Enhancing Intracellular Uptake of Ivermectin through Liposomal Encapsulation
et al., AAPS PharmSciTech, doi:10.1208/s12249-025-03113-8, May 2025
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
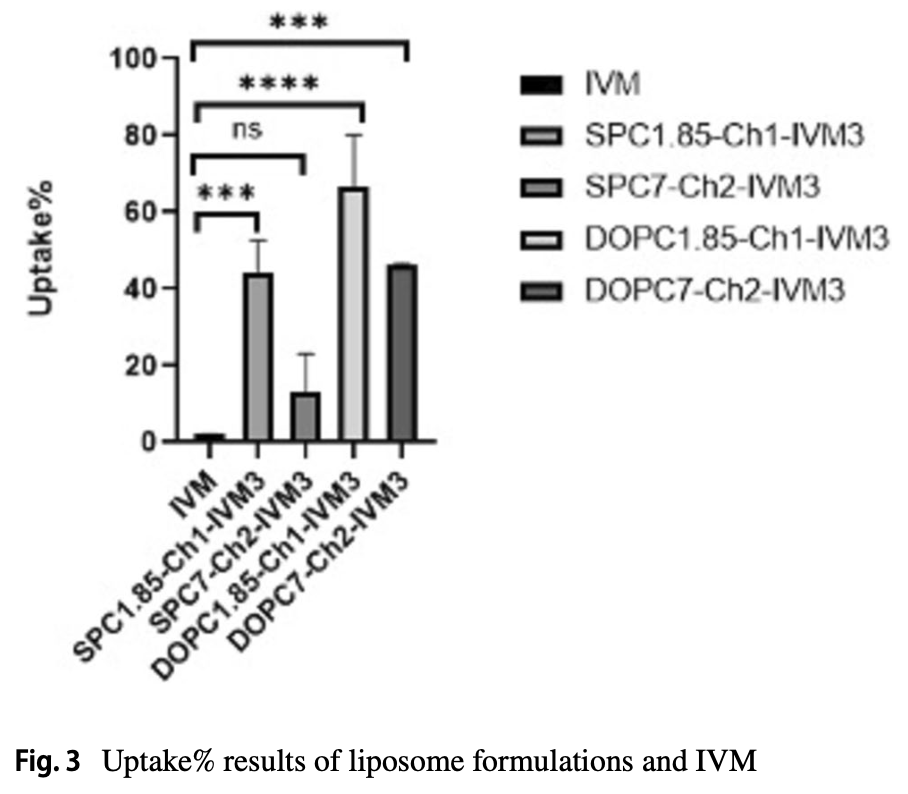
In vitro study showing enhanced intracellular uptake of ivermectin through liposomal encapsulation in Vero E6 cells with reduced cytotoxicity. While free ivermectin showed a half-maximal cytotoxic concentration (CC50) of 10 μM, liposomal formulations exhibited CC50 values exceeding 110 μM, demonstrating significantly reduced toxicity. Cellular uptake of ivermectin-loaded liposomes ranged from 13-60%, compared to only 2% for free ivermectin. This improved delivery approach builds on the authors' previous study that demonstrated enhanced antiviral efficacy of liposomal ivermectin against SARS-CoV-2 in Vero E6 cells. Liposomal encapsulation offers advantages including enhanced solubility for the highly hydrophobic ivermectin, passive targeting of immune cells, sustained release, and improved tissue penetration.
74 preclinical studies support the efficacy of ivermectin for COVID-19:
Ivermectin, better known for antiparasitic activity, is a broad spectrum antiviral with activity against many viruses including H7N771, Dengue37,72,73 , HIV-173, Simian virus 4074, Zika37,75,76 , West Nile76, Yellow Fever77,78, Japanese encephalitis77, Chikungunya78, Semliki Forest virus78, Human papillomavirus57, Epstein-Barr57, BK Polyomavirus79, and Sindbis virus78.
Ivermectin inhibits importin-α/β-dependent nuclear import of viral proteins71,73,74,80 , shows spike-ACE2 disruption at 1nM with microfluidic diffusional sizing38, binds to glycan sites on the SARS-CoV-2 spike protein preventing interaction with blood and epithelial cells and inhibiting hemagglutination41,81, shows dose-dependent inhibition of wildtype and omicron variants36, exhibits dose-dependent inhibition of lung injury61,66, may inhibit SARS-CoV-2 via IMPase inhibition37, may inhibit SARS-CoV-2 induced formation of fibrin clots resistant to degradation9, inhibits SARS-CoV-2 3CLpro54, may inhibit SARS-CoV-2 RdRp activity28, may minimize viral myocarditis by inhibiting NF-κB/p65-mediated inflammation in macrophages60, may be beneficial for COVID-19 ARDS by blocking GSDMD and NET formation82, may interfere with SARS-CoV-2's immune evasion via ORF8 binding4, may inhibit SARS-CoV-2 by disrupting CD147 interaction83-86, may inhibit SARS-CoV-2 attachment to lipid rafts via spike NTD binding2, shows protection against inflammation, cytokine storm, and mortality in an LPS mouse model sharing key pathological features of severe COVID-1959,87, may be beneficial in severe COVID-19 by binding IGF1 to inhibit the promotion of inflammation, fibrosis, and cell proliferation that leads to lung damage8, may minimize SARS-CoV-2 induced cardiac damage40,48, may counter immune evasion by inhibiting NSP15-TBK1/KPNA1 interaction and restoring IRF3 activation88, may disrupt SARS-CoV-2 N and ORF6 protein nuclear transport and their suppression of host interferon responses1, reduces TAZ/YAP nuclear import, relieving SARS-CoV-2-driven suppression of IRF3 and NF-κB antiviral pathways35, increases Bifidobacteria which play a key role in the immune system89, has immunomodulatory51 and anti-inflammatory70,90 properties, and has an extensive and very positive safety profile91.
1.
Gayozo et al., Binding affinities analysis of ivermectin, nucleocapsid and ORF6 proteins of SARS-CoV-2 to human importins α isoforms: A computational approach, Biotecnia, doi:10.18633/biotecnia.v27.2485.
2.
Lefebvre et al., Characterization and Fluctuations of an Ivermectin Binding Site at the Lipid Raft Interface of the N-Terminal Domain (NTD) of the Spike Protein of SARS-CoV-2 Variants, Viruses, doi:10.3390/v16121836.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Bagheri-Far et al., Non-spike protein inhibition of SARS-CoV-2 by natural products through the key mediator protein ORF8, Molecular Biology Research Communications, doi:10.22099/mbrc.2024.50245.2001.
5.
de Oliveira Só et al., In Silico Comparative Analysis of Ivermectin and Nirmatrelvir Inhibitors Interacting with the SARS-CoV-2 Main Protease, Preprints, doi:10.20944/preprints202404.1825.v1.
6.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
7.
Oranu et al., Validation of the binding affinities and stabilities of ivermectin and moxidectin against SARS-CoV-2 receptors using molecular docking and molecular dynamics simulation, GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2024.26.1.0030.
8.
Zhao et al., Identification of the shared gene signatures between pulmonary fibrosis and pulmonary hypertension using bioinformatics analysis, Frontiers in Immunology, doi:10.3389/fimmu.2023.1197752.
9.
Vottero et al., Computational Prediction of the Interaction of Ivermectin with Fibrinogen, Molecular Sciences, doi:10.3390/ijms241411449.
10.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
11.
Umar et al., Inhibitory potentials of ivermectin, nafamostat, and camostat on spike protein and some nonstructural proteins of SARS-CoV-2: Virtual screening approach, Jurnal Teknologi Laboratorium, doi:10.29238/teknolabjournal.v11i1.344.
12.
