Tolerability, Safety, and Pharmacokinetics of Ivermectin After Nasal Application in Healthy Adult Subjects
et al., The Journal of Clinical Pharmacology, doi:10.1002/jcph.70137, EudraCT2022-002670-82, Dec 2025
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Phase 1 RCT of 28 healthy adults showing good tolerability and safety with nasal ivermectin spray (F004). Participants received either 5% ivermectin nasal spray (14mg single dose, then 42mg/day for 5 days) or placebo. Pharmacokinetic analysis showed bioavailability comparable to oral ivermectin solution. Authors hypothesize that nasal application could provide dual action against COVID-19 through local nasal effects and systemic absorption.
1.
Wissel et al., Tolerability, Safety, and Pharmacokinetics of Ivermectin After Nasal Application in Healthy Adult Subjects, The Journal of Clinical Pharmacology, doi:10.1002/jcph.70137.
2.
Mohammed et al., A remodeled ivermectin polycaprolactone-based nanoparticles for inhalation as a promising treatment of pulmonary inflammatory diseases, European Journal of Pharmaceutical Sciences, doi:10.1016/j.ejps.2024.106714.
3.
Saha et al., Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder, Pharmaceutics, doi:10.3390/pharmaceutics14071432.
4.
Albariqi et al., Pharmacokinetics and Safety of Inhaled Ivermectin in Mice as a Potential COVID-19 Treatment, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121688.
5.
Albariqi (B) et al., Preparation and Characterization of Inhalable Ivermectin Powders as a Potential COVID-19 Therapy, Journal of Aerosol Medicine and Pulmonary Drug Delivery, doi:10.1089/jamp.2021.0059.
6.
Mansour et al., Safety of inhaled ivermectin as a repurposed direct drug for treatment of COVID-19: A preclinical tolerance study, International Immunopharmacology, doi:10.1016/j.intimp.2021.108004.
Wissel et al., 17 Dec 2025, Double Blind Randomized Controlled Trial, placebo-controlled, peer-reviewed, mean age 33.5, 6 authors, study period January 2023 - March 2023, trial EudraCT2022-002670-82.
Contact: haeberlein@uni-bonn.de.
