Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2021.625678, Mar 2021
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Modeling study analyzing timing and dosing regimens of hydroxychloroquine, lopinavir/ritonavir, ivermectin, artemisinin, and nitazoxanide. The greatest benefits were seen when treatments were given immediately at the time of diagnosis. Authors state that "For IVM, no results of clinical trials regarding its effectiveness in COVID-19 have been published yet", which is inaccurate - there were 19 peer-reviewed trials published as of Mar 10, 2021 (43 including preprints).
74 preclinical studies support the efficacy of ivermectin for COVID-19:
Ivermectin, better known for antiparasitic activity, is a broad spectrum antiviral with activity against many viruses including H7N771, Dengue37,72,73 , HIV-173, Simian virus 4074, Zika37,75,76 , West Nile76, Yellow Fever77,78, Japanese encephalitis77, Chikungunya78, Semliki Forest virus78, Human papillomavirus57, Epstein-Barr57, BK Polyomavirus79, and Sindbis virus78.
Ivermectin inhibits importin-α/β-dependent nuclear import of viral proteins71,73,74,80 , shows spike-ACE2 disruption at 1nM with microfluidic diffusional sizing38, binds to glycan sites on the SARS-CoV-2 spike protein preventing interaction with blood and epithelial cells and inhibiting hemagglutination41,81, shows dose-dependent inhibition of wildtype and omicron variants36, exhibits dose-dependent inhibition of lung injury61,66, may inhibit SARS-CoV-2 via IMPase inhibition37, may inhibit SARS-CoV-2 induced formation of fibrin clots resistant to degradation9, inhibits SARS-CoV-2 3CLpro54, may inhibit SARS-CoV-2 RdRp activity28, may minimize viral myocarditis by inhibiting NF-κB/p65-mediated inflammation in macrophages60, may be beneficial for COVID-19 ARDS by blocking GSDMD and NET formation82, may interfere with SARS-CoV-2's immune evasion via ORF8 binding4, may inhibit SARS-CoV-2 by disrupting CD147 interaction83-86, may inhibit SARS-CoV-2 attachment to lipid rafts via spike NTD binding2, shows protection against inflammation, cytokine storm, and mortality in an LPS mouse model sharing key pathological features of severe COVID-1959,87, may be beneficial in severe COVID-19 by binding IGF1 to inhibit the promotion of inflammation, fibrosis, and cell proliferation that leads to lung damage8, may minimize SARS-CoV-2 induced cardiac damage40,48, may counter immune evasion by inhibiting NSP15-TBK1/KPNA1 interaction and restoring IRF3 activation88, may disrupt SARS-CoV-2 N and ORF6 protein nuclear transport and their suppression of host interferon responses1, reduces TAZ/YAP nuclear import, relieving SARS-CoV-2-driven suppression of IRF3 and NF-κB antiviral pathways35, increases Bifidobacteria which play a key role in the immune system89, has immunomodulatory51 and anti-inflammatory70,90 properties, and has an extensive and very positive safety profile91.
1.
Gayozo et al., Binding affinities analysis of ivermectin, nucleocapsid and ORF6 proteins of SARS-CoV-2 to human importins α isoforms: A computational approach, Biotecnia, doi:10.18633/biotecnia.v27.2485.
2.
Lefebvre et al., Characterization and Fluctuations of an Ivermectin Binding Site at the Lipid Raft Interface of the N-Terminal Domain (NTD) of the Spike Protein of SARS-CoV-2 Variants, Viruses, doi:10.3390/v16121836.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Bagheri-Far et al., Non-spike protein inhibition of SARS-CoV-2 by natural products through the key mediator protein ORF8, Molecular Biology Research Communications, doi:10.22099/mbrc.2024.50245.2001.
5.
de Oliveira Só et al., In Silico Comparative Analysis of Ivermectin and Nirmatrelvir Inhibitors Interacting with the SARS-CoV-2 Main Protease, Preprints, doi:10.20944/preprints202404.1825.v1.
6.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
7.
Oranu et al., Validation of the binding affinities and stabilities of ivermectin and moxidectin against SARS-CoV-2 receptors using molecular docking and molecular dynamics simulation, GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2024.26.1.0030.
8.
Zhao et al., Identification of the shared gene signatures between pulmonary fibrosis and pulmonary hypertension using bioinformatics analysis, Frontiers in Immunology, doi:10.3389/fimmu.2023.1197752.
9.
Vottero et al., Computational Prediction of the Interaction of Ivermectin with Fibrinogen, Molecular Sciences, doi:10.3390/ijms241411449.
10.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
11.
Umar et al., Inhibitory potentials of ivermectin, nafamostat, and camostat on spike protein and some nonstructural proteins of SARS-CoV-2: Virtual screening approach, Jurnal Teknologi Laboratorium, doi:10.29238/teknolabjournal.v11i1.344.
12.
Alvarado et al., Interaction of the New Inhibitor Paxlovid (PF-07321332) and Ivermectin With the Monomer of the Main Protease SARS-CoV-2: A Volumetric Study Based on Molecular Dynamics, Elastic Networks, Classical Thermodynamics and SPT, Computational Biology and Chemistry, doi:10.1016/j.compbiolchem.2022.107692.
13.
Aminpour et al., In Silico Analysis of the Multi-Targeted Mode of Action of Ivermectin and Related Compounds, Computation, doi:10.3390/computation10040051.
14.
Parvez et al., Insights from a computational analysis of the SARS-CoV-2 Omicron variant: Host–pathogen interaction, pathogenicity, and possible drug therapeutics, Immunity, Inflammation and Disease, doi:10.1002/iid3.639.
15.
Francés-Monerris et al., Microscopic interactions between ivermectin and key human and viral proteins involved in SARS-CoV-2 infection, Physical Chemistry Chemical Physics, doi:10.1039/D1CP02967C.
16.
González-Paz et al., Comparative study of the interaction of ivermectin with proteins of interest associated with SARS-CoV-2: A computational and biophysical approach, Biophysical Chemistry, doi:10.1016/j.bpc.2021.106677.
17.
González-Paz (B) et al., Structural Deformability Induced in Proteins of Potential Interest Associated with COVID-19 by binding of Homologues present in Ivermectin: Comparative Study Based in Elastic Networks Models, Journal of Molecular Liquids, doi:10.1016/j.molliq.2021.117284.
18.
Rana et al., A Computational Study of Ivermectin and Doxycycline Combination Drug Against SARS-CoV-2 Infection, Research Square, doi:10.21203/rs.3.rs-755838/v1.
19.
Muthusamy et al., Virtual Screening Reveals Potential Anti-Parasitic Drugs Inhibiting the Receptor Binding Domain of SARS-CoV-2 Spike protein, Journal of Virology & Antiviral Research, www.scitechnol.com/abstract/virtual-screening-reveals-potential-antiparasitic-drugs-inhibiting-the-receptor-binding-domain-of-sarscov2-spike-protein-16398.html.
20.
Qureshi et al., Mechanistic insights into the inhibitory activity of FDA approved ivermectin against SARS-CoV-2: old drug with new implications, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1906750.
21.
Schöning et al., Highly-transmissible Variants of SARS-CoV-2 May Be More Susceptible to Drug Therapy Than Wild Type Strains, Research Square, doi:10.21203/rs.3.rs-379291/v1.
22.
Bello et al., Elucidation of the inhibitory activity of ivermectin with host nuclear importin α and several SARS-CoV-2 targets, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1911857.
23.
Udofia et al., In silico studies of selected multi-drug targeting against 3CLpro and nsp12 RNA-dependent RNA-polymerase proteins of SARS-CoV-2 and SARS-CoV, Network Modeling Analysis in Health Informatics and Bioinformatics, doi:10.1007/s13721-021-00299-2.