Alvarado et al., Interaction of the New Inhibitor Paxlovid (PF-07321332) and Ivermectin With the Monomer of the Main Protease SARS-CoV-2: A Volumetric Study Based on Molecular Dynamics, Elastic Networks, Classical Thermodynamics and SPT, Computational Biology and Chemistry, doi:10.1016/j.compbiolchem.2022.107692.
13.
Aminpour et al., In Silico Analysis of the Multi-Targeted Mode of Action of Ivermectin and Related Compounds, Computation, doi:10.3390/computation10040051.
14.
Parvez et al., Insights from a computational analysis of the SARS-CoV-2 Omicron variant: Host–pathogen interaction, pathogenicity, and possible drug therapeutics, Immunity, Inflammation and Disease, doi:10.1002/iid3.639.
15.
Francés-Monerris et al., Microscopic interactions between ivermectin and key human and viral proteins involved in SARS-CoV-2 infection, Physical Chemistry Chemical Physics, doi:10.1039/D1CP02967C.
16.
González-Paz et al., Comparative study of the interaction of ivermectin with proteins of interest associated with SARS-CoV-2: A computational and biophysical approach, Biophysical Chemistry, doi:10.1016/j.bpc.2021.106677.
17.
González-Paz (B) et al., Structural Deformability Induced in Proteins of Potential Interest Associated with COVID-19 by binding of Homologues present in Ivermectin: Comparative Study Based in Elastic Networks Models, Journal of Molecular Liquids, doi:10.1016/j.molliq.2021.117284.
18.
Rana et al., A Computational Study of Ivermectin and Doxycycline Combination Drug Against SARS-CoV-2 Infection, Research Square, doi:10.21203/rs.3.rs-755838/v1.
19.
Muthusamy et al., Virtual Screening Reveals Potential Anti-Parasitic Drugs Inhibiting the Receptor Binding Domain of SARS-CoV-2 Spike protein, Journal of Virology & Antiviral Research, www.scitechnol.com/abstract/virtual-screening-reveals-potential-antiparasitic-drugs-inhibiting-the-receptor-binding-domain-of-sarscov2-spike-protein-16398.html.
20.
Qureshi et al., Mechanistic insights into the inhibitory activity of FDA approved ivermectin against SARS-CoV-2: old drug with new implications, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1906750.
21.
Schöning et al., Highly-transmissible Variants of SARS-CoV-2 May Be More Susceptible to Drug Therapy Than Wild Type Strains, Research Square, doi:10.21203/rs.3.rs-379291/v1.
22.
Bello et al., Elucidation of the inhibitory activity of ivermectin with host nuclear importin α and several SARS-CoV-2 targets, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1911857.
23.
Udofia et al., In silico studies of selected multi-drug targeting against 3CLpro and nsp12 RNA-dependent RNA-polymerase proteins of SARS-CoV-2 and SARS-CoV, Network Modeling Analysis in Health Informatics and Bioinformatics, doi:10.1007/s13721-021-00299-2.
24.
Choudhury et al., Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach, Future Medicine, doi:10.2217/fvl-2020-0342.
25.
Kern et al., Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing, Frontiers in Pharmacology, doi:10.3389/fphar.2021.625678.
26.
Saha et al., The Binding mechanism of ivermectin and levosalbutamol with spike protein of SARS-CoV-2, Structural Chemistry, doi:10.1007/s11224-021-01776-0.
27.
Eweas et al., Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2, Frontiers in Microbiology, doi:10.3389/fmicb.2020.592908.
28.
Parvez (B) et al., Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.09.098.
29.
Francés-Monerris (B) et al., Has Ivermectin Virus-Directed Effects against SARS-CoV-2? Rationalizing the Action of a Potential Multitarget Antiviral Agent, ChemRxiv, doi:10.26434/chemrxiv.12782258.v1.
30.
Kalhor et al., Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1824816.
31.
Swargiary, A., Ivermectin as a promising RNA-dependent RNA polymerase inhibitor and a therapeutic drug against SARS-CoV2: Evidence from in silico studies, Research Square, doi:10.21203/rs.3.rs-73308/v1.
32.
Maurya, D., A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID-19 Patients, American Chemical Society (ACS), doi:10.26434/chemrxiv.12630539.v1.
33.
Lehrer et al., Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, In Vivo, 34:5, 3023-3026, doi:10.21873/invivo.12134.
34.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
35.
Kofler et al., M-Motif, a potential non-conventional NLS in YAP/TAZ and other cellular and viral proteins that inhibits classic protein import, iScience, doi:10.1016/j.isci.2025.112105.
36.
Shahin et al., The selective effect of Ivermectin on different human coronaviruses; in-vitro study, Research Square, doi:10.21203/rs.3.rs-4180797/v1.
37.
Jitobaom et al., Identification of inositol monophosphatase as a broad‐spectrum antiviral target of ivermectin, Journal of Medical Virology, doi:10.1002/jmv.29552.
38.
Fauquet et al., Microfluidic Diffusion Sizing Applied to the Study of Natural Products and Extracts That Modulate the SARS-CoV-2 Spike RBD/ACE2 Interaction, Molecules, doi:10.3390/molecules28248072.
39.
García-Aguilar et al., In Vitro Analysis of SARS-CoV-2 Spike Protein and Ivermectin Interaction, International Journal of Molecular Sciences, doi:10.3390/ijms242216392.
40.
Liu et al., SARS-CoV-2 viral genes Nsp6, Nsp8, and M compromise cellular ATP levels to impair survival and function of human pluripotent stem cell-derived cardiomyocytes, Stem Cell Research & Therapy, doi:10.1186/s13287-023-03485-3.
41.
Boschi et al., SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects, bioRxiv, doi:10.1101/2022.11.24.517882.
42.
De Forni et al., Synergistic drug combinations designed to fully suppress SARS-CoV-2 in the lung of COVID-19 patients, PLoS ONE, doi:10.1371/journal.pone.0276751.
43.
Saha (B) et al., Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder, Pharmaceutics, doi:10.3390/pharmaceutics14071432.
44.
Jitobaom (B) et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8.
45.
Croci et al., Liposomal Systems as Nanocarriers for the Antiviral Agent Ivermectin, International Journal of Biomaterials, doi:10.1155/2016/8043983.
46.
Zheng et al., Red blood cell-hitchhiking mediated pulmonary delivery of ivermectin: Effects of nanoparticle properties, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121719.
47.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
48.
Liu (B) et al., Genome-wide analyses reveal the detrimental impacts of SARS-CoV-2 viral gene Orf9c on human pluripotent stem cell-derived cardiomyocytes, Stem Cell Reports, doi:10.1016/j.stemcr.2022.01.014.
49.
Segatori et al., Effect of Ivermectin and Atorvastatin on Nuclear Localization of Importin Alpha and Drug Target Expression Profiling in Host Cells from Nasopharyngeal Swabs of SARS-CoV-2- Positive Patients, Viruses, doi:10.3390/v13102084.
50.
Jitobaom (C) et al., Favipiravir and Ivermectin Showed in Vitro Synergistic Antiviral Activity against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-941811/v1.
51.
Munson et al., Niclosamide and ivermectin modulate caspase-1 activity and proinflammatory cytokine secretion in a monocytic cell line, British Society For Nanomedicine Early Career Researcher Summer Meeting, 2021, web.archive.org/web/20230401070026/https://michealmunson.github.io/COVID.pdf.
52.
Mountain Valley MD, Mountain Valley MD Receives Successful Results From BSL-4 COVID-19 Clearance Trial on Three Variants Tested With Ivectosol™, 5/18, www.globenewswire.com/en/news-release/2021/05/18/2231755/0/en/Mountain-Valley-MD-Receives-Successful-Results-From-BSL-4-COVID-19-Clearance-Trial-on-Three-Variants-Tested-With-Ivectosol.html.