Tolerability, Safety, and Pharmacokinetics of Ivermectin After Nasal Application in Healthy Adult Subjects
The Journal of Clinical Pharmacology, doi:10.1002/jcph.70137
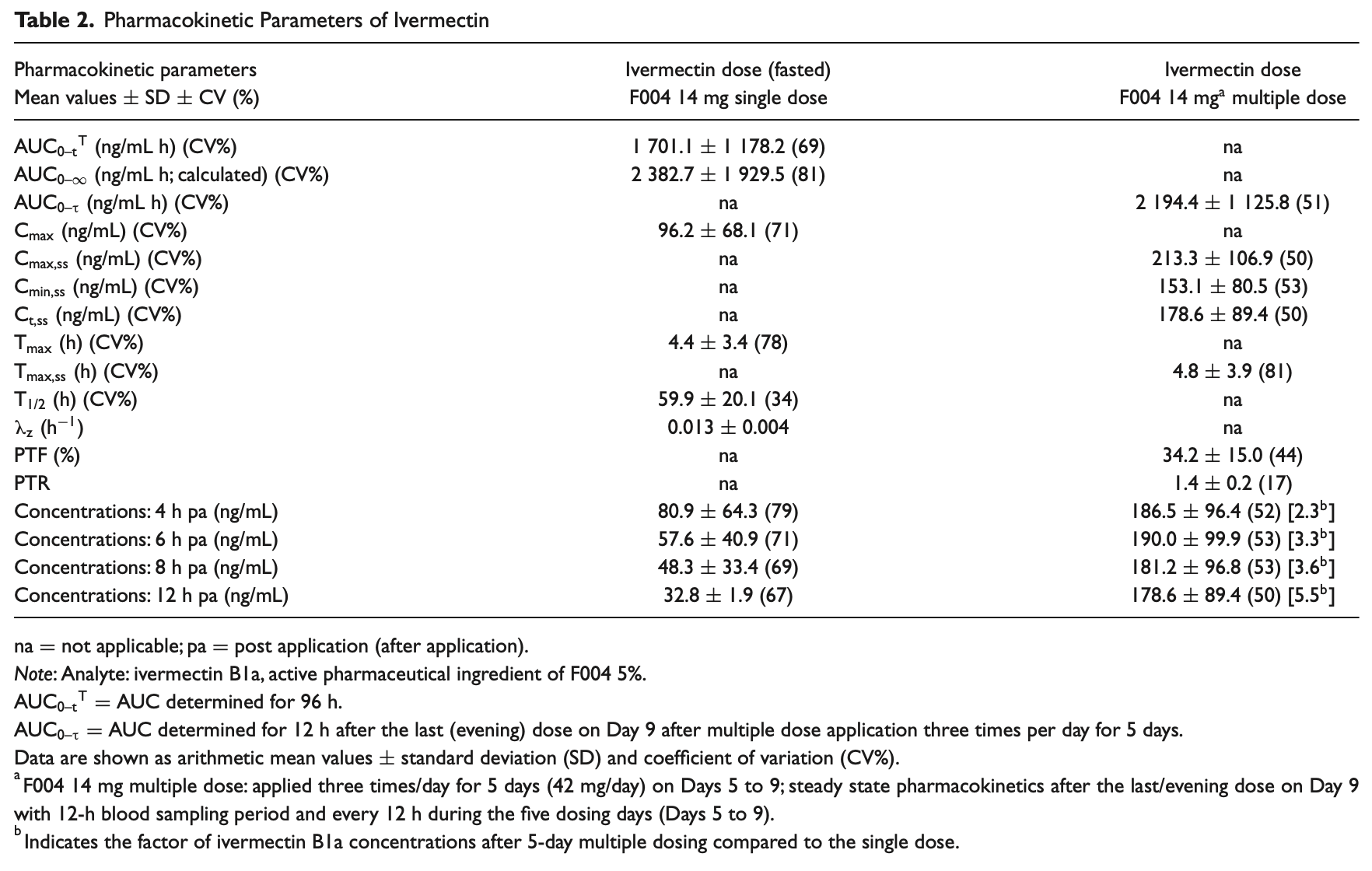
Nasal epithelium is the site of infection for SARS-CoV2 viruses, with interactions of the viral spike protein with the ACE2 receptor of the host cell. Molecular docking studies have shown that ivermectin shields the spike protein and thereby prevents binding to ACE2. Nasal application of high doses of ivermectin could be the right therapeutic approach in the treatment and prevention of COVID-19. Tolerability, safety, and pharmacokinetics of ivermectin, administered nasally as 5% microsuspension (F004), were investigated in a randomized, double-blind, parallel-groups, placebo-controlled phase 1 study in 28 healthy adults. Bioavailability of a single dose of 14 mg ivermectin was determined with AUC 0-t T of 1701.1 ng/mL h (AUC 0-∞ of 2382.7 ng/mL h, calculated), C max of 96.2 ng/mL, T max of 4.4 h, and T 1/2 of 59.9 h. Following 42 mg/day multiple dose (3 × 14 mg every 6 h) administered nasally over 5 days, AUC 0-∞ of 2194.4 ng/mL h was analyzed, and 96% of ivermectin concentrations were still measurable 12 h after the last dose. F004 was safe in this study and well-tolerated. Nine (F004 group) and three (placebo group) of 28 subjects reported 14 symptoms, including a few systemic but mainly local nasal adverse events (AE). The number of subjects reporting AE decreased continuously after both F004 and placebo treatment. All subjects recovered fully with no AE recorded at the end of the study. Nasal examination showed stable patterns of nasal mucosal grading, mucosal bleeding, and crusting of the mucosa. Nasally administered ivermectin is well tolerated in high concentrations and could provide systemic therapeutic benefits in addition to local effects.
Supplemental Information Additional supplemental information can be found by clicking the Supplements link in the PDF toolbar or the Supplemental Information section at the end of web-based version of this article.