24.
Choudhury et al., Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach, Future Medicine, doi:10.2217/fvl-2020-0342.
25.
Kern et al., Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing, Frontiers in Pharmacology, doi:10.3389/fphar.2021.625678.
26.
Saha et al., The Binding mechanism of ivermectin and levosalbutamol with spike protein of SARS-CoV-2, Structural Chemistry, doi:10.1007/s11224-021-01776-0.
27.
Eweas et al., Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2, Frontiers in Microbiology, doi:10.3389/fmicb.2020.592908.
28.
Parvez (B) et al., Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.09.098.
29.
Francés-Monerris (B) et al., Has Ivermectin Virus-Directed Effects against SARS-CoV-2? Rationalizing the Action of a Potential Multitarget Antiviral Agent, ChemRxiv, doi:10.26434/chemrxiv.12782258.v1.
30.
Kalhor et al., Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1824816.
31.
Swargiary, A., Ivermectin as a promising RNA-dependent RNA polymerase inhibitor and a therapeutic drug against SARS-CoV2: Evidence from in silico studies, Research Square, doi:10.21203/rs.3.rs-73308/v1.
32.
Maurya, D., A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID-19 Patients, American Chemical Society (ACS), doi:10.26434/chemrxiv.12630539.v1.
33.
Lehrer et al., Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, In Vivo, 34:5, 3023-3026, doi:10.21873/invivo.12134.
34.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
35.
Kofler et al., M-Motif, a potential non-conventional NLS in YAP/TAZ and other cellular and viral proteins that inhibits classic protein import, iScience, doi:10.1016/j.isci.2025.112105.
36.
Shahin et al., The selective effect of Ivermectin on different human coronaviruses; in-vitro study, Research Square, doi:10.21203/rs.3.rs-4180797/v1.
37.
Jitobaom et al., Identification of inositol monophosphatase as a broad‐spectrum antiviral target of ivermectin, Journal of Medical Virology, doi:10.1002/jmv.29552.
38.
Fauquet et al., Microfluidic Diffusion Sizing Applied to the Study of Natural Products and Extracts That Modulate the SARS-CoV-2 Spike RBD/ACE2 Interaction, Molecules, doi:10.3390/molecules28248072.
39.
García-Aguilar et al., In Vitro Analysis of SARS-CoV-2 Spike Protein and Ivermectin Interaction, International Journal of Molecular Sciences, doi:10.3390/ijms242216392.
40.
Liu et al., SARS-CoV-2 viral genes Nsp6, Nsp8, and M compromise cellular ATP levels to impair survival and function of human pluripotent stem cell-derived cardiomyocytes, Stem Cell Research & Therapy, doi:10.1186/s13287-023-03485-3.
41.
Boschi et al., SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects, bioRxiv, doi:10.1101/2022.11.24.517882.
42.
De Forni et al., Synergistic drug combinations designed to fully suppress SARS-CoV-2 in the lung of COVID-19 patients, PLoS ONE, doi:10.1371/journal.pone.0276751.
43.
Saha (B) et al., Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder, Pharmaceutics, doi:10.3390/pharmaceutics14071432.
44.
Jitobaom (B) et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8.
45.
Croci et al., Liposomal Systems as Nanocarriers for the Antiviral Agent Ivermectin, International Journal of Biomaterials, doi:10.1155/2016/8043983.
46.
Zheng et al., Red blood cell-hitchhiking mediated pulmonary delivery of ivermectin: Effects of nanoparticle properties, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121719.
47.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
48.
Liu (B) et al., Genome-wide analyses reveal the detrimental impacts of SARS-CoV-2 viral gene Orf9c on human pluripotent stem cell-derived cardiomyocytes, Stem Cell Reports, doi:10.1016/j.stemcr.2022.01.014.
49.
Segatori et al., Effect of Ivermectin and Atorvastatin on Nuclear Localization of Importin Alpha and Drug Target Expression Profiling in Host Cells from Nasopharyngeal Swabs of SARS-CoV-2- Positive Patients, Viruses, doi:10.3390/v13102084.
50.
Jitobaom (C) et al., Favipiravir and Ivermectin Showed in Vitro Synergistic Antiviral Activity against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-941811/v1.
51.
Munson et al., Niclosamide and ivermectin modulate caspase-1 activity and proinflammatory cytokine secretion in a monocytic cell line, British Society For Nanomedicine Early Career Researcher Summer Meeting, 2021, web.archive.org/web/20230401070026/https://michealmunson.github.io/COVID.pdf.
52.
Mountain Valley MD, Mountain Valley MD Receives Successful Results From BSL-4 COVID-19 Clearance Trial on Three Variants Tested With Ivectosol™, 5/18, www.globenewswire.com/en/news-release/2021/05/18/2231755/0/en/Mountain-Valley-MD-Receives-Successful-Results-From-BSL-4-COVID-19-Clearance-Trial-on-Three-Variants-Tested-With-Ivectosol.html.
53.
Yesilbag et al., Ivermectin also inhibits the replication of bovine respiratory viruses (BRSV, BPIV-3, BoHV-1, BCoV and BVDV) in vitro, Virus Research, doi:10.1016/j.virusres.2021.198384.
54.
Mody et al., Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents, Communications Biology, doi:10.1038/s42003-020-01577-x.
55.
Jeffreys et al., Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2022.106542.
56.
Surnar et al., Clinically Approved Antiviral Drug in an Orally Administrable Nanoparticle for COVID-19, ACS Pharmacol. Transl. Sci., doi:10.1021/acsptsci.0c00179.
57.
Li et al., Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment, J. Cellular Physiology, doi:10.1002/jcp.30055.
58.
Caly et al., The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research, doi:10.1016/j.antiviral.2020.104787.
59.
Zhang et al., Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflammation Research, doi:10.1007/s00011-008-8007-8.
60.
Gao et al., Ivermectin ameliorates acute myocarditis via the inhibition of importin-mediated nuclear translocation of NF-κB/p65, International Immunopharmacology, doi:10.1016/j.intimp.2024.112073.
61.
Abd-Elmawla et al., Suppression of NLRP3 inflammasome by ivermectin ameliorates bleomycin-induced pulmonary fibrosis, Journal of Zhejiang University-SCIENCE B, doi:10.1631/jzus.B2200385.
62.
Uematsu et al., Prophylactic administration of ivermectin attenuates SARS-CoV-2 induced disease in a Syrian Hamster Model, The Journal of Antibiotics, doi:10.1038/s41429-023-00623-0.
63.
Albariqi et al., Pharmacokinetics and Safety of Inhaled Ivermectin in Mice as a Potential COVID-19 Treatment, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121688.
64.
Errecalde et al., Safety and Pharmacokinetic Assessments of a Novel Ivermectin Nasal Spray Formulation in a Pig Model, Journal of Pharmaceutical Sciences, doi:10.1016/j.xphs.2021.01.017.
65.
Madrid et al., Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation, Heliyon, doi:10.1016/j.heliyon.2020.e05820.
66.
Ma et al., Ivermectin contributes to attenuating the severity of acute lung injury in mice, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2022.113706.
67.
de Melo et al., Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin, EMBO Mol. Med., doi:10.15252/emmm.202114122.
68.
Arévalo et al., Ivermectin reduces in vivo coronavirus infection in a mouse experimental model, Scientific Reports, doi:10.1038/s41598-021-86679-0.
69.
Chaccour et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Scientific Reports, doi:10.1038/s41598-020-74084-y.
70.
Yan et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflammation Research, doi:10.1007/s00011-011-0307-8.
71.
Götz et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Scientific Reports, doi:10.1038/srep23138.
72.
Tay et al., Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Research, doi:10.1016/j.antiviral.2013.06.002.