53.
Yesilbag et al., Ivermectin also inhibits the replication of bovine respiratory viruses (BRSV, BPIV-3, BoHV-1, BCoV and BVDV) in vitro, Virus Research, doi:10.1016/j.virusres.2021.198384.
54.
Mody et al., Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents, Communications Biology, doi:10.1038/s42003-020-01577-x.
55.
Jeffreys et al., Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2022.106542.
56.
Surnar et al., Clinically Approved Antiviral Drug in an Orally Administrable Nanoparticle for COVID-19, ACS Pharmacol. Transl. Sci., doi:10.1021/acsptsci.0c00179.
57.
Li et al., Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment, J. Cellular Physiology, doi:10.1002/jcp.30055.
58.
Caly et al., The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research, doi:10.1016/j.antiviral.2020.104787.
59.
Zhang et al., Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflammation Research, doi:10.1007/s00011-008-8007-8.
60.
Gao et al., Ivermectin ameliorates acute myocarditis via the inhibition of importin-mediated nuclear translocation of NF-κB/p65, International Immunopharmacology, doi:10.1016/j.intimp.2024.112073.
61.
Abd-Elmawla et al., Suppression of NLRP3 inflammasome by ivermectin ameliorates bleomycin-induced pulmonary fibrosis, Journal of Zhejiang University-SCIENCE B, doi:10.1631/jzus.B2200385.
62.
Uematsu et al., Prophylactic administration of ivermectin attenuates SARS-CoV-2 induced disease in a Syrian Hamster Model, The Journal of Antibiotics, doi:10.1038/s41429-023-00623-0.
63.
Albariqi et al., Pharmacokinetics and Safety of Inhaled Ivermectin in Mice as a Potential COVID-19 Treatment, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121688.
64.
Errecalde et al., Safety and Pharmacokinetic Assessments of a Novel Ivermectin Nasal Spray Formulation in a Pig Model, Journal of Pharmaceutical Sciences, doi:10.1016/j.xphs.2021.01.017.
65.
Madrid et al., Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation, Heliyon, doi:10.1016/j.heliyon.2020.e05820.
66.
Ma et al., Ivermectin contributes to attenuating the severity of acute lung injury in mice, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2022.113706.
67.
de Melo et al., Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin, EMBO Mol. Med., doi:10.15252/emmm.202114122.
68.
Arévalo et al., Ivermectin reduces in vivo coronavirus infection in a mouse experimental model, Scientific Reports, doi:10.1038/s41598-021-86679-0.
69.
Chaccour et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Scientific Reports, doi:10.1038/s41598-020-74084-y.
70.
Yan et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflammation Research, doi:10.1007/s00011-011-0307-8.
71.
Götz et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Scientific Reports, doi:10.1038/srep23138.
72.
Tay et al., Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Research, doi:10.1016/j.antiviral.2013.06.002.
73.
Wagstaff et al., Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochemical Journal, doi:10.1042/BJ20120150.
74.
Wagstaff (B) et al., An AlphaScreen®-Based Assay for High-Throughput Screening for Specific Inhibitors of Nuclear Import, SLAS Discovery, doi:10.1177/1087057110390360.
75.
Barrows et al., A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection, Cell Host & Microbe, doi:10.1016/j.chom.2016.07.004.
76.
Yang et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Research, doi:10.1016/j.antiviral.2020.104760.
77.
Mastrangelo et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dks147.
78.
Varghese et al., Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses, Antiviral Research, doi:10.1016/j.antiviral.2015.12.012.
79.
Bennett et al., Role of a nuclear localization signal on the minor capsid Proteins VP2 and VP3 in BKPyV nuclear entry, Virology, doi:10.1016/j.virol.2014.10.013.
80.
Kosyna et al., The importin α/β-specific inhibitor Ivermectin affects HIF-dependent hypoxia response pathways, Biological Chemistry, doi:10.1515/hsz-2015-0171.
81.
Scheim et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
82.
Liu (C) et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
83.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
84.
Scheim (B), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
85.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
86.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
87.
DiNicolantonio et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
88.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
89.
Hazan et al., Treatment with Ivermectin Increases the Population of Bifidobacterium in the Gut, ACG 2023, acg2023posters.eventscribe.net/posterspeakers.asp.
90.
DiNicolantonio (B) et al., Anti-inflammatory activity of ivermectin in late-stage COVID-19 may reflect activation of systemic glycine receptors, Open Heart, doi:10.1136/openhrt-2021-001655.
91.
Descotes, J., Medical Safety of Ivermectin, ImmunoSafe Consultance, web.archive.org/web/20240313025927/https://www.medincell.com/wp-content/uploads/2021/03/Clinical_Safety_of_Ivermectin-March_2021.pdf.
92.
Wissel et al., Tolerability, Safety, and Pharmacokinetics of Ivermectin After Nasal Application in Healthy Adult Subjects, The Journal of Clinical Pharmacology, doi:10.1002/jcph.70137.
93.
Mohammed et al., A remodeled ivermectin polycaprolactone-based nanoparticles for inhalation as a promising treatment of pulmonary inflammatory diseases, European Journal of Pharmaceutical Sciences, doi:10.1016/j.ejps.2024.106714.
Kocas et al., 2 May 2025, peer-reviewed, 4 authors.
Contact: comoglu@pharmacy.ankara.edu.tr.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Enhancing Intracellular Uptake of Ivermectin through Liposomal Encapsulation
AAPS PharmSciTech, doi:10.1208/s12249-025-03113-8
Ivermectin (IVM), an antiparasitic drug approved by the Food and Drug Administration (FDA), is widely used to treat several neglected tropical diseases, including onchocerciasis, helminthiases, and scabies. Additionally, IVM has shown potential as a potent inhibitor of certain RNA viruses, such as SARS-CoV-2. However, IVM is highly hydrophobic, essentially insoluble in water, which limits its bioavailability and therapeutic effectiveness. The use of liposomes as drug carriers offers several advantages, including enhanced solubility for lipophilic drugs, passive targeting of immune system cells, sustained release, and improved tissue penetration. To address the limitations of IVM, including its poor solubility and bioavailability, liposomal formulations were developed using a combination of soyphosphatidylcholine (SPC), dioleylphosphatidylcholine (DOPC), cholesterol (Ch), and diethylphosphate (DCP) in two distinct molar ratios (1.85:1:0.15 and 7:2:1) via the ethanol injection method. The physicochemical properties of the placebo and IVM-loaded liposomes were extensively characterized in our earlier study, including the particle size, polydispersity index, and zeta potential. The present work adds a deeper level of investigation into how to effect cellular uptake and cytotoxicity in vitro of both free IVM and IVM-loaded liposomes in Vero E6 cells. The half-maximal cytotoxic concentrations (CC 50 ) for free IVM and IVM-loaded liposomes were 10 μM and > 110 μM, respectively and the cellular uptake of IVM-loaded liposomes ranged from 13 to 60%, whereas free IVM showed a significantly lower uptake of only 2%. These results demonstrate that liposomal encapsulation effectively enhances IVM's cellular uptake while reducing its cytotoxicity, thus offering a promising strategy for improving the effectiveness of IVM.
