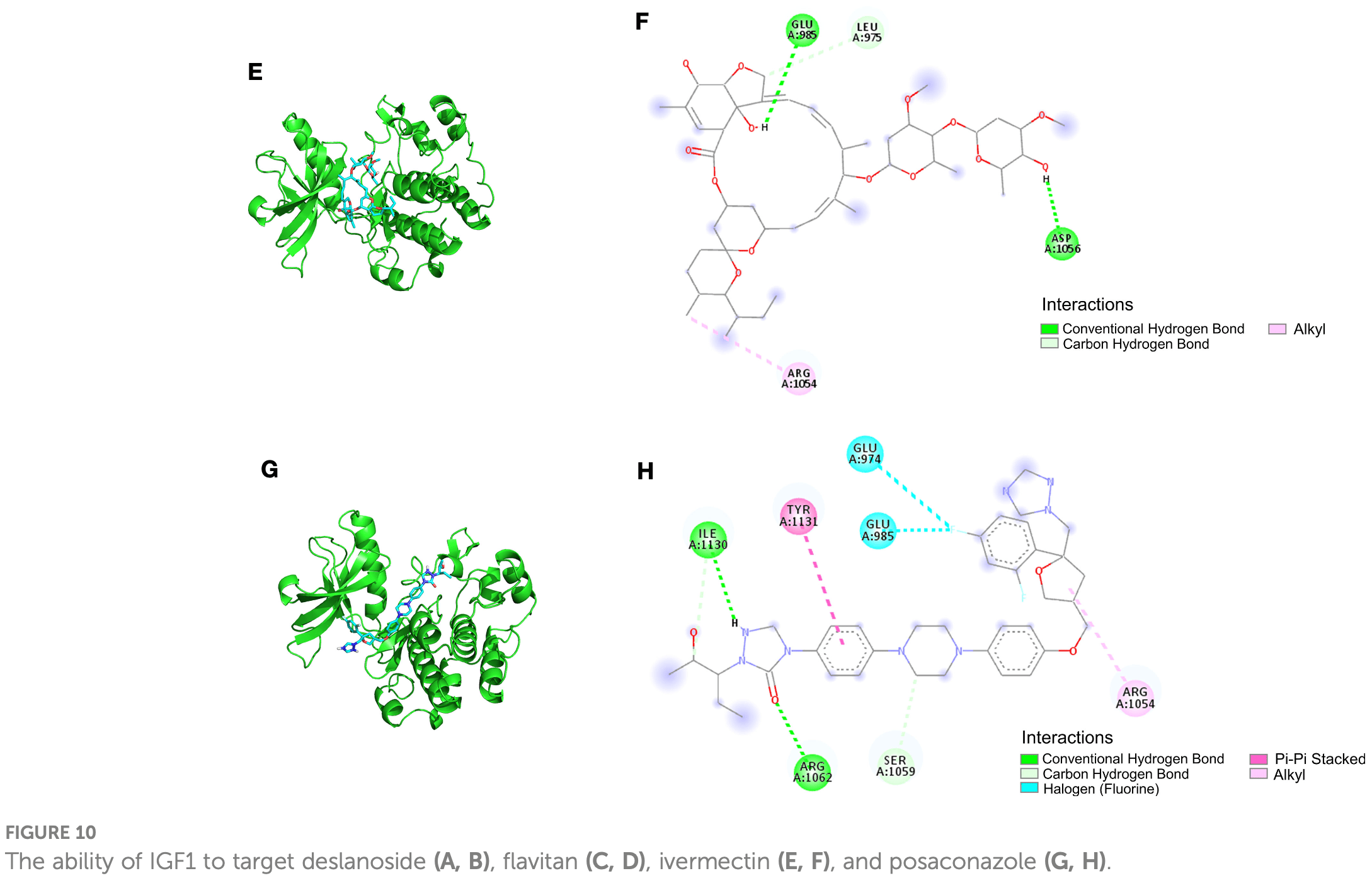
Identification of the shared gene signatures between pulmonary fibrosis and pulmonary hypertension using bioinformatics analysis
et al., Frontiers in Immunology, doi:10.3389/fimmu.2023.1197752, Sep 2023
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In silico study identifying IGF1 as a shared gene between pulmonary fibrosis and hypertension that promotes inflammation, fibrosis, and cell proliferation when overactivated. Molecular docking analysis demonstrated ivermectin directly binds IGF1 through multiple binding modes. This suggests ivermectin may inhibit IGF1 signaling. Since uncontrolled inflammation and lung fibrosis are major issues in severe COVID-19, ivermectin's ability to bind IGF1 indicates it may be able to reduce IGF1-mediated inflammation and fibrosis. By binding and inhibiting IGF1, ivermectin could potentially attenuate damaging effects of hyperactive IGF1 signaling on lung tissues observed in critical COVID-19 cases. This proposed mechanism of action via IGF1 binding provides a rationale for how ivermectin could protect lungs against inflammatory damage in severe COVID-19.
74 preclinical studies support the efficacy of ivermectin for COVID-19:
Ivermectin, better known for antiparasitic activity, is a broad spectrum antiviral with activity against many viruses including H7N771, Dengue37,72,73 , HIV-173, Simian virus 4074, Zika37,75,76 , West Nile76, Yellow Fever77,78, Japanese encephalitis77, Chikungunya78, Semliki Forest virus78, Human papillomavirus57, Epstein-Barr57, BK Polyomavirus79, and Sindbis virus78.
Ivermectin inhibits importin-α/β-dependent nuclear import of viral proteins71,73,74,80 , shows spike-ACE2 disruption at 1nM with microfluidic diffusional sizing38, binds to glycan sites on the SARS-CoV-2 spike protein preventing interaction with blood and epithelial cells and inhibiting hemagglutination41,81, shows dose-dependent inhibition of wildtype and omicron variants36, exhibits dose-dependent inhibition of lung injury61,66, may inhibit SARS-CoV-2 via IMPase inhibition37, may inhibit SARS-CoV-2 induced formation of fibrin clots resistant to degradation9, inhibits SARS-CoV-2 3CLpro54, may inhibit SARS-CoV-2 RdRp activity28, may minimize viral myocarditis by inhibiting NF-κB/p65-mediated inflammation in macrophages60, may be beneficial for COVID-19 ARDS by blocking GSDMD and NET formation82, may interfere with SARS-CoV-2's immune evasion via ORF8 binding4, may inhibit SARS-CoV-2 by disrupting CD147 interaction83-86, may inhibit SARS-CoV-2 attachment to lipid rafts via spike NTD binding2, shows protection against inflammation, cytokine storm, and mortality in an LPS mouse model sharing key pathological features of severe COVID-1959,87, may be beneficial in severe COVID-19 by binding IGF1 to inhibit the promotion of inflammation, fibrosis, and cell proliferation that leads to lung damage8, may minimize SARS-CoV-2 induced cardiac damage40,48, may counter immune evasion by inhibiting NSP15-TBK1/KPNA1 interaction and restoring IRF3 activation88, may disrupt SARS-CoV-2 N and ORF6 protein nuclear transport and their suppression of host interferon responses1, reduces TAZ/YAP nuclear import, relieving SARS-CoV-2-driven suppression of IRF3 and NF-κB antiviral pathways35, increases Bifidobacteria which play a key role in the immune system89, has immunomodulatory51 and anti-inflammatory70,90 properties, and has an extensive and very positive safety profile91.
1.
Gayozo et al., Binding affinities analysis of ivermectin, nucleocapsid and ORF6 proteins of SARS-CoV-2 to human importins α isoforms: A computational approach, Biotecnia, doi:10.18633/biotecnia.v27.2485.
2.