73.
Wagstaff et al., Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochemical Journal, doi:10.1042/BJ20120150.
74.
Wagstaff (B) et al., An AlphaScreen®-Based Assay for High-Throughput Screening for Specific Inhibitors of Nuclear Import, SLAS Discovery, doi:10.1177/1087057110390360.
75.
Barrows et al., A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection, Cell Host & Microbe, doi:10.1016/j.chom.2016.07.004.
76.
Yang et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Research, doi:10.1016/j.antiviral.2020.104760.
77.
Mastrangelo et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dks147.
78.
Varghese et al., Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses, Antiviral Research, doi:10.1016/j.antiviral.2015.12.012.
79.
Bennett et al., Role of a nuclear localization signal on the minor capsid Proteins VP2 and VP3 in BKPyV nuclear entry, Virology, doi:10.1016/j.virol.2014.10.013.
80.
Kosyna et al., The importin α/β-specific inhibitor Ivermectin affects HIF-dependent hypoxia response pathways, Biological Chemistry, doi:10.1515/hsz-2015-0171.
81.
Scheim et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
82.
Liu (C) et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
83.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
84.
Scheim (B), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
85.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
86.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
87.
DiNicolantonio et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
88.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
89.
Hazan et al., Treatment with Ivermectin Increases the Population of Bifidobacterium in the Gut, ACG 2023, acg2023posters.eventscribe.net/posterspeakers.asp.
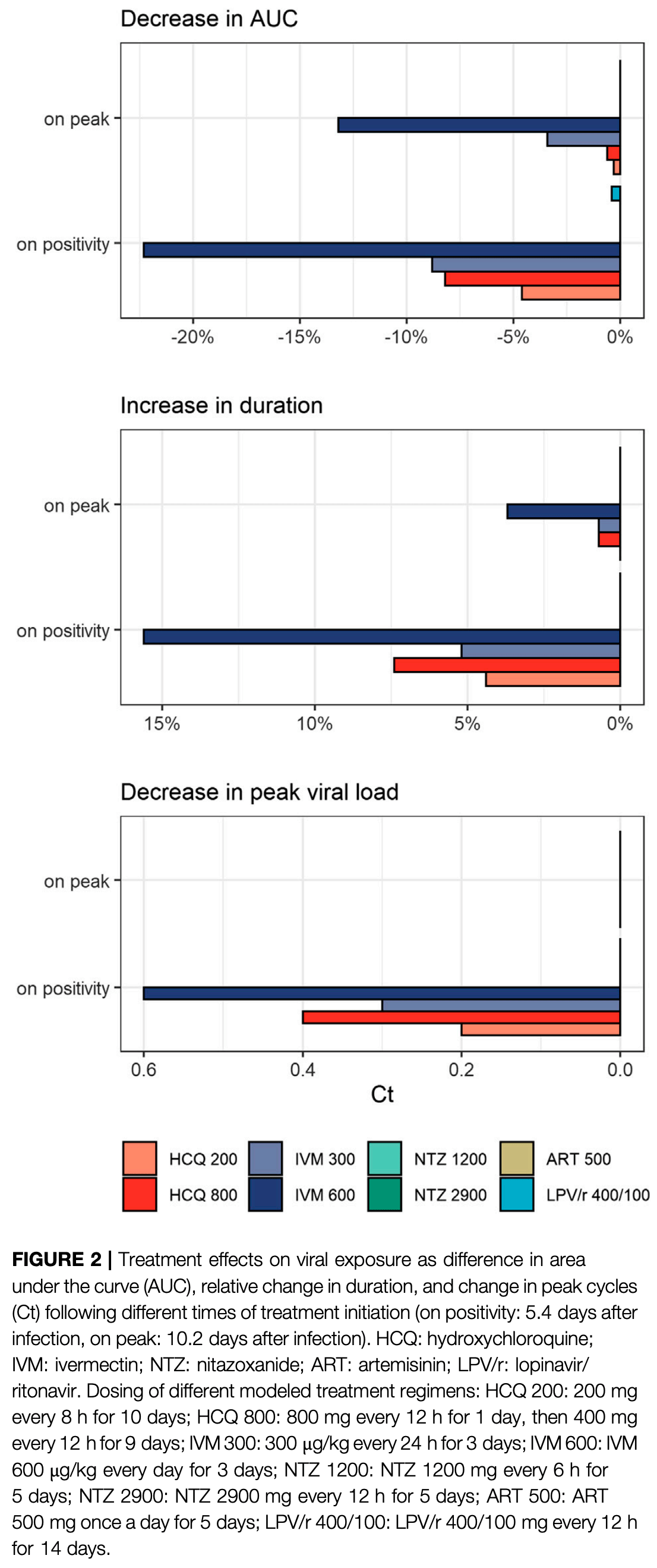
Kern et al., 10 Mar 2021, peer-reviewed, 4 authors.
In silico studies are an important part of preclinical research, however results may be very different in vivo.
Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing
Frontiers in Pharmacology, doi:10.3389/fphar.2021.625678
Several repurposed drugs are currently under investigation in the fight against coronavirus disease 2019 (COVID-19). Candidates are often selected solely by their effective concentrations in vitro, an approach that has largely not lived up to expectations in COVID-19. Cell lines used in in vitro experiments are not necessarily representative of lung tissue. Yet, even if the proposed mode of action is indeed true, viral dynamics in vivo, host response, and concentration-time profiles must also be considered. Here we address the latter issue and describe a model of human SARS-CoV-2 viral kinetics with acquired immune response to investigate the dynamic impact of timing and dosing regimens of hydroxychloroquine, lopinavir/ritonavir, ivermectin, artemisinin, and nitazoxanide. We observed greatest benefits when treatments were given immediately at the time of diagnosis. Even interventions with minor antiviral effect may reduce host exposure if timed correctly. Ivermectin seems to be at least partially effective: given on positivity, peak viral load dropped by 0.3-0.6 log units and exposure by 8.8-22.3%. The other drugs had little to no appreciable effect. Given how well previous clinical trial results for hydroxychloroquine and lopinavir/ritonavir are explained by the models presented here, similar strategies should be considered in future drug candidate prioritization efforts.
AUTHOR CONTRIBUTIONS FH conceived the project; CK, VS, and FH performed the analyses; and FH, CK, and VS wrote the first draft of the manuscript. All authors revised and approved the final manuscript.