Supplementary Information The online version contains supplementary material available at https:// doi . org/ 10. 1208/ s12249-025-03113-8. Author Contributions Meryem Kocas (ORCID: 0000-0002-4165-6191): The author contributed to the conception or design of the study; acquisition, analysis and interpretation of data for the study. She has also drafted or critically revised the manuscript for important intellectual content; and has agreed to be responsible for final approval of the version to be published and for all aspects of the publication. Fumiyoshi Yamashita (ORCID: 0000-0002-3503-8696): The author contributed to the conception or design of the study; acquisition, analysis and interpretation of data for the study. He has also drafted or critically revised the manuscript for important intellectual content; and has agreed to be responsible for final approval of the version to be published and for all aspects of the publication. Tansel Comoglu (ORCID: 0000-0002-4221-5814): The author contributed to the conception or design of the study; acquisition, analysis and interpretation of data for the study. She has also drafted or critically revised the manuscript for important intellectual content; and has agreed to be responsible for final approval of the version to be published and for all aspects of the publication. Qiyue Zhang (ORCID: 0000-0001-8440-2071): The author contributed to the conception or design of the study; acquisition, analysis and interpretation of data for the..
References
Andar, Hood, Vreeland, Devoe, Swaan, Microfluidic preparation of liposomes to determine particle size influence on cellular uptake mechanisms, Pharm Res
Arisoy, Kocas, Comoglu, Guderer, Banerjee, Development of ACE2 loaded decoy liposomes and their effect on SARS-CoV-2 for Covid-19 treatment, Pharm Dev Technol
Bassissi, Lespine, Alvinerie, Assessment of a liposomal formulation of ivermectin in rabbit after a single subcutaneous administration, Parasitol Res
Briuglia, Rotella, Mcfarlane, Lamprou, Influence of cholesterol on liposome stability and on in vitro drug release, Drug Deliv Transl Res
Calvagno, Paolino, Cosco, Iannone, Castelli, Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters and in vitro biological activity of gemcitabine-loaded liposomes, Curr Drug Deliv
Chantarasrivong, Higuchi, Tsuda, Yamane, Hashida et al., Sialyl LewisX mimic-decorated liposomes for anti-angiogenic everolimus delivery to E-selectin expressing endothelial cells, RSC Adv
Chen, Kubo, Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin, J Physiol
Clogston, Patri, Zeta potential measurement. Characterization of nanoparticles intended for drug delivery, doi:10.1007/978-1-60327-198-1_6
Coelho, Ferreira, Alves, Cordeiro, Fonseca et al., Drug delivery systems: Advanced technologies potentially applicable in personalized treatments, Epma J
Croci, Bottaro, Chan, Watanabe, Pezzullo et al., Liposomal systems as nanocarriers for the antiviral agent ivermectin, Int J Biomater
Delandre, Gendrot, Jardot, Bideau, Boxberger et al., Antiviral activity of repurposing ivermectin against a panel of 30 clinical SARS-CoV-2 strains belonging to 14 variants, Pharmaceuticals
Digiacomo, Cardarelli, Pozzi, Palchetti, Digman et al., An apolipoprotein-enriched biomolecular corona switches the cellular uptake mechanism and trafficking pathway of lipid nanoparticles, Nanoscale
Du, Sun, Ethanol injection method for liposome preparation, Methods Mol Biol
Düzgüneş, Nir, Mechanisms and kinetics of liposome-cell interactions, Adv Drug Deliv Rev
El Maghraby, Arafa, Liposomes for enhanced cellular uptake of anticancer agents, Curr Drug Deliv
Eloy, De Souza, Petrilli, Barcellos, Lee et al., Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery, Colloids Surf, B
Feng, Wang, Cai, Bai, Zhu, Ivermectin accelerates autophagic death of glioma cells by inhibiting glycolysis through blocking GLUT4 mediated JAK/STAT signaling pathway activation, Environ Toxicol
Friedrich, Pfeifer, Binder, Aigner, Barbosa et al., Selection and validation of siRNAs preventing uptake and replication of SARS-CoV-2, Front Bioeng Biotechnol
Fujita, Plianchaisuk, Deguchi, Ito, Nao et al., Virological characteristics of a SARS-CoV-2-related bat coronavirus, EBioMedicine
Gandek, Van Der Koog, Nagelkerke, A comparison of cellular uptake mechanisms, delivery efficacy, and intracellular fate between liposomes and extracellular vesicles, Adv Healthcare Mater
Gardikis, Hatziantoniou, Madalina, Fessas, Signorelli et al., New drug delivery nanosystem combining liposomal and dendrimeric technology (liposomal locked-in dendrimers) for cancer therapy, J Pharm Sci
Gong, Wang, Leong, Determination of cellular uptake and endocytic pathways, Bio Protoc
Gouda, Sakr, Nasr, Sammour, Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications, J Drug Deliv Sci Technol
Horowitz, Barenholz, Gabizon, In vitro cytotoxicity of liposome-encapsulated doxorubicin: dependence on liposome composition and drug release, Biochim Biophys Acta (BBA) Biomembr
Hou, Sun, Pan, Gu, Effects of phytosterol butyrate ester on the characteristics of soybean phosphatidylcholine liposomes, J Oleo Sci
Jaafar-Maalej, Diab, Andrieu, Elaissari, Fessi, Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation, J Liposome Res
Jitobaom, Boonarkart, Manopwisedjaroen, Punyadee, Borwornpinyo et al., Favipiravir and ivermectin show in vitro synergistic antiviral activity against SARS-CoV-2, Acta Virol
Johnson-Arbor, Ivermectin: a mini-review, Clin Toxicol
Kapoor, Lee, Tyner, Liposomal drug product development and quality: current us experience and perspective, AAPS J
Kinobe, Owens, A systematic review of experimental evidence for antiviral effects of ivermectin and an in silico analysis of ivermectin's possible mode of action against SARS-CoV-2, Fundam Clin Pharmacol
Kocas, Comoglu, Ozkul, Development and in vitro antiviral activity of ivermectin liposomes as a potential drug carrier system, Arch Pharm
Kulkarni, Shaw, Chapter 10 -Microscopy Techniques
Kullenberg, Degerstedt, Calitz, Pavlović, Balgoma et al., In vitro cell toxicity and intracellular uptake of doxorubicin exposed as a solution or liposomes: implications for treatment of hepatocellular carcinoma, Cells
Lai, Cao, Ou-Yang, Tsai, Lin et al., Different methods of detaching adherent cells and their effects on the cell surface expression of Fas receptor and Fas ligand, Sci Rep
Lakkaraju, Rahman, Dubinsky, Low-density lipoprotein receptor-related protein mediates the endocytosis of anionic liposomes in neurons* 210, J Biol Chem
Large, Abdelmessih, Fink, Auguste, Liposome composition in drug delivery design, synthesis, characterization, and clinical application, Adv Drug Deliv Rev
Lee, Son, Na, Yi, Koo et al., The effects of doxorubicin-loaded liposomes on viability, stem cell surface marker expression and secretion of vascular endothelial growth factor of three-dimensional stem cell spheroids, Exp Ther Med
Liu, Chen, Zhang, A Review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives, Molecules
Lombardo, Kiselev, Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application, Pharmaceutics