Lefebvre et al., Characterization and Fluctuations of an Ivermectin Binding Site at the Lipid Raft Interface of the N-Terminal Domain (NTD) of the Spike Protein of SARS-CoV-2 Variants, Viruses, doi:10.3390/v16121836.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Bagheri-Far et al., Non-spike protein inhibition of SARS-CoV-2 by natural products through the key mediator protein ORF8, Molecular Biology Research Communications, doi:10.22099/mbrc.2024.50245.2001.
5.
de Oliveira Só et al., In Silico Comparative Analysis of Ivermectin and Nirmatrelvir Inhibitors Interacting with the SARS-CoV-2 Main Protease, Preprints, doi:10.20944/preprints202404.1825.v1.
6.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
7.
Oranu et al., Validation of the binding affinities and stabilities of ivermectin and moxidectin against SARS-CoV-2 receptors using molecular docking and molecular dynamics simulation, GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2024.26.1.0030.
8.
Zhao et al., Identification of the shared gene signatures between pulmonary fibrosis and pulmonary hypertension using bioinformatics analysis, Frontiers in Immunology, doi:10.3389/fimmu.2023.1197752.
9.
Vottero et al., Computational Prediction of the Interaction of Ivermectin with Fibrinogen, Molecular Sciences, doi:10.3390/ijms241411449.
10.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
11.
Umar et al., Inhibitory potentials of ivermectin, nafamostat, and camostat on spike protein and some nonstructural proteins of SARS-CoV-2: Virtual screening approach, Jurnal Teknologi Laboratorium, doi:10.29238/teknolabjournal.v11i1.344.
12.
Alvarado et al., Interaction of the New Inhibitor Paxlovid (PF-07321332) and Ivermectin With the Monomer of the Main Protease SARS-CoV-2: A Volumetric Study Based on Molecular Dynamics, Elastic Networks, Classical Thermodynamics and SPT, Computational Biology and Chemistry, doi:10.1016/j.compbiolchem.2022.107692.
13.
Aminpour et al., In Silico Analysis of the Multi-Targeted Mode of Action of Ivermectin and Related Compounds, Computation, doi:10.3390/computation10040051.
14.
Parvez et al., Insights from a computational analysis of the SARS-CoV-2 Omicron variant: Host–pathogen interaction, pathogenicity, and possible drug therapeutics, Immunity, Inflammation and Disease, doi:10.1002/iid3.639.
15.
Francés-Monerris et al., Microscopic interactions between ivermectin and key human and viral proteins involved in SARS-CoV-2 infection, Physical Chemistry Chemical Physics, doi:10.1039/D1CP02967C.
16.
González-Paz et al., Comparative study of the interaction of ivermectin with proteins of interest associated with SARS-CoV-2: A computational and biophysical approach, Biophysical Chemistry, doi:10.1016/j.bpc.2021.106677.
17.
González-Paz (B) et al., Structural Deformability Induced in Proteins of Potential Interest Associated with COVID-19 by binding of Homologues present in Ivermectin: Comparative Study Based in Elastic Networks Models, Journal of Molecular Liquids, doi:10.1016/j.molliq.2021.117284.
18.
Rana et al., A Computational Study of Ivermectin and Doxycycline Combination Drug Against SARS-CoV-2 Infection, Research Square, doi:10.21203/rs.3.rs-755838/v1.
19.
Muthusamy et al., Virtual Screening Reveals Potential Anti-Parasitic Drugs Inhibiting the Receptor Binding Domain of SARS-CoV-2 Spike protein, Journal of Virology & Antiviral Research, www.scitechnol.com/abstract/virtual-screening-reveals-potential-antiparasitic-drugs-inhibiting-the-receptor-binding-domain-of-sarscov2-spike-protein-16398.html.
20.
Qureshi et al., Mechanistic insights into the inhibitory activity of FDA approved ivermectin against SARS-CoV-2: old drug with new implications, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1906750.
21.
Schöning et al., Highly-transmissible Variants of SARS-CoV-2 May Be More Susceptible to Drug Therapy Than Wild Type Strains, Research Square, doi:10.21203/rs.3.rs-379291/v1.
22.
Bello et al., Elucidation of the inhibitory activity of ivermectin with host nuclear importin α and several SARS-CoV-2 targets, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1911857.
23.
Udofia et al., In silico studies of selected multi-drug targeting against 3CLpro and nsp12 RNA-dependent RNA-polymerase proteins of SARS-CoV-2 and SARS-CoV, Network Modeling Analysis in Health Informatics and Bioinformatics, doi:10.1007/s13721-021-00299-2.
24.
Choudhury et al., Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach, Future Medicine, doi:10.2217/fvl-2020-0342.
25.
Kern et al., Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing, Frontiers in Pharmacology, doi:10.3389/fphar.2021.625678.
26.
Saha et al., The Binding mechanism of ivermectin and levosalbutamol with spike protein of SARS-CoV-2, Structural Chemistry, doi:10.1007/s11224-021-01776-0.
27.
Eweas et al., Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2, Frontiers in Microbiology, doi:10.3389/fmicb.2020.592908.
28.
Parvez (B) et al., Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.09.098.
29.
Francés-Monerris (B) et al., Has Ivermectin Virus-Directed Effects against SARS-CoV-2? Rationalizing the Action of a Potential Multitarget Antiviral Agent, ChemRxiv, doi:10.26434/chemrxiv.12782258.v1.
30.
Kalhor et al., Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1824816.
31.
Swargiary, A., Ivermectin as a promising RNA-dependent RNA polymerase inhibitor and a therapeutic drug against SARS-CoV2: Evidence from in silico studies, Research Square, doi:10.21203/rs.3.rs-73308/v1.
32.
Maurya, D., A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID-19 Patients, American Chemical Society (ACS), doi:10.26434/chemrxiv.12630539.v1.
33.
Lehrer et al., Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, In Vivo, 34:5, 3023-3026, doi:10.21873/invivo.12134.
34.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
35.
Kofler et al., M-Motif, a potential non-conventional NLS in YAP/TAZ and other cellular and viral proteins that inhibits classic protein import, iScience, doi:10.1016/j.isci.2025.112105.
36.
Shahin et al., The selective effect of Ivermectin on different human coronaviruses; in-vitro study, Research Square, doi:10.21203/rs.3.rs-4180797/v1.
37.
Jitobaom et al., Identification of inositol monophosphatase as a broad‐spectrum antiviral target of ivermectin, Journal of Medical Virology, doi:10.1002/jmv.29552.
38.
Fauquet et al., Microfluidic Diffusion Sizing Applied to the Study of Natural Products and Extracts That Modulate the SARS-CoV-2 Spike RBD/ACE2 Interaction, Molecules, doi:10.3390/molecules28248072.
39.
García-Aguilar et al., In Vitro Analysis of SARS-CoV-2 Spike Protein and Ivermectin Interaction, International Journal of Molecular Sciences, doi:10.3390/ijms242216392.
40.
Liu et al., SARS-CoV-2 viral genes Nsp6, Nsp8, and M compromise cellular ATP levels to impair survival and function of human pluripotent stem cell-derived cardiomyocytes, Stem Cell Research & Therapy, doi:10.1186/s13287-023-03485-3.
41.
Boschi et al., SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects, bioRxiv, doi:10.1101/2022.11.24.517882.
42.
De Forni et al., Synergistic drug combinations designed to fully suppress SARS-CoV-2 in the lung of COVID-19 patients, PLoS ONE, doi:10.1371/journal.pone.0276751.
43.
Saha (B) et al., Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder, Pharmaceutics, doi:10.3390/pharmaceutics14071432.
44.
Jitobaom (B) et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8.
45.
Croci et al., Liposomal Systems as Nanocarriers for the Antiviral Agent Ivermectin, International Journal of Biomaterials, doi:10.1155/2016/8043983.
46.
Zheng et al., Red blood cell-hitchhiking mediated pulmonary delivery of ivermectin: Effects of nanoparticle properties, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121719.
47.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
48.