SUPPLEMENTARY MATERIAL The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.625678/ full#supplementary-material. Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Copyright © 2021 Kern, Schöning, Chaccour and Hammann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
References
Annie, Sirbu, Frazier, Broce, Lucas, Hydroxychloroquine in hospitalized COVID-19 patients: real world experience assessing mortality, Pharmacotherapy, doi:10.1002/phar.2467
Arshad, Pertinez, Box, Tatham, Rajoli et al., Prioritization of anti-SARS-cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics, Clin. Pharmacol. Ther, doi:10.1002/cpt.1909
Atzori, Villani, Regazzi, Maruzzi, Cargnel, Detection of intrapulmonary concentration of lopinavir in an HIV-infected patient, AIDS, doi:10.1097/01.aids.0000076289.54156.32
Baccam, Beauchemin, Macken, Hayden, Perelson, Kinetics of influenza A virus infection in humans, J Virol, doi:10.1128/JVI.01623-05
Balderas-Acata, Ríos-Rogríguezbueno, Pérez-Becerril, Espinosa-Martínez, Burke-Fraga et al., Bioavailability of two oral-suspension formulations of a single dose of nitazoxanide 500 mg: an open-label, randomized-sequence, two-period crossover, comparison in healthy fasted Mexican adult volunteers, J Bioequiv Availab, doi:10.1016/j.clinthera.2009.08.004
Beauchemin, Handel, A review of mathematical models of influenza A infections within a host or cell culture: lessons learned and challenges ahead, BMC Public Health, doi:10.1186/1471-2458-11-S1-S7
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of covid-19 -preliminary report, N Engl J Med, doi:10.1056/NEJMoa2007764
Birgersson, Van Toi, Truong, Dung, Ashton et al., Population pharmacokinetic properties of artemisinin in healthy male Vietnamese volunteers, Malar J, doi:10.1186/s12936-016-1134-8
Boffito, Hoggard, Lindup, Bonora, Sinicco et al., Lopinavir protein binding in vivo through the 12-hour dosing interval, Ther. Drug Monit, doi:10.1097/00007691-200402000-00008
Boulware, Pullen, Bangdiwala, Pastick, Lofgren et al., A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2016638
Bray, Rayner, Noël, Jans, Wagstaff, Ivermectin and COVID-19: a report in Antiviral Research, widespread interest, an FDA warning, two letters to the editor and the authors' responses, Antiviral Res, doi:10.1016/j.antiviral.2020.104805
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
Canini, Perelson, Viral kinetic modeling: state of the art, J Pharmacokinet Pharmacodyn, doi:10.1007/s10928-014-9363-3
Cao, Hu, Li, Wang, Xu et al., Anti-SARS-CoV-2 potential of artemisinins in vitro, ACS Infect Dis, doi:10.1021/acsinfecdis.0c00522
Cao, Wang, Wen, Liu, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19, New England J. Medicine, doi:10.1056/NEJMoa2001282
Cavalcanti, Zampieri, Rosa, Azevedo, Veiga et al., hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19, N Engl J Med, doi:10.1056/NEJMoa2019014.E
Chaccour, Hammann, Rabinovich, Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety, Malar J, doi:10.1186/s12936-017-1801-4
Chandler, Serious neurological adverse events after ivermectin-do they occur beyond the indication of onchocerciasis?, Am J Trop Med Hyg, doi:10.4269/ajtmh.17-0042
Chandwani, Shuter, Lopinavir/ritonavir in the treatment of HIV-1 infection: a review, Ther Clin Risk Manag, doi:10.2147/tcrm.s3285
Choy, Wong, Kaewpreedee, Sia, Chen et al., Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104786
Chu, Pan, Cheng, Hui, Krishnan et al., Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia, Clin Chem, doi:10.1093/clinchem/hvaa029
Czuppon, Débarre, Gonçalves, Tenaillon, Perelson et al., Predicted success of prophylactic antiviral therapy to block or delay SARS-CoV-2 infection depends on the targeted mechanism, medRxiv, doi:10.1101/2020.05.07.20092965
Degani-Katzav, Klein, Har-Even, Gortler, Tobi et al., Trapping of ivermectin by a pentameric ligand-gated ion channel upon open-to-closed isomerization, Sci Rep, doi:10.1038/srep42481
Dickinson, Boffito, Back, Else, Von Hentig et al., Sequential population pharmacokinetic modeling of lopinavir and ritonavir in healthy volunteers and assessment of different dosing strategies, Antimicrob Agents Chemother, doi:10.1128/AAC.00887-10
Duthaler, Leisegang, Karlsson, Krähenbühl, Hammann, The effect of food on the pharmacokinetics of oral ivermectin, J. Antimicrobial Chemotherapy, doi:10.1093/jac/dkz466
Fajnzylber, Regan, Coxen, Corry, Wong et al., SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat Commun, doi:10.1038/s41467-020-19057-5
Fan, Beitler, Brochard, Calfee, Ferguson et al., COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted?, Lancet Respir Med, doi:10.1016/S2213-2600(20)30304-0
Fda, Prescribing INFORMATION: Alinia ® (nitazoxanide) Tablets (nitazoxanide) for oral suspension
Fda, Prescribing INFORMATION: KALETRA (lopinavir/ritonavir) tablet, film coated for oral use, KALETRA (lopinavir/ritonavir) solution for oral use
Furst, Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases, Lupus
Gautret, Lagier, Parola, Hoang, Meddeb et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Inter. J. Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.105949
Goldman, Gilman, Hollenback, Kato, Premack et al., Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties, Blood, doi:10.1182/blood.v95.11.3460
Gonçalves, Bertrand, Ke, Comets, De Lamballerie et al., Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2 viral load, CPT: Pharm. Syst. Pharm, doi:10.1002/psp4.12543
Gordon, Jang, Bouhaddou, Xu, Obernier et al., A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature, doi:10.1038/s41586-020-2286-9
Guzzo, Furtek, Porras, Chen, Tipping et al., Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J Clin Pharmacol, doi:10.1177/009127002401382731
Hernandez-Vargas, Chapter 3 -model parameter estimation
Hernandez-Vargas, Velasco-Hernandez, In-host mathematical modelling of COVID-19 in humans, Annu Rev Control, doi:10.1016/j.arcontrol.2020.09.006
Hoffmann, Mösbauer, Hofmann-Winkler, Kaul, Kleine-Weber et al., Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2, Nature, doi:10.1038/s41586-020-2575-3
Jagdev, Sidhu, Single-dose, comparative study of venous, capillary and salivary artemisinin concentrations in healthy, male adults, The American Journal of Tropical Medicine and Hygiene, doi:10.4269/ajtmh.1997.56.13
Kim, Ejima, Ito, Iwanami, Ohashi et al., Modelling SARS-CoV-2 dynamics: implications for therapy, MedRxiv, doi:10.1101/2020.03.23.20040493
Kim, Ko, Kim, Kim, Kim et al., Viral load kinetics of SARS-CoV-2 infection in first two patients in korea, J Korean Med Sci, doi:10.3346/jkms.2020.35.e86
Klok, Kruip, Van Der Meer, Arbous, Gommers et al., Incidence of thrombotic complications in critically ill ICU patients with COVID-19, Thromb Res, doi:10.1016/j.thromres.2020.04.013
Klotz, Ogbuokiri, Okonkwo, Ivermectin binds avidly to plasma proteins, Eur J Clin Pharmacol, doi:10.1007/BF00316107
Lauer, Grantz, Bi, Jones, Zheng et al., The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application, Ann Intern Med, doi:10.7326/M20-0504
Li, Chen, Zhang, Guo, Wang et al., Identification of natural compounds with antiviral activities against SARSassociated coronavirus, Antiviral Res, doi:10.1016/j.antiviral.2005.02.007
Li, Li, Zhang, Wang, Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues, Infect Dis Poverty, doi:10.1186/s40249-020-00662-x
Li, Xu, Liu, Zhou, The within-host viral kinetics of SARS-CoV-2, Math Biosci Eng, doi:10.3934/mbe.2020159
Lifschitz, Virkel, Sallovitz, Sutra, Galtier et al., Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle, Vet Parasitol, doi:10.1016/s0304-4017(99)00175-2
Lim, Im, Cho, Bae, Klein et al., Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax, Antimicrob Agents Chemother, doi:10.1128/AAC.00339-08
Liu, Cao, Xu, Wang, Zhang et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov, doi:10.1038/s41421-020-0156-0