References
Algorta, Krolewiecki, Pinto, Gold, Muñoz, Pharmacokinetic characterization and comparative bioavailability of an innovative orodispersible fixed-dose combination of ivermectin and albendazole: A single dose, open label, sequence randomized, crossover clinical trial in healthy volunteers, Front Pharmacol, doi:10.3389/fphar.2022.914886
Andrews, Herman, Gandhi, Treatments for COVID-19, Annu Rev Med, doi:10.1146/annurev-med-052422-020316
Baraka, Mahmoud, Marschke, Geary, Homeida et al., Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus, Eur J Clin Pharmacol, doi:10.1007/s002280050131
Brown, Ricci, Ottesen, Ivermectin: effectiveness in lymphatic filariasis, Parasitology, doi:10.1017/s0031182000006570
Caly, Druce, Catton, Jans, Wagstaff, Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
Canga, Prieto, Liébana, Martínez, Vega et al., The pharmacokinetics and interactions of ivermectin in humans-A mini-review, AAPS J, doi:10.1208/s12248-007-9000-9
Dayer, Coronavirus, -nCoV) Deactivation via Spike Glycoprotein Shielding by Old Drugs: A Bioinformatic Study, Preprints, doi:10.20944/preprints202005.0020.v1
Edwards, Dingsdale, Helsby, Orme, Breckenridge, The relative systemic availability of ivermectin after administration as capsule, tablet, and oral solution, Eur J Clin Pharmacol, doi:10.1007/BF00637608
Eweas, Alhossary, As, Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2, Front Microbiol, doi:10.3389/fmicb.2020.592908
Glp, Principles of Good Laboratory Practice as Specified by National (German Chemicals Law
Goa, Mctavish, Clissold, Ivermectin. A review of its antifilarial activity, pharmacokinetic properties and clinical efficacy in onchocerciasis, Drugs, doi:10.2165/00003495-199142040-00007
Guzzo, Furtek, Porras, Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J Clin Pharmacol, doi:10.1177/009127002401382731
Kerr, Cadegiani, Baldi, Ivermectin Prophylaxis Used for COVID-19: A Citywide, Prospective, Observational Study of 223,128 Subjects Using Propensity Score Matching, Cureus, doi:10.7759/cureus.21272
Mathachan, Sardana, Khurana, Current use of ivermectin in dermatology, tropical medicine, and COVID-19. An update on pharmacology, uses, proven and varied proposed mechanistic action, Indian Dermatol Online J, doi:10.4103/idoj.idoj_298_21
Mody, Ho, Wills, Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents, Commun Biol, doi:10.1038/s42003-020-01577-x
Mudatsir, Yufika, Nainu, Antiviral Activity of Ivermectin Against SARS-CoV-2: An Old-Fashioned Dog with a New Trick-A Literature Review, Sci Pharm, doi:10.3390/scipharm88030036
Muñoz, Ballester, Antonijoan, Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers, PLoS Negl Trop Dis, doi:10.1371/journal.pntd.0006020
Raedler, Soolantra (ivermectin) 1% cream: a novel, antibiotic-free agent approved for the treatment of patients with rosacea, Am Health Drug Benefits
Song, Shi, Zhang, Ivermectin for the treatment of COVID-19: A systematic review and meta-analysis, Heliyon, doi:10.1016/j.heliyon.2024.e27647
Surnar, Kamran, Shah, Dhar, Clinically Approved Antiviral Drug in an Orally Administrable Nanoparticle for COVID-19, ACS Pharmacol Transl Sci, doi:10.1021/acsptsci.0c00179
Wulan, Heydet, Walker, Gahan, Ghildyal, Nucleocytoplasmic transport of nucleocapsid proteins of enveloped RNA viruses, Front Microbiol, doi:10.3389/fmicb.2015.00553
Yang, Atkinson, Wang, The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Res, doi:10.1016/j.antiviral.2020.104760
DOI record:
{
"DOI": "10.1002/jcph.70137",
"ISSN": [
"0091-2700",
"1552-4604"
],