Liu (B) et al., Genome-wide analyses reveal the detrimental impacts of SARS-CoV-2 viral gene Orf9c on human pluripotent stem cell-derived cardiomyocytes, Stem Cell Reports, doi:10.1016/j.stemcr.2022.01.014.
49.
Segatori et al., Effect of Ivermectin and Atorvastatin on Nuclear Localization of Importin Alpha and Drug Target Expression Profiling in Host Cells from Nasopharyngeal Swabs of SARS-CoV-2- Positive Patients, Viruses, doi:10.3390/v13102084.
50.
Jitobaom (C) et al., Favipiravir and Ivermectin Showed in Vitro Synergistic Antiviral Activity against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-941811/v1.
51.
Munson et al., Niclosamide and ivermectin modulate caspase-1 activity and proinflammatory cytokine secretion in a monocytic cell line, British Society For Nanomedicine Early Career Researcher Summer Meeting, 2021, web.archive.org/web/20230401070026/https://michealmunson.github.io/COVID.pdf.
52.
Mountain Valley MD, Mountain Valley MD Receives Successful Results From BSL-4 COVID-19 Clearance Trial on Three Variants Tested With Ivectosol™, 5/18, www.globenewswire.com/en/news-release/2021/05/18/2231755/0/en/Mountain-Valley-MD-Receives-Successful-Results-From-BSL-4-COVID-19-Clearance-Trial-on-Three-Variants-Tested-With-Ivectosol.html.
53.
Yesilbag et al., Ivermectin also inhibits the replication of bovine respiratory viruses (BRSV, BPIV-3, BoHV-1, BCoV and BVDV) in vitro, Virus Research, doi:10.1016/j.virusres.2021.198384.
54.
Mody et al., Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents, Communications Biology, doi:10.1038/s42003-020-01577-x.
55.
Jeffreys et al., Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2022.106542.
56.
Surnar et al., Clinically Approved Antiviral Drug in an Orally Administrable Nanoparticle for COVID-19, ACS Pharmacol. Transl. Sci., doi:10.1021/acsptsci.0c00179.
57.
Li et al., Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment, J. Cellular Physiology, doi:10.1002/jcp.30055.
58.
Caly et al., The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research, doi:10.1016/j.antiviral.2020.104787.
59.
Zhang et al., Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflammation Research, doi:10.1007/s00011-008-8007-8.
60.
Gao et al., Ivermectin ameliorates acute myocarditis via the inhibition of importin-mediated nuclear translocation of NF-κB/p65, International Immunopharmacology, doi:10.1016/j.intimp.2024.112073.
61.
Abd-Elmawla et al., Suppression of NLRP3 inflammasome by ivermectin ameliorates bleomycin-induced pulmonary fibrosis, Journal of Zhejiang University-SCIENCE B, doi:10.1631/jzus.B2200385.
62.
Uematsu et al., Prophylactic administration of ivermectin attenuates SARS-CoV-2 induced disease in a Syrian Hamster Model, The Journal of Antibiotics, doi:10.1038/s41429-023-00623-0.
63.
Albariqi et al., Pharmacokinetics and Safety of Inhaled Ivermectin in Mice as a Potential COVID-19 Treatment, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121688.
64.
Errecalde et al., Safety and Pharmacokinetic Assessments of a Novel Ivermectin Nasal Spray Formulation in a Pig Model, Journal of Pharmaceutical Sciences, doi:10.1016/j.xphs.2021.01.017.
65.
Madrid et al., Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation, Heliyon, doi:10.1016/j.heliyon.2020.e05820.
66.
Ma et al., Ivermectin contributes to attenuating the severity of acute lung injury in mice, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2022.113706.
67.
de Melo et al., Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin, EMBO Mol. Med., doi:10.15252/emmm.202114122.
68.
Arévalo et al., Ivermectin reduces in vivo coronavirus infection in a mouse experimental model, Scientific Reports, doi:10.1038/s41598-021-86679-0.
69.
Chaccour et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Scientific Reports, doi:10.1038/s41598-020-74084-y.
70.
Yan et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflammation Research, doi:10.1007/s00011-011-0307-8.
71.
Götz et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Scientific Reports, doi:10.1038/srep23138.
72.
Tay et al., Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Research, doi:10.1016/j.antiviral.2013.06.002.
73.
Wagstaff et al., Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochemical Journal, doi:10.1042/BJ20120150.
74.
Wagstaff (B) et al., An AlphaScreen®-Based Assay for High-Throughput Screening for Specific Inhibitors of Nuclear Import, SLAS Discovery, doi:10.1177/1087057110390360.
75.
Barrows et al., A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection, Cell Host & Microbe, doi:10.1016/j.chom.2016.07.004.
76.
Yang et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Research, doi:10.1016/j.antiviral.2020.104760.
77.
Mastrangelo et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dks147.
78.
Varghese et al., Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses, Antiviral Research, doi:10.1016/j.antiviral.2015.12.012.
79.
Bennett et al., Role of a nuclear localization signal on the minor capsid Proteins VP2 and VP3 in BKPyV nuclear entry, Virology, doi:10.1016/j.virol.2014.10.013.
80.
Kosyna et al., The importin α/β-specific inhibitor Ivermectin affects HIF-dependent hypoxia response pathways, Biological Chemistry, doi:10.1515/hsz-2015-0171.
81.
Scheim et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
82.
Liu (C) et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
83.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
84.
Scheim (B), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
85.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
86.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
87.
DiNicolantonio et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
88.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
89.
Hazan et al., Treatment with Ivermectin Increases the Population of Bifidobacterium in the Gut, ACG 2023, acg2023posters.eventscribe.net/posterspeakers.asp.
Zhao et al., 4 Sep 2023, peer-reviewed, 12 authors.
Contact: wenhui5621006@126.com, pandyyuan@tongji.edu.cn, gongsugang@tongji.edu.cn.
In silico studies are an important part of preclinical research, however results may be very different in vivo.
Identification of the shared gene signatures between pulmonary fibrosis and pulmonary hypertension using bioinformatics analysis
Frontiers in Immunology, doi:10.3389/fimmu.2023.1197752
Pulmonary fibrosis (PF) and pulmonary hypertension (PH) have common pathophysiological features, such as the significant remodeling of pulmonary parenchyma and vascular wall. There is no effective specific drug in clinical treatment for these two diseases, resulting in a worse prognosis and higher mortality. This study aimed to screen the common key genes and immune characteristics of PF and PH by means of bioinformatics to find new common therapeutic targets. Expression profiles are
Ethics statement The studies involving humans were approved by the Ethics Committee of Shanghai Pulmonary Hospital (numbers: K22-137Y). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions HZ, LW, and YY investigated the literature research, got the data, and analyzed the data. Q-HZ and JH wrote the article. RJ and C-JL modified the figures. H-LQ and Y-QM revised the article. W-HW, PY, and S-GG conceived the idea of the study, designed the steps of the study, and directed the data analysis. All authors contributed to the article and approved the submitted version.