Long, Liu, Deng, Wu, Deng et al., Antibody responses to SARS-CoV-2 in patients with COVID-19, Nat Med, doi:10.1038/s41591-020-0897-1
Magleby, Westblade, Trzebucki, Simon, Rajan et al., Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019, Clin Infect Dis, doi:10.1093/cid/ciaa851
Maisonnasse, Guedj, Contreras, Behillil, Solas et al., Hydroxychloroquine in the treatment and prophylaxis of SARS-CoV-2 infection in non-human primates, Virology, doi:10.21203/rs.3.rs-27223/v1
Mao, Jin, Wang, Hu, Chen et al., Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China, JAMA Neurol, doi:10.1001/jamaneurol.2020.1127
Martins-Filho, Barreto-Alves, Fakhouri, Potential role for nitazoxanide in treating SARS-CoV-2 infection, American Journal of Physiology-Lung Cellular and Molecular Physiology, doi:10.1152/ajplung.00170.2020
Mastrangelo, Pezzullo, De Burghgraeve, Kaptein, Pastorino et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, J Antimicrob Chemother, doi:10.1093/jac/dks147
Molento, COVID-19 and the rush for self-medication and self-dosing with ivermectin: a word of caution, One Health, doi:10.1016/j.onehlt.2020.100148
Molina, Delaugerre, Le Goff, Mela-Lima, Ponscarme et al., No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection, Med Mal Infect, doi:10.1016/j.medmal.2020.03.006
Nair, Huang, Fidock, Polyak, Wagoner et al., Artemisia annua L. extracts prevent in vitro replication of SARS-CoV-2, bioRxiv, doi:10.1101/2021.01.08.425825
Nordling, Unproven herbal remedy against COVID-19 could fuel drugresistant malaria, scientists warn
Oakes, Fuchs, Gardner, Lazartigues, Yue, Nicotine and the renin-angiotensin system, Am. J. physiol. Regul. Integr. Comp. Physiol, doi:10.1152/ajpregu.00099.2018
Owens, Excitement around hydroxychloroquine for treating COVID-19 causes challenges for rheumatology, Lancet Rheumatol, doi:10.1016/S2665-9913(20)30089-8
Pan, Peto, Karim, Alejandria, Henao-Restrepo et al., Repurposed antiviral drugs for COVID-19 -interim WHO SOLIDARITY trial results, medRxiv, doi:10.1101/2020.10.15.20209817
Quiros Roldan, Biasiotto, Magro, Zanella, The possible mechanisms of action of 4-aminoquinolines (chloroquine/ hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): a role for iron homeostasis?, Pharmacol Res, doi:10.1016/j.phrs.2020.104904
Rajasingham, Bangdiwala, Nicol, Skipper, Pastick et al., Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial, Clin. Infect. Dis, doi:10.1093/cid/ciaa1571
Rajoli, Pertinez, Arshad, Box, Tatham et al., Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis, medRxiv, doi:10.1101/2020.05.01.20087130
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review, JAMA, doi:10.1001/jama.2020.6019
Schmith, Zhou, Lohmer, The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19, Cli. Pharmacol. Ther, doi:10.1002/cpt.1889
Sehailia, Chemat, Antimalarial-agent artemisinin and derivatives portray more potent binding to Lys353 and Lys31-binding hotspots of SARS-CoV-2 spike protein than hydroxychloroquine: potential repurposing of artenimol for COVID-19, J Biomol Struct Dyn, doi:10.1080/07391102.2020.1796809
Struyf, Deeks, Dinnes, Takwoingi, Davenport et al., Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease, Cochrane Database Syst Rev, doi:10.1002/14651858.CD013665
Sze, Pan, Nevill, Gray, Martin et al., Ethnicity and clinical outcomes in COVID-19: a systematic review and metaanalysis, EClinicalMedicine, doi:10.1016/j.eclinm.2020.100630
Tang, Cao, Han, Wang, Chen et al., Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial, BMJ, doi:10.1136/bmj.m1849
Tang, Liu, Zhang, Xu, Ji et al., Cytokine storm in COVID-19: the current evidence and treatment strategies, Frontiers in Immunology, doi:10.3389/fimmu.2020.01708
To, Tsang, Leung, Tam, Wu et al., Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30196-1
Tripathy, Dassarma, Roy, Chabalala, Matsabisa, A review on possible modes of action of chloroquine/hydroxychloroquine: repurposing against SAR-CoV-2 (COVID-19) pandemic, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.106028
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J, doi:10.1042/BJ20120150
Walsh, Jordan, Clyne, Rohde, Drummond et al., SARS-CoV-2 detection, viral load and infectivity over the course of an infection, J. Infect, doi:10.1016/j.jinf.2020.06.067
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wang, Xu, Gao, Lu, Han et al., Detection of SARS-CoV-2 in different types of clinical specimens, JAMA, doi:10.1001/jama.2020.3786
Welle, COVID-19: Tests for 'miracle cure' herb Artemisia begin
Who, Emergence and spread of artemisinin resistance calls for intensified efforts to withdraw oral artemisinin monotherapy from the market
Who, Solidarity" clinical trial for COVID-19 treatments
Wu, Wang, Kuo, Shannar, Peter et al., An update on current therapeutic drugs treating COVID-19, Current Pharmacology Reports, doi:10.1007/s40495-020-00216-7
Yamasmith, Saleh-Arong, .-H, Avirutnan, Angkasekwinai et al., Efficacy and safety of ivermectin against dengue infection: a phase III, randomized, double-blind, placebo-controlled trial
Young, Ong, Kalimuddin, Low, Tan et al., Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore, JAMA, doi:10.1001/jama.2020.3204
Zhang, Xiao, Zhang, Xia, Cao et al., Coagulopathy and antiphospholipid antibodies in patients with covid-19
DOI record:
{
"DOI": "10.3389/fphar.2021.625678",
"ISSN": [
"1663-9812"
],
"URL": "http://dx.doi.org/10.3389/fphar.2021.625678",
"abstract": "<jats:p>Several repurposed drugs are currently under investigation in the fight against coronavirus disease 2019 (COVID-19). Candidates are often selected solely by their effective concentrations <jats:italic>in vitro</jats:italic>, an approach that has largely not lived up to expectations in COVID-19. Cell lines used in <jats:italic>in vitro</jats:italic> experiments are not necessarily representative of lung tissue. Yet, even if the proposed mode of action is indeed true, viral dynamics <jats:italic>in vivo</jats:italic>, host response, and concentration-time profiles must also be considered. Here we address the latter issue and describe a model of human SARS-CoV-2 viral kinetics with acquired immune response to investigate the dynamic impact of timing and dosing regimens of hydroxychloroquine, lopinavir/ritonavir, ivermectin, artemisinin, and nitazoxanide. We observed greatest benefits when treatments were given immediately at the time of diagnosis. Even interventions with minor antiviral effect may reduce host exposure if timed correctly. Ivermectin seems to be at least partially effective: given on positivity, peak viral load dropped by 0.3–0.6 log units and exposure by 8.8–22.3%. The other drugs had little to no appreciable effect. Given how well previous clinical trial results for hydroxychloroquine and lopinavir/ritonavir are explained by the models presented here, similar strategies should be considered in future drug candidate prioritization efforts.</jats:p>",
"alternative-id": [
"10.3389/fphar.2021.625678"
],
"author": [
{
"affiliation": [],
"family": "Kern",
"given": "Charlotte",
"sequence": "first"
},
{
"affiliation": [],
"family": "Schöning",
"given": "Verena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaccour",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hammann",
"given": "Felix",
"sequence": "additional"
}
],
"container-title": "Frontiers in Pharmacology",
"container-title-short": "Front. Pharmacol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2021,
3,
10
]
],
"date-time": "2021-03-10T08:54:38Z",
"timestamp": 1615366478000
},
"deposited": {
"date-parts": [
[
2021,
3,
10
]
],
"date-time": "2021-03-10T08:55:12Z",
"timestamp": 1615366512000
},
"indexed": {
"date-parts": [
[
2024,
3,
4
]
],
"date-time": "2024-03-04T02:32:38Z",
"timestamp": 1709519558209
},
"is-referenced-by-count": 11,
"issued": {
"date-parts": [
[
2021,
3,
10
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
10
]
],