"URL": "http://dx.doi.org/10.1002/jcph.70137",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>\n Nasal epithelium is the site of infection for SARS‐CoV2 viruses, with interactions of the viral spike protein with the ACE2 receptor of the host cell. Molecular docking studies have shown that ivermectin shields the spike protein and thereby prevents binding to ACE2. Nasal application of high doses of ivermectin could be the right therapeutic approach in the treatment and prevention of COVID‐19. Tolerability, safety, and pharmacokinetics of ivermectin, administered nasally as 5% microsuspension (F004), were investigated in a randomized, double‐blind, parallel‐groups, placebo‐controlled phase 1 study in 28 healthy adults. Bioavailability of a single dose of 14 mg ivermectin was determined with AUC\n <jats:sub>0–t</jats:sub>\n <jats:sup>T</jats:sup>\n of 1701.1 ng/mL h (AUC\n <jats:sub>0–∞</jats:sub>\n of 2382.7 ng/mL h, calculated), C\n <jats:sub>max</jats:sub>\n of 96.2 ng/mL, T\n <jats:sub>max</jats:sub>\n of 4.4 h, and T\n <jats:sub>1/2</jats:sub>\n of 59.9 h. Following 42 mg/day multiple dose (3 × 14 mg every 6 h) administered nasally over 5 days, AUC\n <jats:sub>0‐∞</jats:sub>\n of 2194.4 ng/mL h was analyzed, and 96% of ivermectin concentrations were still measurable 12 h after the last dose. F004 was safe in this study and well‐tolerated. Nine (F004 group) and three (placebo group) of 28 subjects reported 14 symptoms, including a few systemic but mainly local nasal adverse events (AE). The number of subjects reporting AE decreased continuously after both F004 and placebo treatment. All subjects recovered fully with no AE recorded at the end of the study. Nasal examination showed stable patterns of nasal mucosal grading, mucosal bleeding, and crusting of the mucosa. Nasally administered ivermectin is well tolerated in high concentrations and could provide systemic therapeutic benefits in addition to local effects.\n </jats:p>",
"alternative-id": [
"10.1002/jcph.70137"
],
"article-number": "e70137",
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2025-07-21"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2025-11-14"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2025-12-17"
}
],
"author": [
{
"affiliation": [
{
"name": "HWI Pharma Services GmbH Rülzheim Germany"
}
],
"family": "Wissel",
"given": "Stefan",
"sequence": "first"
},
{
"affiliation": [
{
"name": "HWI Pharma Services GmbH Rülzheim Germany"
}
],
"family": "Wissel",
"given": "Philipp",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HWI Pharma Services GmbH Rülzheim Germany"
}
],
"family": "Rischer",
"given": "Matthias",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HWI Pharma Services GmbH Rülzheim Germany"
}
],
"family": "Häberlein",
"given": "Felix",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dr. Riethmüller M/R/S GmbH Frankfurt am Main Germany"
}
],
"family": "Riethmüller‐Winzen",
"given": "Hilde",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Biochemistry and Molecular Biology Medical Faculty University of Bonn Bonn Germany"
}
],
"family": "Häberlein",
"given": "Hanns",
"sequence": "additional"
}
],
"container-title": "The Journal of Clinical Pharmacology",
"container-title-short": "The Journal of Clinical Pharma",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"accp1.onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2025,
12,
17
]
],
"date-time": "2025-12-17T13:14:42Z",
"timestamp": 1765977282000
},
"deposited": {
"date-parts": [
[
2025,
12,
17
]
],
"date-time": "2025-12-17T13:14:44Z",
"timestamp": 1765977284000
},
"indexed": {
"date-parts": [
[
2025,
12,
17
]
],
"date-time": "2025-12-17T13:17:14Z",
"timestamp": 1765977434519,
"version": "3.48.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
12,
17
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2026,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
12,
17
]
],
"date-time": "2025-12-17T00:00:00Z",
"timestamp": 1765929600000
}
}
],
"link": [
{
"URL": "https://accp1.onlinelibrary.wiley.com/doi/pdf/10.1002/jcph.70137",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2025,
12,
17
]
]
},
"published-online": {
"date-parts": [
[
2025,
12,
17
]
]
},
"published-print": {
"date-parts": [
[
2026,
1
]
]
},
"publisher": "Wiley",
"reference": [
{
"author": "World Health Organization",
"key": "e_1_2_10_2_1",
"volume-title": "WHO COVID‐19 Dashboard, Number of COVID‐19 Deaths Reported to WHO (Cumulative Total)",
"year": "2025"
},
{
"DOI": "10.1146/annurev‐med‐052422‐020316",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"DOI": "10.20944/preprints202005.0020.v1",