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1197752/ full#supplementary-material
References
Azuaje, Zhang, Jeanty, Puhl, Rodius et al., Analysis of a gene co-expression network establishes robust association between Col5a2 and ischemic heart disease, BMC Med Genomics, doi:10.1186/1755-8794-6-13
Bilbao, Luciani, Johannesson, Piszczek, Rosenthal, Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease, EMBO Mol Med, doi:10.15252/emmm.201303376
Bindea, Mlecnik, Hackl, Charoentong, Tosolini et al., ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks, Bioinformatics, doi:10.1093/bioinformatics/btp101
Birjandi, Palchevskiy, Xue, Nunez, Kern et al., CD4(+) CD25(hi)Foxp3(+) cells exacerbate bleomycin-induced pulmonary fibrosis, Am J Pathol, doi:10.1016/j.ajpath.2016.03.020
Bloor, Knight, Kedia, Spiteri, Allen, Differential mRNA expression of insulin-like growth factor-1 splice variants in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis, Am J Respir Crit Care Med, doi:10.1164/ajrccm.164.2.2003114
Bourgeois, Bonnet, Breuils-Bonnet, Habbout, Paradis et al., Inhibition of CHK 1 (Checkpoint kinase 1) elicits therapeutic effects in pulmonary arterial hypertension, Arterioscler Thromb Vasc Biol, doi:10.1161/ATVBAHA.119.312537
Brummelman, Pilipow, Lugli, The single-cell phenotypic identity of human CD8(+) and CD4(+) T cells, Int Rev Cell Mol Biol, doi:10.1016/bs.ircmb.2018.05.007
Celada, Kropski, Herazo-Maya, Luo, Creecy et al., PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-b1 production, Sci Transl Med, doi:10.1126/scitranslmed.aar8356
Chen, Collum, Luo, Weng, Le et al., Macrophage bone morphogenic protein receptor 2 depletion in idiopathic pulmonary fibrosis and Group III pulmonary hypertension, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00142.2016
Chen, Liu, Sun, Zeng, Cai et al., Foxf2 and Smad6 co-regulation of collagen 5A2 transcription is involved in the pathogenesis of intrauterine adhesion, J Cell Mol Med, doi:10.1111/jcmm.14708
Choi, Lee, Sunde, Huizar, Haugk et al., Insulinlike growth factor-I receptor blockade improves outcome in mouse model of lung injury, Am J Respir Crit Care Med, doi:10.1164/rccm.200802-228OC
Collum, Amione-Guerra, As, Difrancesco, Hernandez et al., Pulmonary hypertension associated with idiopathic pulmonary fibrosis: current and future perspectives, Can Respir J, doi:10.1155/2017/1430350
Depianto, Chandriani, Abbas, Jia, 'diaye et al., Heterogeneous gene expression signatures correspond to distinct lung pathologies and biomarkers of disease severity in idiopathic pulmonary fibrosis, Thorax, doi:10.1136/thoraxjnl-2013-204596
Echeverria-Esnal, Martıń-Ontiyuelo, Navarrete-Rouco, Barcelo-Vidal, Conde-Estevez et al., Pharmacological management of antifungal agents in pulmonary aspergillosis: an updated review, Expert Rev Anti Infect Ther, doi:10.1080/14787210.2021.1962292
Formiga, Leblanc, De Souza Reboucas, Farias, De Oliveira et al., Ivermectin: an award-winning drug with expected antiviral activity against COVID-19, J Control Release, doi:10.1016/j.jconrel.2020.10.009
Garibaldi, 'alessio, Mock, Files, Chau et al., Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment, Am J Respir Cell Mol Biol, doi:10.1165/rcmb.2012-0198OC
George, Patterson, Reed, Thillai, Lung transplantation for idiopathic pulmonary fibrosis, Lancet Respir Med, doi:10.1016/S2213-2600(18)30502-2
Gilani, Vuga, Lindell, Gibson, Xue et al., CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis, PloS One, doi:10.1371/journal.pone.0008959
Govindarajan, Duraiyan, Kaliyappan, Palanisamy, Microarray and its applications, J Pharm Bioallied Sci, doi:10.4103/0975-7406.100283
Grant, Mimche, Paine R 3rd, Liou, Qian et al., Enhanced epithelial sodium channel activity in neonatal Scnn1b mouse lung attenuates high oxygen-induced lung injury, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00538.2020
Gu, Kumar, Lee, Mickael, Graham, Common genetic variants in pulmonary arterial hypertension, Lancet Respir Med, doi:10.1016/S2213-2600(18)30448-X
Hautefort, Girerd, Montani, Cohen-Kaminsky, Price et al., T-helper 17 cell polarization in pulmonary arterial hypertension, Chest, doi:10.1378/chest.14-1678
Heukels, Moor, Der Thüsen, Wijsenbeek, Kool, Inflammation and immunity in IPF pathogenesis and treatment, Respir Med, doi:10.1016/j.rmed.2018.12.015
Humbert, Kovacs, Hoeper, Badagliacca, Berger et al., ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension, Eur Respir J, doi:10.1183/13993003.00879-2022
Ichikawa, Hirahara, Kokubo, Kiuchi, Aoki et al., CD103 (hi) T(reg) cells constrain lung fibrosis induced by CD103(lo) tissue-resident pathogenic CD4 T cells, Nat Immunol, doi:10.1038/s41590-019-0494-y
Kolahian, Fernandez, Eickelberg, Hartl, Immune mechanisms in pulmonary fibrosis, Am J Respir Cell Mol Biol, doi:10.1165/rcmb.2016-0121TR
Kotsianidis, Nakou, Bouchliou, Tzouvelekis, Spanoudakis et al., Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis, Am J Respir Crit Care Med, doi:10.1164/rccm.200812-1936OC
Kundranda, Gracian, Zafar, Meiri, Bendell et al., Randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE), Ann Oncol, doi:10.1016/j.annonc.2019.09.004
Langfelder, Horvath, WGCNA: an R package for weighted correlation network analysis, BMC Bioinf, doi:10.1186/1471-2105-9-559
Lettieri, Nathan, Barnett, Ahmad, Shorr, Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis, Chest, doi:10.1378/chest.129.3.746
Liu, Huang, Li, Li, Zhang, The cardiac glycoside deslanoside exerts anticancer activity in prostate cancer cells by modulating multiple signaling pathways, Cancers, doi:10.3390/cancers13225809
Liu, Shi, Jiang, Liu, He et al., Regulatory T cellrelated gene indicators in pulmonary hypertension, Front Pharmacol, doi:10.3389/fphar.2022.908783
Lu, Li, Hu, Wang, Expression of immune related genes and possible regulatory mechanisms in Alzheimer's disease, Front Immunol, doi:10.3389/fimmu.2021.768966
Ma, Qin, Zhong, Liao, Su et al., Flavine adenine dinucleotide inhibits pathological cardiac hypertrophy and fibrosis through activating short chain acyl-CoA dehydrogenase, Biochem Pharmacol, doi:10.1016/j.bcp.2020.114100
Martinez, Collard, Pardo, Raghu, Richeldi et al., Idiopathic pulmonary fibrosis, Nat Rev Dis Primers, doi:10.1038/nrdp.2017.74
Meng, Shi, Zeng, Chen, Wu, The role of COL5A2 in patients with muscle-invasive bladder cancer: A bioinformatics analysis of public datasets involving 787 subjects and 29 cell lines, Front Oncol, doi:10.3389/fonc.2018.00659
Milara, Ballester, Morell, Ortiz, Escriváj et al., JAK2 mediates lung fibrosis, pulmonary vascular remodelling and hypertension in idiopathic pulmonary fibrosis: an experimental study, Thorax, doi:10.1136/thoraxjnl-2017-210728
Mirdamadi, Bommhardt, Goihl, Guttek, Zouboulis et al., Insulin and Insulin-like growth factor-1 can activate the phosphoinositide-3-kinase /Akt/FoxO1 pathway in T cells in vitro, Dermatoendocrinol, doi:10.1080/19381980.2017.1356518
Mura, Cecchini, Joseph, Granton, Osteopontin lung gene expression is a marker of disease severity in pulmonary arterial hypertension, Respirology, doi:10.1111/resp.13557
Murphy, Durum, Longo, Human growth hormone promotes engraftment of murine or human T cells in severe combined immunodeficient mice, Proc Natl Acad Sci U.S.A, doi:10.1073/pnas.89.10.4481
Nathan, Barbera, Gaine, Harari, Martinez et al., Pulmonary hypertension in chronic lung disease and hypoxia, Eur Respir J, doi:10.1183/13993003.01914-2018
Nathan, Waxman, Rajagopal, Case, Johri et al., Inhaled treprostinil and forced vital capacity in patients with interstitial lung disease and associated pulmonary hypertension: a post-hoc analysis of the INCREASE study, Lancet Respir Med, doi:10.1016/S2213-2600(21)00165-X
