstructured": "Schmith VD, Zhou J, Lohmer LRL. The approved dose of Ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin Pharmacol Ther. 2020;108:762–5.",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1016/S0304-4017(99)00175-2",
"author": "A Lifschitz",
"doi-asserted-by": "publisher",
"first-page": "327",
"journal-title": "Vet Parasitol",
"key": "580_CR57",
"unstructured": "Lifschitz A, Virkel G, Sallovitz J, Sutra JF, Galtier P, Alvinerie M, et al. Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle. Vet Parasitol. 2000;87:327–38.",
"volume": "87",
"year": "2000"
},
{
"DOI": "10.1021/jf00101a015",
"author": "SHL Chiu",
"doi-asserted-by": "publisher",
"first-page": "2072",
"journal-title": "J Agric Food Chem",
"key": "580_CR58",
"unstructured": "Chiu SHL, Green ML, Baylis FP, Eline D, Rosegay A, Meriwether H, et al. Absorption, tissue distribution, and excretion of tritium-labeled ivermectin in cattle, sheep, and rat. J Agric Food Chem. 1990;38:2072–8.",
"volume": "38",
"year": "1990"
},
{
"DOI": "10.1371/journal.pntd.0008835",
"author": "K Kongmanas",
"doi-asserted-by": "publisher",
"first-page": "e0008835",
"journal-title": "PLoS Negl Trop Dis",
"key": "580_CR59",
"unstructured": "Kongmanas K, Punyadee N, Wasuworawong K, Songjaeng A, Prommool T, Pewkliang Y, et al. Immortalized stem cell-derived hepatocyte-like cells: an alternative model for studying dengue pathogenesis and therapy. PLoS Negl Trop Dis. 2020;14:e0008835.",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1332",
"author": "Y Suputtamongkol",
"doi-asserted-by": "publisher",
"first-page": "e586",
"journal-title": "Clin Infect Dis",
"key": "580_CR60",
"unstructured": "Suputtamongkol Y, Avirutnan P, Mairiang D, Angkasekwinai N, Niwattayakul K, Yamasmith E, et al. Ivermectin accelerates circulating nonstructural protein 1 (NS1) clearance in adult dengue patients: a combined phase 2/3 randomized double-blinded placebo controlled trial. Clin Infect Dis. 2021;72:e586–93.",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1016/j.ymthe.2020.12.016",
"author": "T Bobrowski",
"doi-asserted-by": "publisher",
"first-page": "873",
"journal-title": "Mol Ther",
"key": "580_CR61",
"unstructured": "Bobrowski T, Chen L, Eastman RT, Itkin Z, Shinn P, Chen CZ, et al. Synergistic and antagonistic drug combinations against SARS-CoV-2. Mol Ther. 2021;29:873–85.",
"volume": "29",
"year": "2021"
}
],
"reference-count": 61,
"references-count": 61,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcpharmacoltoxicol.biomedcentral.com/articles/10.1186/s40360-022-00580-8"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "23"
}