"date-time": "2021-03-10T00:00:00Z",
"timestamp": 1615334400000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2021.625678/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2021,
3,
10
]
]
},
"published-online": {
"date-parts": [
[
2021,
3,
10
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1002/phar.2467",
"article-title": "Hydroxychloroquine in hospitalized COVID-19 patients: real world experience assessing mortality",
"author": "Annie",
"doi-asserted-by": "publisher",
"first-page": "1072",
"journal-title": "Pharmacotherapy",
"key": "B1",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1909",
"article-title": "Prioritization of anti-SARS-cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics",
"author": "Arshad",
"doi-asserted-by": "publisher",
"first-page": "775",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "B2",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1097/01.aids.0000076289.54156.32",
"article-title": "Detection of intrapulmonary concentration of lopinavir in an HIV-infected patient",
"author": "Atzori",
"doi-asserted-by": "publisher",
"first-page": "1710",
"journal-title": "AIDS",
"key": "B3",
"volume": "17",
"year": "2003"
},
{
"DOI": "10.1128/JVI.01623-05",
"article-title": "Kinetics of influenza A virus infection in humans",
"author": "Baccam",
"doi-asserted-by": "publisher",
"first-page": "7590",
"journal-title": "J Virol",
"key": "B4",
"volume": "80",
"year": "2006"
},
{
"DOI": "10.1016/j.clinthera.2009.08.004",
"article-title": "Bioavailability of two oral-suspension formulations of a single dose of nitazoxanide 500 mg: an open-label, randomized-sequence, two-period crossover, comparison in healthy fasted Mexican adult volunteers",
"author": "Balderas-Acata",
"doi-asserted-by": "publisher",
"first-page": "43",
"journal-title": "J Bioequiv Availab",
"key": "B5",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.1186/1471-2458-11-S1-S7",
"article-title": "A review of mathematical models of influenza A infections within a host or cell culture: lessons learned and challenges ahead",
"author": "Beauchemin",
"doi-asserted-by": "publisher",
"first-page": "S7",
"journal-title": "BMC Public Health",
"key": "B6",
"volume": "11",
"year": "2011"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of covid-19 - preliminary report",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "B7",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1186/s12936-016-1134-8",
"article-title": "Population pharmacokinetic properties of artemisinin in healthy male Vietnamese volunteers",
"author": "Birgersson",
"doi-asserted-by": "publisher",
"first-page": "90",
"journal-title": "Malar J.",
"key": "B8",
"volume": "15",
"year": "2016"
},
{
"DOI": "10.1097/00007691-200402000-00008",
"article-title": "Lopinavir protein binding in vivo through the 12-hour dosing interval",
"author": "Boffito",
"doi-asserted-by": "publisher",
"first-page": "35",
"journal-title": "Ther. Drug Monit.",
"key": "B9",
"volume": "26",
"year": "2004"
},
{
"DOI": "10.1056/NEJMoa2016638",
"article-title": "A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19",
"author": "Boulware",
"doi-asserted-by": "publisher",
"first-page": "517",
"journal-title": "New England Journal of Medicine",
"key": "B10",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104805",
"article-title": "Ivermectin and COVID-19: a report in Antiviral Research, widespread interest, an FDA warning, two letters to the editor and the authors' responses",
"author": "Bray",
"doi-asserted-by": "publisher",
"first-page": "104805",
"journal-title": "Antiviral Res.",
"key": "B11",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "publisher",
"first-page": "104787",
"journal-title": "Antiviral Res.",
"key": "B12",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1007/s10928-014-9363-3",
"article-title": "Viral kinetic modeling: state of the art",
"author": "Canini",
"doi-asserted-by": "publisher",
"first-page": "431",
"journal-title": "J Pharmacokinet Pharmacodyn",
"key": "B13",
"volume": "41",
"year": "2014"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19",
"author": "Cao",
"doi-asserted-by": "publisher",
"first-page": "1787",
"journal-title": "New England J. Medicine",
"key": "B14",
"volume": "382",
"year": ""
},
{
"DOI": "10.1021/acsinfecdis.0c00522",
"article-title": "Anti-SARS-CoV-2 potential of artemisinins in vitro",
"author": "Cao",
"doi-asserted-by": "publisher",
"first-page": "2524",
"journal-title": "ACS Infect Dis.",
"key": "B15",
"volume": "6",
"year": ""
},
{
"DOI": "10.1056/NEJMoa2019014.E",
"article-title": "hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19",
"author": "Cavalcanti",
"doi-asserted-by": "publisher",
"first-page": "2041",
"journal-title": "N Engl J Med.",
"key": "B16",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1186/s12936-017-1801-4",
"article-title": "Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety",
"author": "Chaccour",
"doi-asserted-by": "publisher",
"first-page": "161",
"journal-title": "Malar J",
"key": "B17",
"volume": "16",
"year": "2017"
},
{
"DOI": "10.4269/ajtmh.17-0042",
"article-title": "Serious neurological adverse events after ivermectin-do they occur beyond the indication of onchocerciasis?",
"author": "Chandler",
"doi-asserted-by": "publisher",
"first-page": "382",
"journal-title": "Am J Trop Med Hyg",
"key": "B18",
"volume": "98",
"year": "2018"
},
{
"DOI": "10.2147/tcrm.s3285",
"article-title": "Lopinavir/ritonavir in the treatment of HIV-1 infection: a review",
"author": "Chandwani",
"doi-asserted-by": "publisher",
"first-page": "1023",
"journal-title": "Ther Clin Risk Manag.",
"key": "B19",
"volume": "4",
"year": "2008"
},
{
"DOI": "10.1016/j.antiviral.2020.104786",
"article-title": "Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro",
"author": "Choy",
"doi-asserted-by": "publisher",
"first-page": "104786",
"journal-title": "Antiviral Res.",
"key": "B20",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1093/clinchem/hvaa029",
"article-title": "Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia",
"author": "Chu",
"doi-asserted-by": "publisher",
"first-page": "549",
"journal-title": "Clin Chem.",
"key": "B21",
"volume": "66",
"year": "2020"
},
{
"DOI": "10.1101/2020.05.07.20092965",
"article-title": "Predicted success of prophylactic antiviral therapy to block or delay SARS-CoV-2 infection depends on the targeted mechanism",
"author": "Czuppon",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "B22",
"year": "2020"
},
{
"DOI": "10.1038/srep42481",
"article-title": "Trapping of ivermectin by a pentameric ligand-gated ion channel upon open-to-closed isomerization",
"author": "Degani-Katzav",
"doi-asserted-by": "publisher",
"first-page": "42481",
"journal-title": "Sci Rep.",
"key": "B23",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1128/AAC.00887-10",
"article-title": "Sequential population pharmacokinetic modeling of lopinavir and ritonavir in healthy volunteers and assessment of different dosing strategies",
"author": "Dickinson",
"doi-asserted-by": "publisher",
"first-page": "2775",
"journal-title": "Antimicrob Agents Chemother.",
"key": "B24",
"volume": "55",
"year": "2011"
},
{
"DOI": "10.1093/jac/dkz466",
"article-title": "The effect of food on the pharmacokinetics of oral ivermectin",
"author": "Duthaler",
"doi-asserted-by": "publisher",
"first-page": "438",
"journal-title": "J. Antimicrobial Chemotherapy",
"key": "B25",
"volume": "75",
"year": "2019"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"article-title": "SARS-CoV-2 viral load is associated with increased disease severity and mortality",
"author": "Fajnzylber",
"doi-asserted-by": "publisher",
"first-page": "5493",
"journal-title": "Nat Commun.",
"key": "B26",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30304-0",
"article-title": "COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted?",
"author": "Fan",