No, Imam, Nadeem, Al-Harbi, Korashy et al., Riboflavin attenuates lipopolysaccharide-induced lung injury in rats, Toxicol Mech Methods, doi:10.3109/15376516.2015.1045662
Oherle, Acker, Bonfield, Wang, Gray et al., Insulin-like growth factor 1 supports a pulmonary niche that promotes type 3 innate lymphoid cell development in newborn lungs, Immunity, doi:10.1016/j.immuni.2020.01.005
Pahal, Sharma, Idiopathic Pulmonary Artery Hypertension
Raghu, Amatto, Behr, Stowasser, Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review, Eur Respir J, doi:10.1183/13993003.02316-2014
Rajagopal, Bryant, Sahay, Wareing, Zhou et al., Idiopathic pulmonary fibrosis and pulmonary hypertension: Heracles meets the Hydra, Br J Pharmacol, doi:10.1111/bph.15036
Rajkumar, Konishi, Richards, Ishizawar, Wiechert et al., Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension, Am J Physiol Heart Circ Physiol, doi:10.1152/ajpheart.00254.2009
Savai, Pullamsetti, Kolbe, Bieniek, Voswinckel et al., Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension, Am J Respir Crit Care Med, doi:10.1164/rccm.201202-0335OC
Sharma, Maclean, Pinto, Kradin, The effect of an anti-CD3 monoclonal antibody on bleomycin-induced lymphokine production and lung injury, Am J Respir Crit Care Med, doi:10.1164/ajrccm.154.1.8680680
Stacher, Graham, Hunt, Gandjeva, Groshong et al., Modern age pathology of pulmonary arterial hypertension, Am J Respir Crit Care Med, doi:10.1164/rccm.201201-0164OC
Sun, Ramchandran, Chen, Yang, Raj, Smooth muscle insulin-like growth factor-1 mediates hypoxia-induced pulmonary hypertension in neonatal mice, Am J Respir Cell Mol Biol, doi:10.1165/rcmb.2015-0388OC
Tamosiuniene, Manouvakhova, Mesange, Saito, Qian et al., Dominant role for regulatory T cells in protecting females against pulmonary hypertension, Circ Res, doi:10.1161/CIRCRESAHA.117.312058
Tamosiuniene, Tian, Dhillon, Wang, Sung et al., Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension, Circ Res, doi:10.1161/CIRCRESAHA.110.236927
Teodoro, De, Queiroz, Santos, Catanozi et al., Proposition of a novel animal model of systemic sclerosis induced by type V collagen in C57BL/6 mice that reproduces fibrosis, vasculopathy and autoimmunity, Arthritis Res Ther, doi:10.1186/s13075-019-2052-2
Tian, Jiang, Jiang, Tamosiuniene, Kim et al., The role of regulatory T cells in pulmonary arterial hypertension, Front Immunol, doi:10.3389/fimmu.2021.684657
Tomaszewski, Bębnowska, Hrynkiewicz, Dworzyński, Niedzẃiedzka-Rystwej et al., Role of the immune system elements in pulmonary arterial hypertension, J Clin Med, doi:10.3390/jcm10163757
Vanderbeke, Janssen, Bergmans, Bourgeois, Buil et al., Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): a randomised, open-label, proof-of-concept trial, Intensive Care Med, doi:10.1007/s00134-021-06431-0
Vaz, Hwang, Kagiampakis, Phallen, Patil et al., Chronic cigarette smoke-induced epigenomic changes precede sensitization of bronchial epithelial cells to single-step transformation by KRAS mutations, Cancer Cell, doi:10.1016/j.ccell.2017.08.006
Vignali, Collison, Workman, How regulatory T cells work, Nat Rev Immunol, doi:10.1038/nri2343
Wang, Lee, Dhandapani, Baek, Kim et al., 8-paradol from ginger exacerbates PINK1/Parkin mediated mitophagy to induce apoptosis in human gastric adenocarcinoma, Pharmacol Res, doi:10.1016/j.phrs.2022.106610
Weigel, Malempati, Reid, Voss, Cho et al., Phase 2 trial of cixutumumab in children, adolescents, and young adults with refractory solid tumors: a report from the Children's Oncology Group, Pediatr Blood Cancer, doi:10.1002/pbc.24605
Wu, Bonnet, Shimauchi, Toro, Grobs et al., Potential for inhibition of checkpoint kinases 1/2 in pulmonary fibrosis and secondary pulmonary hypertension, Thorax, doi:10.1136/thoraxjnl-2021-217377
Xu, Janocha, Erzurum, Metabolism in pulmonary hypertension, Annu Rev Physiol, doi:10.1146/annurev-physiol-031620-123956
Yan, Ci, Chen, Chen, Li et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflammation Res, doi:10.1007/s00011-011-0307-8
Yan, He, Jiang, Wang, Chen et al., DNA methyltransferase 3B deficiency unveils a new pathological mechanism of pulmonary hypertension, Sci Adv, doi:10.1126/sciadv.aba2470
Yang, Sun, Ramchandran, Raj, IGF-1 signaling in neonatal hypoxiainduced pulmonary hypertension: Role of epigenetic regulation, Vascul Pharmacol, doi:10.1016/j.vph.2015.04.005
Yao, Zhang, Gao, Wang, Dai et al., Exploration of the shared gene signatures and molecular mechanisms between systemic lupus erythematosus and pulmonary arterial hypertension: evidence from transcriptome data, Front Immunol, doi:10.3389/fimmu.2021.658341
Zeng, Liu, Zhang, Identification of potential biomarkers and immune infiltration characteristics in idiopathic pulmonary arterial hypertension using bioinformatics analysis, Front Cardiovasc Med, doi:10.3389/fcvm.2021.624714
Zhang, Song, Ci, Ju, Li, Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflammation Res, doi:10.1007/s00011-008-8007-8
Zhu, Liu, Hao, Feng, Chen et al., Dietary geranylgeranyl pyrophosphate counteracts the benefits of statin therapy in experimental pulmonary h y p e r t e n s i o n . C i r c u l a t i o n ( 2 0 2 1 ), 1, doi:10.1161/CIRCULATIONAHA.120.046542
DOI record:
{
"DOI": "10.3389/fimmu.2023.1197752",
"ISSN": [
"1664-3224"
],
"URL": "http://dx.doi.org/10.3389/fimmu.2023.1197752",
"abstract": "<jats:p>Pulmonary fibrosis (PF) and pulmonary hypertension (PH) have common pathophysiological features, such as the significant remodeling of pulmonary parenchyma and vascular wall. There is no effective specific drug in clinical treatment for these two diseases, resulting in a worse prognosis and higher mortality. This study aimed to screen the common key genes and immune characteristics of PF and PH by means of bioinformatics to find new common therapeutic targets. Expression profiles are downloaded from the Gene Expression Database. Weighted gene co-expression network analysis is used to identify the co-expression modules related to PF and PH. We used the ClueGO software to enrich and analyze the common genes in PF and PH and obtained the protein–protein interaction (PPI) network. Then, the differential genes were screened out in another cohort of PF and PH, and the shared genes were crossed. Finally, RT-PCR verification and immune infiltration analysis were performed on the intersection genes. In the result, the positive correlation module with the highest correlation between PF and PH was determined, and it was found that lymphocyte activation is a common feature of the pathophysiology of PF and PH. Eight common characteristic genes (<jats:italic>ACTR2, COL5A2, COL6A3, CYSLTR1, IGF1, RSPO3, SCARNA17</jats:italic> and <jats:italic>SEL1L</jats:italic>) were gained. Immune infiltration showed that compared with the control group, resting CD4 memory T cells were upregulated in PF and PH. Combining the results of crossing characteristic genes in ImmPort database and RT-PCR, the important gene <jats:italic>IGF1</jats:italic> was obtained. Knocking down <jats:italic>IGF1</jats:italic> could significantly reduce the proliferation and apoptosis resistance in pulmonary microvascular endothelial cells, pulmonary smooth muscle cells, and fibroblasts induced by hypoxia, platelet-derived growth factor-BB (PDGF-BB), and transforming growth factor-β1 (TGF-β1), respectively. Our work identified the common biomarkers of PF and PH and provided a new candidate gene for the potential therapeutic targets of PF and PH in the future.</jats:p>",