Miles, Cullen, Kenaan, Gu, Andrews et al., Unravelling the interactions between small molecules and liposomal bilayers via molecular dynamics and thermodynamic modelling, Int J Pharm
Montizaan, Yang, Reker-Smit, Salvati, Comparison of the uptake mechanisms of zwitterionic and negatively charged liposomes by HeLa cells, Nanomedicine: Nanotechnol Biol Med
Nakhaei, Margiana, Bokov, Abdelbasset, Kouhbanani et al., Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol, Front Bioeng Biotechnol
Nir, Nieva, Uptake of Liposomes by Cells: Experimental procedures and modeling, methods in enzymology, doi:10.1016/S0076-6879(03)72013-8
Nsairat, Khater, Sayed, Odeh, Bawab et al., Liposomes: structure, composition, types, and clinical applications, Heliyon
Omura, Crump, Ivermectin: panacea for resource-poor communities?, Trends Parasitol
Rolim, Santos, Chaves, Gonçalves, Freitas-Neto et al., Preformulation study of ivermectin raw material, J Therm Anal Calorim
Saddiqi, Kadir, Abdullah, Bakar Zakaria, Banke, Preparation, characterization and in vitro cytotoxicity evaluation of free and liposome-encapsulated tylosin, Open-Nano
Sakai-Kato, Yoshida, Izutsu, Effect of surface charge on the size-dependent cellular internalization of liposomes, Chem Phys Lipids
Samad, Sultana, Aqil, Liposomal drug delivery systems: an update review, Curr Drug Deliv
Schulz, Neodo, Coulibaly, Keiser, Development and validation of a LC-MS/MS method for ivermectin quantification in dried blood spots: application to a pharmacokinetic study in Trichuris trichiura-infected adults, Anal Methods
Shah, Nguyen, Patel, Cote, Al-Fatease et al., Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes, Nanomedicine: Nanotechnol Biol Med
Shailesh, Neelam, Sandeep, Gupta, Liposomes: a review, J Pharm Res
Soni, Saini, Formulation design and optimization of cationic-charged liposomes of brimonidine tartrate for effective ocular drug delivery by design of experiment (DoE) approach, Drug Dev Ind Pharm
Strachan, Dyett, Nasa, Valery, Conn, Toxicity and cellular uptake of lipid nanoparticles of different structure and composition, J Colloid Interface Sci
Syama, Jakubek, Chen, Zaifman, Tam et al., Development of lipid nanoparticles and liposomes reference materials (II): cytotoxic profiles, Sci Rep
Tang, Hu, Wang, Yao, Zhang et al., Ivermectin, a potential anticancer drug derived from an antiparasitic drug, Pharmacol Res
Ugwu, Conradie, Anticancer properties of complexes derived from bidentate ligands, J Inorg Biochem
Vishvakarma, Sharma, LIPOSOMES: AN OVERVIEW, Journal of Drug Delivery and Therapeutics, doi:10.22270/jddt.v0i0.843
Zhang, Chen, Swaroop, Xu, Wang et al., Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro, Cell Discov
Zhang, Ni, Zhang, Xu, Gao et al., Ivermectin confers its cytotoxic effects by inducing AMPK/mTOR-mediated autophagy and DNA damage, Chemosphere
Zhaorigetu, Rodriguez-Aguayo, Sood, Lopez-Berestein, Walton, Delivery of negatively charged liposomes into the atherosclerotic plaque of apolipoprotein E-deficient mouse aortic tissue, J Liposome Res
Zhou, Wu, Ning, Wu, Xu et al., Ivermectin has new application in inhibiting colorectal cancer cell growth, Front Pharmacol
Zhu, Li, Hou, Liu, Liu et al., Preparation and quality evaluation of ivermectin liposome, J Northwest A & F Univ-Nat Sci Ed
Çağdaş, Sezer, Bucak, Liposomes as potential drug carrier systems for drug delivery, Appl Nanotechnol Drug Deliv
DOI record:
{
"DOI": "10.1208/s12249-025-03113-8",
"ISSN": [
"1530-9932"
],
"URL": "http://dx.doi.org/10.1208/s12249-025-03113-8",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Ivermectin (IVM), an antiparasitic drug approved by the Food and Drug Administration (FDA), is widely used to treat several neglected tropical diseases, including onchocerciasis, helminthiases, and scabies. Additionally, IVM has shown potential as a potent inhibitor of certain RNA viruses, such as SARS-CoV-2. However, IVM is highly hydrophobic, essentially insoluble in water, which limits its bioavailability and therapeutic effectiveness. The use of liposomes as drug carriers offers several advantages, including enhanced solubility for lipophilic drugs, passive targeting of immune system cells, sustained release, and improved tissue penetration. To address the limitations of IVM, including its poor solubility and bioavailability, liposomal formulations were developed using a combination of soyphosphatidylcholine (SPC), dioleylphosphatidylcholine (DOPC), cholesterol (Ch), and diethylphosphate (DCP) in two distinct molar ratios (1.85:1:0.15 and 7:2:1) via the ethanol injection method. The physicochemical properties of the placebo and IVM-loaded liposomes were extensively characterized in our earlier study, including the particle size, polydispersity index, and zeta potential. The present work adds a deeper level of investigation into how to effect cellular uptake and cytotoxicity <jats:italic>in vitro</jats:italic> of both free IVM and IVM-loaded liposomes in Vero E6 cells. The half-maximal cytotoxic concentrations (CC<jats:sub>50</jats:sub>) for free IVM and IVM-loaded liposomes were 10 μM and > 110 μM, respectively and the cellular uptake of IVM-loaded liposomes ranged from 13 to 60%, whereas free IVM showed a significantly lower uptake of only 2%. These results demonstrate that liposomal encapsulation effectively enhances IVM’s cellular uptake while reducing its cytotoxicity, thus offering a promising strategy for improving the effectiveness of IVM.</jats:p>\n <jats:p>\n <jats:bold>Graphical Abstract</jats:bold>\n </jats:p>",
"alternative-id": [
"3113"
],
"article-number": "123",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "19 November 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "10 April 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "2 May 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare no conflict of interest."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-4165-6191",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kocas",
"given": "Meryem",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-3503-8696",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yamashita",
"given": "Fumiyoshi",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4221-5814",
"affiliation": [],
"authenticated-orcid": false,
"family": "Comoglu",
"given": "Tansel",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8440-2071",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhang",
"given": "Qiyue",
"sequence": "additional"
}
],
"container-title": "AAPS PharmSciTech",
"container-title-short": "AAPS PharmSciTech",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T16:07:57Z",
"timestamp": 1746202077000
},
"deposited": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T17:02:50Z",
"timestamp": 1746205370000
},
"funder": [
{
"name": "Social Science University of Ankara"
}
],
"indexed": {
"date-parts": [
[
2025,
5,
3
]
],
"date-time": "2025-05-03T04:09:38Z",
"timestamp": 1746245378192,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2025,
5,
2
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2025,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T00:00:00Z",
"timestamp": 1746144000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T00:00:00Z",