"doi-asserted-by": "crossref",
"key": "e_1_2_10_4_1",
"unstructured": "DayerMR.Coronavirus (2019‐nCoV) Deactivation via Spike Glycoprotein Shielding by Old Drugs: A Bioinformatic Study.Preprints.2020. doi:10.20944/preprints202005.0020.v1"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.1021/acsptsci.0c00179",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1038/s42003‐020‐01577‐x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.3389/fmicb.2020.592908",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1016/j.antiviral.2020.104760",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.3390/scipharm88030036",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.3389/fmicb.2015.00553",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.7759/cureus.21272",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1016/j.heliyon.2024.e27647",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"author": "GLP",
"key": "e_1_2_10_14_1",
"volume-title": "Principles of Good Laboratory Practice as Specified by National (German Chemicals Law, Annex 1, 28 August 2013) and International",
"year": "1998"
},
{
"key": "e_1_2_10_15_1",
"unstructured": "International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) European Medicines Agency (EMA). Guideline for good clinical practice E6 (R2). EMA/CHMP/ICH/135/1995.2016. Accessed July 1 2025.https://www.ema.europa.eu/en/documents/scientific‐guideline/ich‐e‐6‐r2‐guideline‐good‐clinical‐practice‐step‐5_en."
},
{
"key": "e_1_2_10_16_1",
"unstructured": "Reflection Paper for Laboratories that Perform the Analysis or Evaluation of Clinical Trial Samples (EMA/INS/GCP/532137/2010) February 28 2012. Accessed July 1 2025.https://www.ema.europa.eu/en/documents/regulatory‐procedural‐guideline/reflection‐paper‐laboratories‐perform‐analysis‐or‐evaluation‐clinical‐trial‐samples_en.pdf."
},
{
"DOI": "10.4103/idoj.idoj_298_21",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.2165/00003495‐199142040‐00007",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1017/s0031182000006570",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"article-title": "Soolantra (ivermectin) 1% cream: a novel, antibiotic‐free agent approved for the treatment of patients with rosacea",
"author": "Raedler LA",
"first-page": "122",
"journal-title": "Am Health Drug Benefits",
"key": "e_1_2_10_20_1",
"volume": "8",
"year": "2015"
},
{
"DOI": "10.1177/009127002401382731",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.1371/journal.pntd.0006020",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"DOI": "10.1007/BF00637608",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_23_1"
},
{
"key": "e_1_2_10_24_1",
"unstructured": "FDA.Center for Drug Evaluation and Research. Application Number: 050742. Merck Research Laboratories. 11/22/96 Stromectol (ivermectin 6 mg tablet) Review. Merck Research Laboratories. November 22 1996. Accessed July 1 2025.https://www.accessdata.fda.gov/drugsatfda_docs/nda/96/050742ap.pdf.https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=050742."
},
{
"key": "e_1_2_10_25_1",
"unstructured": "Public Assessment Report. Scientific Discussion. Ivermectin Substipharm 3 mg Tablets (ivermectin). NL/H/3952/001/DC. College ter Beoordeling van Geneesmiddelen–Medicines Evaluation Board (CBG‐MEB). July 19 2018. Accessed July 1 2025.https://db.cbg‐meb.nl/pars/h120488.pdf."
},
{
"DOI": "10.3389/fphar.2022.914886",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_1"
},
{
"DOI": "10.1208/s12248‐007‐9000‐9",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"DOI": "10.1007/s002280050131",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_1"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://accp1.onlinelibrary.wiley.com/doi/10.1002/jcph.70137"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Tolerability, Safety, and Pharmacokinetics of Ivermectin After Nasal Application in Healthy Adult Subjects",
"type": "journal-article",
"update-policy": "https://doi.org/10.1002/crossmark_policy",
"volume": "66"
}