"alternative-id": [
"10.3389/fimmu.2023.1197752"
],
"author": [
{
"affiliation": [],
"family": "Zhao",
"given": "Hui",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wang",
"given": "Lan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yan",
"given": "Yi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Qin-Hua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "He",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiang",
"given": "Rong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luo",
"given": "Ci-Jun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qiu",
"given": "Hong-Ling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miao",
"given": "Yu-Qing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gong",
"given": "Su-Gang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yuan",
"given": "Ping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Wen-Hui",
"sequence": "additional"
}
],
"container-title": "Frontiers in Immunology",
"container-title-short": "Front. Immunol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
9,
4
]
],
"date-time": "2023-09-04T08:54:51Z",
"timestamp": 1693817691000
},
"deposited": {
"date-parts": [
[
2023,
9,
4
]
],
"date-time": "2023-09-04T08:54:57Z",
"timestamp": 1693817697000
},
"indexed": {
"date-parts": [
[
2023,
9,
7
]
],
"date-time": "2023-09-07T21:09:21Z",
"timestamp": 1694120961713
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
9,
4
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
9,
4
]
],
"date-time": "2023-09-04T00:00:00Z",
"timestamp": 1693785600000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2023.1197752/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
9,
4
]
]
},
"published-online": {
"date-parts": [
[
2023,
9,
4
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1183/13993003.00879-2022",
"article-title": "ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension",
"author": "Humbert",
"doi-asserted-by": "publisher",
"first-page": "2200879",
"journal-title": "Eur Respir J",
"key": "B1",
"volume": "61",
"year": "2022"
},
{
"DOI": "10.1183/13993003.01914-2018",
"article-title": "Pulmonary hypertension in chronic lung disease and hypoxia",
"author": "Nathan",
"doi-asserted-by": "publisher",
"first-page": "1801914",
"journal-title": "Eur Respir J",
"key": "B2",
"volume": "53",
"year": "2019"
},
{
"DOI": "10.1183/13993003.02316-2014",
"article-title": "Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review",
"author": "Raghu",
"doi-asserted-by": "publisher",
"journal-title": "Eur Respir J",
"key": "B3",
"volume": "46",
"year": "2015"
},
{
"article-title": "Idiopathic Pulmonary Artery Hypertension",
"author": "Pahal",
"key": "B4",
"volume-title": "StatPearls",
"year": "2022"
},
{
"DOI": "10.1038/nrdp.2017.74",
"article-title": "Idiopathic pulmonary fibrosis",
"author": "Martinez",
"doi-asserted-by": "publisher",
"first-page": "17074",
"journal-title": "Nat Rev Dis Primers",
"key": "B5",
"volume": "3",
"year": "2017"
},
{
"DOI": "10.1111/bph.15036",
"article-title": "Idiopathic pulmonary fibrosis and pulmonary hypertension: Heracles meets the Hydra",
"author": "Rajagopal",
"doi-asserted-by": "publisher",
"journal-title": "Br J Pharmacol",
"key": "B6",
"volume": "178",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(18)30502-2",
"article-title": "Lung transplantation for idiopathic pulmonary fibrosis",
"author": "George",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med",
"key": "B7",
"volume": "7",
"year": "2019"
},
{
"DOI": "10.1378/chest.129.3.746",
"article-title": "Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis",
"author": "Lettieri",
"doi-asserted-by": "publisher",
"journal-title": "Chest",
"key": "B8",
"volume": "129",
"year": "2006"
},
{
"DOI": "10.1155/2017/1430350",
"article-title": "Pulmonary hypertension associated with idiopathic pulmonary fibrosis: current and future perspectives",
"author": "Collum",
"doi-asserted-by": "publisher",
"first-page": "1430350",
"journal-title": "Can Respir J",
"key": "B9",
"volume": "2017",
"year": "2017"
},
{
"DOI": "10.1136/thoraxjnl-2017-210728",
"article-title": "JAK2 mediates lung fibrosis, pulmonary vascular remodelling and hypertension in idiopathic pulmonary fibrosis: an experimental study",
"author": "Milara",
"doi-asserted-by": "publisher",
"journal-title": "Thorax",
"key": "B10",
"volume": "73",
"year": "2018"
},
{
"DOI": "10.1152/ajplung.00142.2016",
"article-title": "Macrophage bone morphogenic protein receptor 2 depletion in idiopathic pulmonary fibrosis and Group III pulmonary hypertension",
"author": "Chen",
"doi-asserted-by": "publisher",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "B11",
"volume": "311",
"year": "2016"
},
{
"DOI": "10.1152/ajpheart.00254.2009",
"article-title": "Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension",
"author": "Rajkumar",
"doi-asserted-by": "publisher",
"journal-title": "Am J Physiol Heart Circ Physiol",
"key": "B12",
"volume": "298",
"year": "2010"
},
{
"DOI": "10.1136/thoraxjnl-2021-217377",
"article-title": "Potential for inhibition of checkpoint kinases 1/2 in pulmonary fibrosis and secondary pulmonary hypertension",
"author": "Wu",
"doi-asserted-by": "publisher",
"journal-title": "Thorax",
"key": "B13",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1161/ATVBAHA.119.312537",
"article-title": "Inhibition of CHK 1 (Checkpoint kinase 1) elicits therapeutic effects in pulmonary arterial hypertension",
"author": "Bourgeois",
"doi-asserted-by": "publisher",
"journal-title": "Arterioscler Thromb Vasc Biol",
"key": "B14",
"volume": "39",
"year": "2019"
},
{
"DOI": "10.4103/0975-7406.100283",
"article-title": "Microarray and its applications",
"author": "Govindarajan",
"doi-asserted-by": "publisher",
"journal-title": "J Pharm Bioallied Sci",
"key": "B15",
"volume": "4",
"year": "2012"
},
{
"DOI": "10.3389/fimmu.2021.658341",
"article-title": "Exploration of the shared gene signatures and molecular mechanisms between systemic lupus erythematosus and pulmonary arterial hypertension: evidence from transcriptome data",
"author": "Yao",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B16",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1186/1471-2105-9-559",
"article-title": "WGCNA: an R package for weighted correlation network analysis",
"author": "Langfelder",
"doi-asserted-by": "publisher",
"first-page": "559",
"journal-title": "BMC Bioinf",
"key": "B17",
"volume": "9",
"year": "2008"
},
{
"DOI": "10.1093/bioinformatics/btp101",
"article-title": "ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks",
"author": "Bindea",
"doi-asserted-by": "publisher",
"journal-title": "Bioinformatics",
"key": "B18",
"volume": "25",
"year": "2009"
},
{
"DOI": "10.3389/fimmu.2021.768966",
"article-title": "Expression of immune related genes and possible regulatory mechanisms in Alzheimer's disease",
"author": "Lu",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B19",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1136/thoraxjnl-2013-204596",
"article-title": "Heterogeneous gene expression signatures correspond to distinct lung pathologies and biomarkers of disease severity in idiopathic pulmonary fibrosis",
"author": "DePianto",
"doi-asserted-by": "publisher",
"first-page": "48",
"journal-title": "Thorax",
"key": "B20",
"volume": "70",
"year": "2015"
},
{
"DOI": "10.1111/resp.13557",
"article-title": "Osteopontin lung gene expression is a marker of disease severity in pulmonary arterial hypertension",
"author": "Mura",
"doi-asserted-by": "publisher",
"journal-title": "Respirology",
"key": "B21",