"timestamp": 1746144000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1208/s12249-025-03113-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1208/s12249-025-03113-8/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1208/s12249-025-03113-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1208",
"published": {
"date-parts": [
[
2025,
5,
2
]
]
},
"published-online": {
"date-parts": [
[
2025,
5,
2
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.pt.2014.07.005",
"author": "S Omura",
"doi-asserted-by": "publisher",
"first-page": "445",
"issue": "9",
"journal-title": "Trends Parasitol",
"key": "3113_CR1",
"unstructured": "Omura S, Crump A. Ivermectin: panacea for resource-poor communities? Trends Parasitol. 2014;30(9):445–55.",
"volume": "30",
"year": "2014"
},
{
"DOI": "10.1016/j.phrs.2020.105207",
"author": "M Tang",
"doi-asserted-by": "publisher",
"first-page": "105207",
"journal-title": "Pharmacol Res",
"key": "3113_CR2",
"unstructured": "Tang M, Hu X, Wang Y, Yao X, Zhang W, Yu C, et al. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol Res. 2021;163:105207.",
"volume": "163",
"year": "2021"
},
{
"DOI": "10.1113/JP275236",
"author": "IS Chen",
"doi-asserted-by": "publisher",
"first-page": "1833",
"issue": "10",
"journal-title": "J Physiol",
"key": "3113_CR3",
"unstructured": "Chen IS, Kubo Y. Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J Physiol. 2018;596(10):1833–45.",
"volume": "596",
"year": "2018"
},
{
"DOI": "10.1080/15563650.2022.2043338",
"author": "K Johnson-Arbor",
"doi-asserted-by": "publisher",
"first-page": "571",
"issue": "5",
"journal-title": "Clin Toxicol",
"key": "3113_CR4",
"unstructured": "Johnson-Arbor K. Ivermectin: a mini-review. Clin Toxicol. 2022;60(5):571–5.",
"volume": "60",
"year": "2022"
},
{
"DOI": "10.1007/s00436-005-0073-z",
"author": "F Bassissi",
"doi-asserted-by": "publisher",
"first-page": "244",
"issue": "3",
"journal-title": "Parasitol Res",
"key": "3113_CR5",
"unstructured": "Bassissi F, Lespine A, Alvinerie M. Assessment of a liposomal formulation of ivermectin in rabbit after a single subcutaneous administration. Parasitol Res. 2006;98(3):244–9.",
"volume": "98",
"year": "2006"
},
{
"DOI": "10.1155/2016/8043983",
"author": "R Croci",
"doi-asserted-by": "publisher",
"first-page": "8043983",
"journal-title": "Int J Biomater",
"key": "3113_CR6",
"unstructured": "Croci R, Bottaro E, Chan KWK, Watanabe S, Pezzullo M, Mastrangelo E, et al. Liposomal systems as nanocarriers for the antiviral agent ivermectin. Int J Biomater. 2016;2016:8043983.",
"volume": "2016",
"year": "2016"
},
{
"author": "X Zhu",
"first-page": "24",
"issue": "4",
"journal-title": "J Northwest A & F Univ- Nat Sci Ed",
"key": "3113_CR7",
"unstructured": "Zhu X, Li Y, Hou B, Liu L, Liu A, Xiong Y, et al. Preparation and quality evaluation of ivermectin liposome. J Northwest A & F Univ- Nat Sci Ed. 2010;38(4):24–30.",
"volume": "38",
"year": "2010"
},
{
"DOI": "10.1016/j.heliyon.2022.e09394",
"author": "H Nsairat",
"doi-asserted-by": "publisher",
"first-page": "e09394",
"issue": "5",
"journal-title": "Heliyon",
"key": "3113_CR8",
"unstructured": "Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8(5):e09394.",
"volume": "8",
"year": "2022"
},
{
"author": "S Shailesh",
"first-page": "1163",
"issue": "7",
"journal-title": "J Pharm Res",
"key": "3113_CR9",
"unstructured": "Shailesh S, Neelam S, Sandeep K, Gupta G. Liposomes: a review. J Pharm Res. 2009;2(7):1163–7.",
"volume": "2",
"year": "2009"
},
{
"DOI": "10.1080/10837450.2022.2042557",
"author": "S Arisoy",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Pharm Dev Technol",
"key": "3113_CR10",
"unstructured": "Arisoy S, Kocas M, Comoglu T, Guderer I, Banerjee S. Development of ACE2 loaded decoy liposomes and their effect on SARS-CoV-2 for Covid-19 treatment. Pharm Dev Technol. 2022;27:1–11.",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1007/s13167-010-0001-x",
"author": "JF Coelho",
"doi-asserted-by": "publisher",
"first-page": "164",
"issue": "1",
"journal-title": "Epma J",
"key": "3113_CR11",
"unstructured": "Coelho JF, Ferreira PC, Alves P, Cordeiro R, Fonseca AC, Góis JR, et al. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. Epma J. 2010;1(1):164–209.",
"volume": "1",
"year": "2010"
},
{
"author": "M Çağdaş",
"first-page": "1",
"journal-title": "Appl Nanotechnol Drug Deliv",
"key": "3113_CR12",
"unstructured": "Çağdaş M, Sezer AD, Bucak S. Liposomes as potential drug carrier systems for drug delivery. Appl Nanotechnol Drug Deliv. 2014;1:1–50.",
"volume": "1",
"year": "2014"
},
{
"DOI": "10.3389/fbioe.2021.705886",
"author": "P Nakhaei",
"doi-asserted-by": "publisher",
"first-page": "705886",
"journal-title": "Front Bioeng Biotechnol",
"key": "3113_CR13",
"unstructured": "Nakhaei P, Margiana R, Bokov DO, Abdelbasset WK, Jadidi Kouhbanani MA, Varma RS, et al. Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front Bioeng Biotechnol. 2021;9:705886.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.colsurfb.2014.09.029",
"author": "JO Eloy",
"doi-asserted-by": "publisher",
"first-page": "345",
"journal-title": "Colloids Surf, B",
"key": "3113_CR14",
"unstructured": "Eloy JO, de Souza MC, Petrilli R, Barcellos JPA, Lee RJ, Marchetti JM. Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery. Colloids Surf, B. 2014;123:345–63.",
"volume": "123",
"year": "2014"
},
{
"DOI": "10.3390/pharmaceutics14030543",
"author": "D Lombardo",
"doi-asserted-by": "publisher",
"first-page": "543",
"issue": "3",
"journal-title": "Pharmaceutics",
"key": "3113_CR15",
"unstructured": "Lombardo D, Kiselev MA. Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics. 2022;14(3):543.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1208/s12248-017-0049-9",
"author": "M Kapoor",
"doi-asserted-by": "publisher",
"first-page": "632",
"issue": "3",
"journal-title": "AAPS J",
"key": "3113_CR16",
"unstructured": "Kapoor M, Lee SL, Tyner KM. Liposomal drug product development and quality: current us experience and perspective. AAPS J. 2017;19(3):632–41.",
"volume": "19",
"year": "2017"
},
{
"DOI": "10.2174/156720107782151269",
"author": "A Samad",
"doi-asserted-by": "publisher",
"first-page": "297",
"issue": "4",
"journal-title": "Curr Drug Deliv",
"key": "3113_CR17",
"unstructured": "Samad A, Sultana Y, Aqil M. Liposomal drug delivery systems: an update review. Curr Drug Deliv. 2007;4(4):297–305.",
"volume": "4",
"year": "2007"
},
{
"DOI": "10.3390/molecules27041372",
"author": "P Liu",
"doi-asserted-by": "publisher",
"first-page": "1372",
"issue": "4",
"journal-title": "Molecules.",
"key": "3113_CR18",
"unstructured": "Liu P, Chen G, Zhang J. A Review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. 2022;27(4):1372.",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1007/978-1-0716-2954-3_5",
"author": "G Du",
"doi-asserted-by": "publisher",
"first-page": "65",
"journal-title": "Methods Mol Biol",
"key": "3113_CR19",
"unstructured": "Du G, Sun X. Ethanol injection method for liposome preparation. Methods Mol Biol. 2023;2622:65–70.",
"volume": "2622",
"year": "2023"
},
{
"DOI": "10.3109/08982100903347923",
"author": "C Jaafar-Maalej",