"doi-asserted-by": "publisher",
"first-page": "816",
"journal-title": "Lancet Respir Med",
"key": "B27",
"volume": "8",
"year": "2020"
},
{
"key": "B28",
"volume-title": "Prescribing INFORMATION: Alinia® (nitazoxanide) Tablets (nitazoxanide) for oral suspension",
"year": "2020"
},
{
"key": "B29",
"volume-title": "Prescribing INFORMATION: KALETRA (lopinavir/ritonavir) tablet, film coated for oral use, KALETRA (lopinavir/ritonavir) solution for oral use",
"year": "2020"
},
{
"DOI": "10.1177/0961203396005001041",
"article-title": "Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases",
"author": "Furst",
"doi-asserted-by": "crossref",
"first-page": "S11",
"journal-title": "Lupus",
"key": "B30",
"volume": "5",
"year": "1996"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"article-title": "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial",
"author": "Gautret",
"doi-asserted-by": "publisher",
"first-page": "105949",
"journal-title": "Inter. J. Antimicrobial Agents",
"key": "B31",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1182/blood.v95.11.3460",
"article-title": "Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties",
"author": "Goldman",
"doi-asserted-by": "publisher",
"first-page": "3460",
"journal-title": "Blood",
"key": "B32",
"volume": "95",
"year": "2000"
},
{
"DOI": "10.1002/psp4.12543",
"article-title": "Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2 viral load",
"author": "Gonçalves",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "CPT: Pharm. Syst. Pharm.",
"key": "B33",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"article-title": "A SARS-CoV-2 protein interaction map reveals targets for drug repurposing",
"author": "Gordon",
"doi-asserted-by": "publisher",
"first-page": "459",
"journal-title": "Nature",
"key": "B34",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1177/009127002401382731",
"article-title": "Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects",
"author": "Guzzo",
"doi-asserted-by": "publisher",
"first-page": "1122",
"journal-title": "J Clin Pharmacol",
"key": "B35",
"volume": "42",
"year": "2002"
},
{
"DOI": "10.1016/j.arcontrol.2020.09.006",
"article-title": "In-host mathematical modelling of COVID-19 in humans",
"author": "Hernandez-Vargas",
"doi-asserted-by": "publisher",
"first-page": "448",
"journal-title": "Annu Rev Control",
"key": "B36",
"volume": "50",
"year": "2020"
},
{
"article-title": "Chapter 3 - model parameter estimation",
"author": "Hernandez-Vargas",
"first-page": "35",
"key": "B37",
"volume-title": "Modeling and control of infectious diseases in the host",
"year": "2019"
},
{
"DOI": "10.1038/s41586-020-2575-3",
"article-title": "Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "588",
"journal-title": "Nature",
"key": "B38",
"volume": "585",
"year": "2020"
},
{
"DOI": "10.4269/ajtmh.1997.56.13",
"article-title": "Single-dose, comparative study of venous, capillary and salivary artemisinin concentrations in healthy, male adults",
"author": "Jagdev",
"doi-asserted-by": "publisher",
"first-page": "13",
"journal-title": "The American Journal of Tropical Medicine and Hygiene",
"key": "B39",
"volume": "56",
"year": "1997"
},
{
"DOI": "10.3346/jkms.2020.35.e86",
"article-title": "Viral load kinetics of SARS-CoV-2 infection in first two patients in korea",
"author": "Kim",
"doi-asserted-by": "publisher",
"first-page": "e86",
"journal-title": "J Korean Med Sci.",
"key": "B40",
"volume": "35",
"year": ""
},
{
"DOI": "10.1101/2020.03.23.20040493",
"article-title": "Modelling SARS-CoV-2 dynamics: implications for therapy",
"author": "Kim",
"doi-asserted-by": "publisher",
"journal-title": "MedRxiv",
"key": "B41",
"year": ""
},
{
"DOI": "10.1016/j.thromres.2020.04.013",
"article-title": "Incidence of thrombotic complications in critically ill ICU patients with COVID-19",
"author": "Klok",
"doi-asserted-by": "publisher",
"first-page": "145",
"journal-title": "Thromb Res.",
"key": "B42",
"volume": "191",
"year": "2020"
},
{
"DOI": "10.1007/BF00316107",
"article-title": "Ivermectin binds avidly to plasma proteins",
"author": "Klotz",
"doi-asserted-by": "publisher",
"first-page": "607",
"journal-title": "Eur J Clin Pharmacol.",
"key": "B43",
"volume": "39",
"year": "1990"
},
{
"DOI": "10.7326/M20-0504",
"article-title": "The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application",
"author": "Lauer",
"doi-asserted-by": "publisher",
"first-page": "577",
"journal-title": "Ann Intern Med.",
"key": "B44",
"volume": "172",
"year": "2020"
},
{
"DOI": "10.3934/mbe.2020159",
"article-title": "The within-host viral kinetics of SARS-CoV-2",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "2853",
"journal-title": "Math Biosci Eng.",
"key": "B45",
"volume": "17",
"year": ""
},
{
"DOI": "10.1186/s40249-020-00662-x",
"article-title": "Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "45",
"journal-title": "Infect Dis Poverty",
"key": "B46",
"volume": "9",
"year": ""
},
{
"DOI": "10.1016/j.antiviral.2005.02.007",
"article-title": "Identification of natural compounds with antiviral activities against SARS-associated coronavirus",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "18",
"journal-title": "Antiviral Res.",
"key": "B47",
"volume": "67",
"year": "2005"
},
{
"DOI": "10.1016/s0304-4017(99)00175-2",
"article-title": "Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle",
"author": "Lifschitz",
"doi-asserted-by": "publisher",
"first-page": "327",
"journal-title": "Vet Parasitol.",
"key": "B48",
"volume": "87",
"year": "2000"
},
{
"DOI": "10.1128/AAC.00339-08",
"article-title": "Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax",
"author": "Lim",
"doi-asserted-by": "publisher",
"first-page": "1468",
"journal-title": "Antimicrob Agents Chemother.",
"key": "B49",
"volume": "53",
"year": "2009"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"article-title": "Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "16",
"journal-title": "Cell Discov.",
"key": "B50",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0897-1",
"article-title": "Antibody responses to SARS-CoV-2 in patients with COVID-19",
"author": "Long",
"doi-asserted-by": "publisher",
"first-page": "845",
"journal-title": "Nat Med.",
"key": "B51",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa851",
"article-title": "Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019",
"author": "Magleby",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis.",
"key": "B52",
"year": "2020"
},
{
"DOI": "10.21203/rs.3.rs-27223/v1",
"article-title": "Hydroxychloroquine in the treatment and prophylaxis of SARS-CoV-2 infection in non-human primates",
"author": "Maisonnasse",
"doi-asserted-by": "publisher",
"journal-title": "Virology.",
"key": "B53",
"year": "2020"
},
{
"DOI": "10.1001/jamaneurol.2020.1127",
"article-title": "Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China",
"author": "Mao",
"doi-asserted-by": "publisher",
"first-page": "683",
"journal-title": "JAMA Neurol.",
"key": "B54",
"volume": "77",
"year": "2020"
},
{
"DOI": "10.1152/ajplung.00170.2020",
"article-title": "Potential role for nitazoxanide in treating SARS-CoV-2 infection",
"author": "Martins-Filho",
"doi-asserted-by": "publisher",
"first-page": "L35",
"journal-title": "American Journal of Physiology-Lung Cellular and Molecular Physiology",
"key": "B55",
"volume": "319",
"year": "2020"
},
{
"DOI": "10.1093/jac/dks147",
"article-title": "Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug",
"author": "Mastrangelo",
"doi-asserted-by": "publisher",
"first-page": "1884",
"journal-title": "J Antimicrob Chemother.",
"key": "B56",
"volume": "67",
"year": "2012"
},
{
"DOI": "10.1016/j.onehlt.2020.100148",
"article-title": "COVID-19 and the rush for self-medication and self-dosing with ivermectin: a word of caution",
"author": "Molento",
"doi-asserted-by": "publisher",
"first-page": "100148",
"journal-title": "One Health",