"volume": "24",
"year": "2019"
},
{
"DOI": "10.1016/j.phrs.2022.106610",
"article-title": "8-paradol from ginger exacerbates PINK1/Parkin mediated mitophagy to induce apoptosis in human gastric adenocarcinoma",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "106610",
"journal-title": "Pharmacol Res",
"key": "B22",
"volume": "187",
"year": "2023"
},
{
"DOI": "10.1016/S2213-2600(21)00165-X",
"article-title": "Inhaled treprostinil and forced vital capacity in patients with interstitial lung disease and associated pulmonary hypertension: a post-hoc analysis of the INCREASE study",
"author": "Nathan",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med",
"key": "B23",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.3389/fcvm.2021.624714",
"article-title": "Identification of potential biomarkers and immune infiltration characteristics in idiopathic pulmonary arterial hypertension using bioinformatics analysis",
"author": "Zeng",
"doi-asserted-by": "publisher",
"journal-title": "Front Cardiovasc Med",
"key": "B24",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(18)30448-X",
"article-title": "Common genetic variants in pulmonary arterial hypertension",
"author": "Gu",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med",
"key": "B25",
"volume": "7",
"year": "2019"
},
{
"DOI": "10.1146/annurev-physiol-031620-123956",
"article-title": "Metabolism in pulmonary hypertension",
"author": "Xu",
"doi-asserted-by": "publisher",
"journal-title": "Annu Rev Physiol",
"key": "B26",
"volume": "83",
"year": "2021"
},
{
"DOI": "10.1126/sciadv.aba2470",
"article-title": "DNA methyltransferase 3B deficiency unveils a new pathological mechanism of pulmonary hypertension",
"author": "Yan",
"doi-asserted-by": "publisher",
"first-page": "eaba2470",
"journal-title": "Sci Adv",
"key": "B27",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.ccell.2017.08.006",
"article-title": "Chronic cigarette smoke-induced epigenomic changes precede sensitization of bronchial epithelial cells to single-step transformation by KRAS mutations",
"author": "Vaz",
"doi-asserted-by": "publisher",
"first-page": "360",
"journal-title": "Cancer Cell",
"key": "B28",
"volume": "32",
"year": "2017"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.046542",
"article-title": "Dietary geranylgeranyl pyrophosphate counteracts the benefits of statin therapy in experimental pulmonary hypertension",
"author": "Zhu",
"doi-asserted-by": "publisher",
"journal-title": "Circulation",
"key": "B29",
"volume": "143",
"year": "2021"
},
{
"DOI": "10.1164/rccm.201201-0164OC",
"article-title": "Modern age pathology of pulmonary arterial hypertension",
"author": "Stacher",
"doi-asserted-by": "publisher",
"journal-title": "Am J Respir Crit Care Med",
"key": "B30",
"volume": "186",
"year": "2012"
},
{
"DOI": "10.1165/rcmb.2016-0121TR",
"article-title": "Immune mechanisms in pulmonary fibrosis",
"author": "Kolahian",
"doi-asserted-by": "publisher",
"journal-title": "Am J Respir Cell Mol Biol",
"key": "B31",
"volume": "55",
"year": "2016"
},
{
"DOI": "10.1164/ajrccm.154.1.8680680",
"article-title": "The effect of an anti-CD3 monoclonal antibody on bleomycin-induced lymphokine production and lung injury",
"author": "Sharma",
"doi-asserted-by": "publisher",
"first-page": "193",
"journal-title": "Am J Respir Crit Care Med",
"key": "B32",
"volume": "154",
"year": "1996"
},
{
"DOI": "10.1016/bs.ircmb.2018.05.007",
"article-title": "The single-cell phenotypic identity of human CD8(+) and CD4(+) T cells",
"author": "Brummelman",
"doi-asserted-by": "publisher",
"first-page": "63",
"journal-title": "Int Rev Cell Mol Biol",
"key": "B33",
"volume": "341",
"year": "2018"
},
{
"DOI": "10.1378/chest.14-1678",
"article-title": "T-helper 17 cell polarization in pulmonary arterial hypertension",
"author": "Hautefort",
"doi-asserted-by": "publisher",
"journal-title": "Chest",
"key": "B34",
"volume": "147",
"year": "2015"
},
{
"DOI": "10.1371/journal.pone.0008959",
"article-title": "CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis",
"author": "Gilani",
"doi-asserted-by": "publisher",
"journal-title": "PloS One",
"key": "B35",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.3389/fimmu.2021.684657",
"article-title": "The role of regulatory T cells in pulmonary arterial hypertension",
"author": "Tian",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B36",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1164/rccm.201202-0335OC",
"article-title": "Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension",
"author": "Savai",
"doi-asserted-by": "publisher",
"first-page": "897",
"journal-title": "Am J Respir Crit Care Med",
"key": "B37",
"volume": "186",
"year": "2012"
},
{
"DOI": "10.1126/scitranslmed.aar8356",
"article-title": "PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production",
"author": "Celada",
"doi-asserted-by": "publisher",
"first-page": "eaar8356",
"journal-title": "Sci Transl Med",
"key": "B38",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.3390/jcm10163757",
"article-title": "Role of the immune system elements in pulmonary arterial hypertension",
"author": "Tomaszewski",
"doi-asserted-by": "publisher",
"first-page": "3757",
"journal-title": "J Clin Med",
"key": "B39",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1161/CIRCRESAHA.117.312058",
"article-title": "Dominant role for regulatory T cells in protecting females against pulmonary hypertension",
"author": "Tamosiuniene",
"doi-asserted-by": "publisher",
"journal-title": "Circ Res",
"key": "B40",
"volume": "122",
"year": "2018"
},
{
"DOI": "10.1161/CIRCRESAHA.110.236927",
"article-title": "Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension",
"author": "Tamosiuniene",
"doi-asserted-by": "publisher",
"journal-title": "Circ Res",
"key": "B41",
"volume": "109",
"year": "2011"
},
{
"DOI": "10.3389/fphar.2022.908783",
"article-title": "Regulatory T cell-related gene indicators in pulmonary hypertension",
"author": "Liu",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "B42",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.rmed.2018.12.015",
"article-title": "Inflammation and immunity in IPF pathogenesis and treatment",
"author": "Heukels",
"doi-asserted-by": "publisher",
"first-page": "79",
"journal-title": "Respir Med",
"key": "B43",
"volume": "147",
"year": "2019"
},
{
"DOI": "10.1038/nri2343",
"article-title": "How regulatory T cells work",
"author": "Vignali",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Immunol",
"key": "B44",
"volume": "8",
"year": "2008"
},
{
"DOI": "10.1164/rccm.200812-1936OC",
"article-title": "Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis",
"author": "Kotsianidis",
"doi-asserted-by": "publisher",
"journal-title": "Am J Respir Crit Care Med",
"key": "B45",
"volume": "179",
"year": "2009"
},
{
"DOI": "10.1165/rcmb.2012-0198OC",
"article-title": "Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment",
"author": "Garibaldi",
"doi-asserted-by": "publisher",
"first-page": "35",
"journal-title": "Am J Respir Cell Mol Biol",
"key": "B46",
"volume": "48",
"year": "2013"
},
{
"DOI": "10.1038/s41590-019-0494-y",
"article-title": "CD103(hi) T(reg) cells constrain lung fibrosis induced by CD103(lo) tissue-resident pathogenic CD4 T cells",
"author": "Ichikawa",
"doi-asserted-by": "publisher",
"journal-title": "Nat Immunol",
"key": "B47",
"volume": "20",
"year": "2019"
},
{
"DOI": "10.1016/j.ajpath.2016.03.020",
"article-title": "CD4(+)CD25(hi)Foxp3(+) cells exacerbate bleomycin-induced pulmonary fibrosis",
"author": "Birjandi",
"doi-asserted-by": "publisher",
"journal-title": "Am J Pathol",