"doi-asserted-by": "publisher",
"first-page": "228",
"issue": "3",
"journal-title": "J Liposome Res",
"key": "3113_CR20",
"unstructured": "Jaafar-Maalej C, Diab R, Andrieu V, Elaissari A, Fessi H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J Liposome Res. 2010;20(3):228–43.",
"volume": "20",
"year": "2010"
},
{
"DOI": "10.1016/j.jddst.2020.102174",
"author": "A Gouda",
"doi-asserted-by": "publisher",
"first-page": "102174",
"journal-title": "J Drug Deliv Sci Technol",
"key": "3113_CR21",
"unstructured": "Gouda A, Sakr OS, Nasr M, Sammour O. Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications. J Drug Deliv Sci Technol. 2021;61:102174.",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.2174/156720107779314749",
"author": "M Grazia Calvagno",
"doi-asserted-by": "publisher",
"first-page": "89",
"issue": "1",
"journal-title": "Curr Drug Deliv",
"key": "3113_CR22",
"unstructured": "Grazia Calvagno M, Celia C, Paolino D, Cosco D, Iannone M, Castelli F, et al. Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters and in vitro biological activity of gemcitabine-loaded liposomes. Curr Drug Deliv. 2007;4(1):89–101.",
"volume": "4",
"year": "2007"
},
{
"DOI": "10.1016/j.addr.2021.113851",
"author": "DE Large",
"doi-asserted-by": "publisher",
"first-page": "113851",
"journal-title": "Adv Drug Deliv Rev",
"key": "3113_CR23",
"unstructured": "Large DE, Abdelmessih RG, Fink EA, Auguste DT. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851.",
"volume": "176",
"year": "2021"
},
{
"DOI": "10.1016/S0169-409X(99)00037-X",
"author": "N Düzgüneş",
"doi-asserted-by": "publisher",
"first-page": "3",
"issue": "1",
"journal-title": "Adv Drug Deliv Rev",
"key": "3113_CR24",
"unstructured": "Düzgüneş N, Nir S. Mechanisms and kinetics of liposome–cell interactions. Adv Drug Deliv Rev. 1999;40(1):3–18.",
"volume": "40",
"year": "1999"
},
{
"DOI": "10.1016/j.jcis.2020.05.002",
"author": "JB Strachan",
"doi-asserted-by": "publisher",
"first-page": "241",
"journal-title": "J Colloid Interface Sci",
"key": "3113_CR25",
"unstructured": "Strachan JB, Dyett BP, Nasa Z, Valery C, Conn CE. Toxicity and cellular uptake of lipid nanoparticles of different structure and composition. J Colloid Interface Sci. 2020;576:241–51.",
"volume": "576",
"year": "2020"
},
{
"DOI": "10.1002/ardp.202300708",
"author": "M Kocas",
"doi-asserted-by": "publisher",
"first-page": "2300708",
"issue": "8",
"journal-title": "Arch Pharm",
"key": "3113_CR26",
"unstructured": "Kocas M, Comoglu T, Ozkul A. Development and in vitro antiviral activity of ivermectin liposomes as a potential drug carrier system. Arch Pharm. 2024;357(8):2300708.",
"volume": "357",
"year": "2024"
},
{
"DOI": "10.3390/ph15040445",
"author": "O Delandre",
"doi-asserted-by": "publisher",
"first-page": "445",
"issue": "4",
"journal-title": "Pharmaceuticals",
"key": "3113_CR27",
"unstructured": "Delandre O, Gendrot M, Jardot P, Le Bideau M, Boxberger M, Boschi C, et al. Antiviral activity of repurposing ivermectin against a panel of 30 clinical SARS-CoV-2 strains belonging to 14 variants. Pharmaceuticals. 2022;15(4):445.",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1016/j.ebiom.2024.105181",
"author": "S Fujita",
"doi-asserted-by": "publisher",
"first-page": "105181",
"journal-title": "EBioMedicine",
"key": "3113_CR28",
"unstructured": "Fujita S, Plianchaisuk A, Deguchi S, Ito H, Nao N, Wang L, et al. Virological characteristics of a SARS-CoV-2-related bat coronavirus, BANAL-20-236. EBioMedicine. 2024;104:105181.",
"volume": "104",
"year": "2024"
},
{
"DOI": "10.1016/B978-0-12-801024-2.00010-8",
"doi-asserted-by": "crossref",
"key": "3113_CR29",
"unstructured": "Kulkarni VS, Shaw C. Chapter 10 - Microscopy Techniques. In: Kulkarni VS, Shaw C, editors. Essential Chemistry for Formulators of Semisolid and Liquid Dosages. Boston: Academic Press; 2016. p. 183-92."
},
{
"DOI": "10.1039/C8AY00828K",
"author": "JD Schulz",
"doi-asserted-by": "publisher",
"first-page": "2901",
"issue": "24",
"journal-title": "Anal Methods",
"key": "3113_CR30",
"unstructured": "Schulz JD, Neodo A, Coulibaly JT, Keiser J. Development and validation of a LC-MS/MS method for ivermectin quantification in dried blood spots: application to a pharmacokinetic study in Trichuris trichiura-infected adults. Anal Methods. 2018;10(24):2901–9.",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.1111/fcp.12644",
"author": "RT Kinobe",
"doi-asserted-by": "publisher",
"first-page": "260",
"issue": "2",
"journal-title": "Fundam Clin Pharmacol",
"key": "3113_CR31",
"unstructured": "Kinobe RT, Owens L. A systematic review of experimental evidence for antiviral effects of ivermectin and an in silico analysis of ivermectin’s possible mode of action against SARS-CoV-2. Fundam Clin Pharmacol. 2021;35(2):260–76.",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-23013-2",
"author": "K Syama",
"doi-asserted-by": "publisher",
"first-page": "18071",
"issue": "1",
"journal-title": "Sci Rep",
"key": "3113_CR32",
"unstructured": "Syama K, Jakubek ZJ, Chen S, Zaifman J, Tam YYC, Zou S. Development of lipid nanoparticles and liposomes reference materials (II): cytotoxic profiles. Sci Rep. 2022;12(1):18071.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3389/av.2023.12265",
"author": "K Jitobaom",
"doi-asserted-by": "publisher",
"first-page": "12265",
"journal-title": "Acta Virol",
"key": "3113_CR33",
"unstructured": "Jitobaom K, Boonarkart C, Manopwisedjaroen S, Punyadee N, Borwornpinyo S, Thitithanyanont A, et al. Favipiravir and ivermectin show in vitro synergistic antiviral activity against SARS-CoV-2. Acta Virol. 2023;67:12265.",
"volume": "67",
"year": "2023"
},
{
"author": "H Lee",
"first-page": "4950",
"issue": "6",
"journal-title": "Exp Ther Med",
"key": "3113_CR34",
"unstructured": "Lee H, Son J, Na CB, Yi G, Koo H, Park JB. The effects of doxorubicin-loaded liposomes on viability, stem cell surface marker expression and secretion of vascular endothelial growth factor of three-dimensional stem cell spheroids. Exp Ther Med. 2018;15(6):4950–60.",
"volume": "15",
"year": "2018"
},
{
"DOI": "10.1039/C9RA01943J",
"author": "C Chantarasrivong",
"doi-asserted-by": "publisher",
"first-page": "20518",
"journal-title": "RSC Adv",
"key": "3113_CR35",
"unstructured": "Chantarasrivong C, Higuchi Y, Tsuda M, Yamane Y, Hashida M, Konishi M, et al. Sialyl LewisX mimic-decorated liposomes for anti-angiogenic everolimus delivery to E-selectin expressing endothelial cells. RSC Adv. 2019;9:20518–27.",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1016/j.jinorgbio.2023.112268",
"author": "DI Ugwu",
"doi-asserted-by": "publisher",
"first-page": "112268",
"journal-title": "J Inorg Biochem",
"key": "3113_CR36",
"unstructured": "Ugwu DI, Conradie J. Anticancer properties of complexes derived from bidentate ligands. J Inorg Biochem. 2023;246:112268.",
"volume": "246",
"year": "2023"
},
{
"DOI": "10.3389/fbioe.2022.801870",
"author": "M Friedrich",
"doi-asserted-by": "publisher",
"first-page": "801870",
"journal-title": "Front Bioeng Biotechnol",
"key": "3113_CR37",