"key": "B57",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.medmal.2020.03.006",
"article-title": "No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection",
"author": "Molina",
"doi-asserted-by": "publisher",
"first-page": "384",
"journal-title": "Med Mal Infect.",
"key": "B58",
"volume": "50",
"year": "2020"
},
{
"DOI": "10.1101/2021.01.08.425825",
"article-title": "Artemisia annua L. extracts prevent in vitro replication of SARS-CoV-2",
"author": "Nair",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "B59",
"year": "2021"
},
{
"key": "B60",
"unstructured": "Unproven herbal remedy against COVID-19 could fuel drug-resistant malaria, scientists warn\n NordlingL.\n 2020"
},
{
"DOI": "10.1152/ajpregu.00099.2018",
"article-title": "Nicotine and the renin-angiotensin system",
"author": "Oakes",
"doi-asserted-by": "publisher",
"first-page": "R895",
"journal-title": "Am. J. physiol. Regul. Integr. Comp. Physiol.",
"key": "B61",
"volume": "315",
"year": "2018"
},
{
"DOI": "10.1016/S2665-9913(20)30089-8",
"article-title": "Excitement around hydroxychloroquine for treating COVID-19 causes challenges for rheumatology",
"author": "Owens",
"doi-asserted-by": "publisher",
"first-page": "e257",
"journal-title": "Lancet Rheumatol.",
"key": "B62",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1101/2020.10.15.20209817",
"article-title": "Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results",
"author": "Pan",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "B63",
"year": "2020"
},
{
"DOI": "10.1016/j.phrs.2020.104904",
"article-title": "The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): a role for iron homeostasis?",
"author": "Quiros Roldan",
"doi-asserted-by": "publisher",
"first-page": "104904",
"journal-title": "Pharmacol Res.",
"key": "B64",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1571",
"article-title": "Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial",
"author": "Rajasingham",
"doi-asserted-by": "publisher",
"journal-title": "Clin. Infect. Dis.",
"key": "B65",
"year": "2020"
},
{
"DOI": "10.1101/2020.05.01.20087130",
"article-title": "Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis",
"author": "Rajoli",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "B66",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6019",
"article-title": "Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review",
"author": "Sanders",
"doi-asserted-by": "publisher",
"first-page": "1824",
"journal-title": "JAMA",
"key": "B67",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1889",
"article-title": "The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19",
"author": "Schmith",
"doi-asserted-by": "publisher",
"first-page": "762",
"journal-title": "Cli. Pharmacol. Ther.",
"key": "B68",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1080/07391102.2020.1796809",
"article-title": "Antimalarial-agent artemisinin and derivatives portray more potent binding to Lys353 and Lys31-binding hotspots of SARS-CoV-2 spike protein than hydroxychloroquine: potential repurposing of artenimol for COVID-19",
"author": "Sehailia",
"doi-asserted-by": "publisher",
"journal-title": "J Biomol Struct Dyn",
"key": "B69",
"year": "2020"
},
{
"DOI": "10.1002/14651858.CD013665",
"article-title": "Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease",
"author": "Struyf",
"doi-asserted-by": "publisher",
"first-page": "CD013665",
"journal-title": "Cochrane Database Syst Rev.",
"key": "B70",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2020.100630",
"article-title": "Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis",
"author": "Sze",
"doi-asserted-by": "publisher",
"first-page": "100630",
"journal-title": "EClinicalMedicine",
"key": "B71",
"volume": "29",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1849",
"article-title": "Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial",
"author": "Tang",
"doi-asserted-by": "publisher",
"first-page": "m1849",
"journal-title": "BMJ",
"key": "B72",
"volume": "369",
"year": ""
},
{
"DOI": "10.3389/fimmu.2020.01708",
"article-title": "Cytokine storm in COVID-19: the current evidence and treatment strategies",
"author": "Tang",
"doi-asserted-by": "publisher",
"first-page": "1708",
"journal-title": "Frontiers in Immunology",
"key": "B73",
"volume": "11",
"year": ""
},
{
"DOI": "10.1016/S1473-3099(20)30196-1",
"article-title": "Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study",
"author": "To",
"doi-asserted-by": "publisher",
"first-page": "565",
"journal-title": "Lancet Infect Dis.",
"key": "B74",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106028",
"article-title": "A review on possible modes of action of chloroquine/hydroxychloroquine: repurposing against SAR-CoV-2 (COVID-19) pandemic",
"author": "Tripathy",
"doi-asserted-by": "publisher",
"first-page": "106028",
"journal-title": "Int J Antimicrob Agents",
"key": "B75",
"volume": "56",
"year": "2020"
},
{
"key": "B76",
"volume-title": "Statement from the chief investigators of the randomised evaluation of COVid-19 thERapY (RECOVERY) trial on lopinavir-ritonavir",
"year": "2020"
},
{
"DOI": "10.1042/BJ20120150",
"article-title": "Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus",
"author": "Wagstaff",
"doi-asserted-by": "publisher",
"first-page": "851",
"journal-title": "Biochem J.",
"key": "B77",
"volume": "443",
"year": "2012"
},
{
"DOI": "10.1016/j.jinf.2020.06.067",
"article-title": "SARS-CoV-2 detection, viral load and infectivity over the course of an infection",
"author": "Walsh",
"doi-asserted-by": "publisher",
"first-page": "357",
"journal-title": "J. Infect.",
"key": "B78",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "269",
"journal-title": "Cell Res.",
"key": "B79",
"volume": "30",
"year": ""
},
{
"DOI": "10.1001/jama.2020.3786",
"article-title": "Detection of SARS-CoV-2 in different types of clinical specimens",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "1843",
"journal-title": "JAMA",
"key": "B80",
"volume": "323",
"year": ""
},
{
"key": "B81",
"unstructured": "COVID-19: Tests for ‘miracle cure' herb Artemisia begin\n WelleD.\n 2020"
},
{
"key": "B82",
"volume-title": "Emergence and spread of artemisinin resistance calls for intensified efforts to withdraw oral artemisinin monotherapy from the market",
"year": "2014"
},
{
"key": "B83",
"volume-title": "“Solidarity” clinical trial for COVID-19 treatments",
"year": "2020"
},
{
"DOI": "10.1007/s40495-020-00216-7",
"article-title": "An update on current therapeutic drugs treating COVID-19",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "56",
"journal-title": "Current Pharmacology Reports",
"key": "B84",
"volume": "6",
"year": "2020"
},
{
"article-title": "Efficacy and safety of ivermectin against dengue infection: a phase III, randomized, double-blind, placebo-controlled trial",
"author": "Yamasmith",
"key": "B85",
"volume-title": "The 34th annual meeting the royal college of physicians of Thailand 'internal medicine and one health",
"year": "2018"
},
{
"DOI": "10.1001/jama.2020.3204",
"article-title": "Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore",
"author": "Young",
"doi-asserted-by": "publisher",
"first-page": "1488",
"journal-title": "JAMA",
"key": "B86",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2007575",
"article-title": "Coagulopathy and antiphospholipid antibodies in patients with covid-19",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "e38",
"journal-title": "N Engl J Med.",
"key": "B87",
"volume": "382",
"year": "2020"
}
],
"reference-count": 87,
"references-count": 87,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.21203/rs.3.rs-153281/v2",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.21203/rs.3.rs-153281/v3",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.21203/rs.3.rs-153281/v1",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2021.625678/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "12"
}