"key": "B48",
"volume": "186",
"year": "2016"
},
{
"DOI": "10.1073/pnas.89.10.4481",
"article-title": "Human growth hormone promotes engraftment of murine or human T cells in severe combined immunodeficient mice",
"author": "Murphy",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A.",
"key": "B49",
"volume": "89",
"year": "1992"
},
{
"DOI": "10.1080/19381980.2017.1356518",
"article-title": "Insulin and Insulin-like growth factor-1 can activate the phosphoinositide-3-kinase /Akt/FoxO1 pathway in T cells in vitro",
"author": "Mirdamadi",
"doi-asserted-by": "publisher",
"journal-title": "Dermatoendocrinol",
"key": "B50",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.15252/emmm.201303376",
"article-title": "Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease",
"author": "Bilbao",
"doi-asserted-by": "publisher",
"journal-title": "EMBO Mol Med",
"key": "B51",
"volume": "6",
"year": "2014"
},
{
"DOI": "10.1016/j.immuni.2020.01.005",
"article-title": "Insulin-like growth factor 1 supports a pulmonary niche that promotes type 3 innate lymphoid cell development in newborn lungs",
"author": "Oherle",
"doi-asserted-by": "publisher",
"first-page": "275",
"journal-title": "Immunity",
"key": "B52",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1164/ajrccm.164.2.2003114",
"article-title": "Differential mRNA expression of insulin-like growth factor-1 splice variants in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis",
"author": "Bloor",
"doi-asserted-by": "publisher",
"journal-title": "Am J Respir Crit Care Med",
"key": "B53",
"volume": "164",
"year": "2001"
},
{
"DOI": "10.1016/j.vph.2015.04.005",
"article-title": "IGF-1 signaling in neonatal hypoxia-induced pulmonary hypertension: Role of epigenetic regulation",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "20",
"journal-title": "Vascul Pharmacol",
"key": "B54",
"volume": "73",
"year": "2015"
},
{
"DOI": "10.1165/rcmb.2015-0388OC",
"article-title": "Smooth muscle insulin-like growth factor-1 mediates hypoxia-induced pulmonary hypertension in neonatal mice",
"author": "Sun",
"doi-asserted-by": "publisher",
"journal-title": "Am J Respir Cell Mol Biol",
"key": "B55",
"volume": "55",
"year": "2016"
},
{
"DOI": "10.1164/rccm.200802-228OC",
"article-title": "Insulin-like growth factor-I receptor blockade improves outcome in mouse model of lung injury",
"author": "Choi",
"doi-asserted-by": "publisher",
"journal-title": "Am J Respir Crit Care Med",
"key": "B56",
"volume": "179",
"year": "2009"
},
{
"DOI": "10.1002/pbc.24605",
"article-title": "Phase 2 trial of cixutumumab in children, adolescents, and young adults with refractory solid tumors: a report from the Children's Oncology Group",
"author": "Weigel",
"doi-asserted-by": "publisher",
"journal-title": "Pediatr Blood Cancer",
"key": "B57",
"volume": "61",
"year": "2014"
},
{
"DOI": "10.1016/j.annonc.2019.09.004",
"article-title": "Randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE)",
"author": "Kundranda",
"doi-asserted-by": "publisher",
"first-page": "79",
"journal-title": "Ann Oncol",
"key": "B58",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1111/jcmm.14708",
"article-title": "Foxf2 and Smad6 co-regulation of collagen 5A2 transcription is involved in the pathogenesis of intrauterine adhesion",
"author": "Chen",
"doi-asserted-by": "publisher",
"journal-title": "J Cell Mol Med",
"key": "B59",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1186/s13075-019-2052-2",
"article-title": "Proposition of a novel animal model of systemic sclerosis induced by type V collagen in C57BL/6 mice that reproduces fibrosis, vasculopathy and autoimmunity",
"author": "Teodoro",
"doi-asserted-by": "publisher",
"first-page": "278",
"journal-title": "Arthritis Res Ther",
"key": "B60",
"volume": "21",
"year": "2019"
},
{
"DOI": "10.3389/fonc.2018.00659",
"article-title": "The role of COL5A2 in patients with muscle-invasive bladder cancer: A bioinformatics analysis of public datasets involving 787 subjects and 29 cell lines",
"author": "Meng",
"doi-asserted-by": "publisher",
"journal-title": "Front Oncol",
"key": "B61",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.1186/1755-8794-6-13",
"article-title": "Analysis of a gene co-expression network establishes robust association between Col5a2 and ischemic heart disease",
"author": "Azuaje",
"doi-asserted-by": "publisher",
"first-page": "13",
"journal-title": "BMC Med Genomics",
"key": "B62",
"volume": "6",
"year": "2013"
},
{
"DOI": "10.3390/cancers13225809",
"article-title": "The cardiac glycoside deslanoside exerts anticancer activity in prostate cancer cells by modulating multiple signaling pathways",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "5809",
"journal-title": "Cancers (Basel)",
"key": "B63",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1152/ajplung.00538.2020",
"article-title": "Enhanced epithelial sodium channel activity in neonatal Scnn1b mouse lung attenuates high oxygen-induced lung injury",
"author": "Grant",
"doi-asserted-by": "publisher",
"first-page": "L29",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "B64",
"volume": "321",
"year": "2021"
},
{
"DOI": "10.1016/j.bcp.2020.114100",
"article-title": "Flavine adenine dinucleotide inhibits pathological cardiac hypertrophy and fibrosis through activating short chain acyl-CoA dehydrogenase",
"author": "Ma",
"doi-asserted-by": "publisher",
"first-page": "114100",
"journal-title": "Biochem Pharmacol",
"key": "B65",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.3109/15376516.2015.1045662",
"article-title": "Riboflavin attenuates lipopolysaccharide-induced lung injury in rats",
"author": "Al-Harbi",
"doi-asserted-by": "publisher",
"journal-title": "Toxicol Mech Methods",
"key": "B66",
"volume": "25",
"year": "2015"
},
{
"DOI": "10.1016/j.jconrel.2020.10.009",
"article-title": "Ivermectin: an award-winning drug with expected antiviral activity against COVID-19",
"author": "Formiga",
"doi-asserted-by": "publisher",
"journal-title": "J Control Release",
"key": "B67",
"volume": "329",
"year": "2021"
},
{
"DOI": "10.1007/s00011-011-0307-8",
"article-title": "Anti-inflammatory effects of ivermectin in mouse model of allergic asthma",
"author": "Yan",
"doi-asserted-by": "publisher",
"journal-title": "Inflammation Res",
"key": "B68",
"volume": "60",
"year": "2011"
},
{
"DOI": "10.1007/s00011-008-8007-8",
"article-title": "Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice",
"author": "Zhang",
"doi-asserted-by": "publisher",
"journal-title": "Inflammation Res",
"key": "B69",
"volume": "57",
"year": "2008"
},
{
"DOI": "10.1007/s00134-021-06431-0",
"article-title": "Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): a randomised, open-label, proof-of-concept trial",
"author": "Vanderbeke",
"doi-asserted-by": "publisher",
"journal-title": "Intensive Care Med",
"key": "B70",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1080/14787210.2021.1962292",
"article-title": "Pharmacological management of antifungal agents in pulmonary aspergillosis: an updated review",
"author": "Echeverria-Esnal",
"doi-asserted-by": "publisher",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "B71",
"volume": "20",
"year": "2022"
}
],
"reference-count": 71,
"references-count": 71,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2023.1197752/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Immunology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Identification of the shared gene signatures between pulmonary fibrosis and pulmonary hypertension using bioinformatics analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "14"
}