"unstructured": "Friedrich M, Pfeifer G, Binder S, Aigner A, Vollmer Barbosa P, Makert GR, et al. Selection and validation of siRNAs preventing uptake and replication of SARS-CoV-2. Front Bioeng Biotechnol. 2022;10:801870.",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2021.717529",
"author": "S Zhou",
"doi-asserted-by": "publisher",
"first-page": "717529",
"journal-title": "Front Pharmacol",
"key": "3113_CR38",
"unstructured": "Zhou S, Wu H, Ning W, Wu X, Xu X, Ma Y, et al. Ivermectin has new application in inhibiting colorectal cancer cell growth. Front Pharmacol. 2021;12:717529.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3390/cells10071717",
"author": "F Kullenberg",
"doi-asserted-by": "publisher",
"first-page": "1717",
"issue": "7",
"journal-title": "Cells",
"key": "3113_CR39",
"unstructured": "Kullenberg F, Degerstedt O, Calitz C, Pavlović N, Balgoma D, Gråsjö J, et al. In vitro cell toxicity and intracellular uptake of doxorubicin exposed as a solution or liposomes: implications for treatment of hepatocellular carcinoma. Cells. 2021;10(7):1717.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-09605-y",
"author": "T-Y Lai",
"doi-asserted-by": "publisher",
"first-page": "5713",
"issue": "1",
"journal-title": "Sci Rep",
"key": "3113_CR40",
"unstructured": "Lai T-Y, Cao J, Ou-Yang P, Tsai C-Y, Lin C-W, Chen C-C, et al. Different methods of detaching adherent cells and their effects on the cell surface expression of Fas receptor and Fas ligand. Sci Rep. 2022;12(1):5713.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1038/s41421-020-00222-5",
"author": "Q Zhang",
"doi-asserted-by": "publisher",
"first-page": "80",
"issue": "1",
"journal-title": "Cell Discov",
"key": "3113_CR41",
"unstructured": "Zhang Q, Chen CZ, Swaroop M, Xu M, Wang L, Lee J, et al. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 2020;6(1):80.",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.21769/BioProtoc.3169",
"author": "J Gong",
"doi-asserted-by": "publisher",
"first-page": "e3169",
"issue": "4",
"journal-title": "Bio Protoc",
"key": "3113_CR42",
"unstructured": "Gong J, Wang HX, Leong KW. Determination of cellular uptake and endocytic pathways. Bio Protoc. 2019;9(4):e3169.",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1080/03639045.2022.2070198",
"author": "PK Soni",
"doi-asserted-by": "publisher",
"first-page": "1847",
"issue": "11",
"journal-title": "Drug Dev Ind Pharm",
"key": "3113_CR43",
"unstructured": "Soni PK, Saini TR. Formulation design and optimization of cationic-charged liposomes of brimonidine tartrate for effective ocular drug delivery by design of experiment (DoE) approach. Drug Dev Ind Pharm. 2021;47(11):1847–66.",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1016/j.nano.2019.02.019",
"author": "VM Shah",
"doi-asserted-by": "publisher",
"first-page": "146",
"journal-title": "Nanomedicine: Nanotechnol Biol Med",
"key": "3113_CR44",
"unstructured": "Shah VM, Nguyen DX, Patel P, Cote B, Al-Fatease A, Pham Y, et al. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomedicine: Nanotechnol Biol Med. 2019;18:146–56.",
"volume": "18",
"year": "2019"
},
{
"DOI": "10.1016/j.ijpharm.2024.124367",
"author": "CM Miles",
"doi-asserted-by": "publisher",
"first-page": "124367",
"journal-title": "Int J Pharm",
"key": "3113_CR45",
"unstructured": "Miles CM, Cullen S, Kenaan H, Gu W, Andrews GP, Sosso GC, et al. Unravelling the interactions between small molecules and liposomal bilayers via molecular dynamics and thermodynamic modelling. Int J Pharm. 2024;660:124367.",
"volume": "660",
"year": "2024"
},
{
"DOI": "10.1007/s13346-015-0220-8",
"author": "ML Briuglia",
"doi-asserted-by": "publisher",
"first-page": "231",
"issue": "3",
"journal-title": "Drug Deliv Transl Res",
"key": "3113_CR46",
"unstructured": "Briuglia ML, Rotella C, McFarlane A, Lamprou DA. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv Transl Res. 2015;5(3):231–42.",
"volume": "5",
"year": "2015"
},
{
"DOI": "10.1007/978-1-60327-198-1_6",
"doi-asserted-by": "publisher",
"key": "3113_CR47",
"unstructured": "Clogston JD, Patri AK. Zeta potential measurement. Characterization of nanoparticles intended for drug delivery. 2011;63–70. https://doi.org/10.1007/978-1-60327-198-1_6."
},
{
"DOI": "10.1016/j.chemosphere.2020.127448",
"author": "P Zhang",
"doi-asserted-by": "publisher",
"first-page": "127448",
"journal-title": "Chemosphere",
"key": "3113_CR48",
"unstructured": "Zhang P, Ni H, Zhang Y, Xu W, Gao J, Cheng J, et al. Ivermectin confers its cytotoxic effects by inducing AMPK/mTOR-mediated autophagy and DNA damage. Chemosphere. 2020;259:127448.",
"volume": "259",
"year": "2020"
},
{
"DOI": "10.2174/1567201817666200708113131",
"author": "MG El Maghraby",
"doi-asserted-by": "publisher",
"first-page": "861",
"issue": "10",
"journal-title": "Curr Drug Deliv",
"key": "3113_CR49",
"unstructured": "El Maghraby MG, Arafa FM. Liposomes for enhanced cellular uptake of anticancer agents. Curr Drug Deliv. 2020;17(10):861–73.",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1016/0005-2736(92)90084-Y",
"author": "AT Horowitz",
"doi-asserted-by": "publisher",
"first-page": "203",
"issue": "2",
"journal-title": "Biochim Biophys Acta (BBA) Biomembr",
"key": "3113_CR50",
"unstructured": "Horowitz AT, Barenholz Y, Gabizon AA. In vitro cytotoxicity of liposome-encapsulated doxorubicin: dependence on liposome composition and drug release. Biochim Biophys Acta (BBA) Biomembr. 1992;1109(2):203–9.",
"volume": "1109",
"year": "1992"
},
{
"DOI": "10.1007/s10973-014-3691-9",
"author": "LA Rolim",
"doi-asserted-by": "publisher",
"first-page": "807",
"issue": "1",
"journal-title": "J Therm Anal Calorim",
"key": "3113_CR51",
"unstructured": "Rolim LA, dos Santos FCM, Chaves LL, Gonçalves MLCM, Freitas-Neto JL, da Silva do Nascimento AL, et al. Preformulation study of ivermectin raw material. J Therm Anal Calorim. 2015;120(1):807–16.",
"volume": "120",
"year": "2015"
},
{
"DOI": "10.5650/jos.ess21033",
"author": "L Hou",
"doi-asserted-by": "publisher",
"first-page": "1295",
"issue": "9",
"journal-title": "J Oleo Sci",
"key": "3113_CR52",
"unstructured": "Hou L, Sun X, Pan L, Gu K. Effects of phytosterol butyrate ester on the characteristics of soybean phosphatidylcholine liposomes. J Oleo Sci. 2021;70(9):1295–306.",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1002/jps.22121",
"author": "K Gardikis",
"doi-asserted-by": "publisher",
"first-page": "3561",
"journal-title": "J Pharm Sci",
"key": "3113_CR53",
"unstructured": "Gardikis K, Hatziantoniou S, Madalina B, Fessas D, Signorelli M, Felekis T, et al. New drug delivery nanosystem combining liposomal and dendrimeric technology (liposomal locked-in dendrimers) for cancer therapy. J Pharm Sci. 2010;99:3561–71.",
"volume": "99",
"year": "2010"
},
{
"DOI": "10.1002/adhm.202300319",
"author": "TB Gandek",
"doi-asserted-by": "publisher",
"first-page": "2300319",
"issue": "25",
"journal-title": "Adv Healthcare Mater",
"key": "3113_CR54",
"unstructured": "Gandek TB, van der Koog L, Nagelkerke A. A comparison of cellular uptake mechanisms, delivery efficacy, and intracellular fate between liposomes and extracellular vesicles. Adv Healthcare Mater. 2023;12(25):2300319.",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.22270/jddt.v0i0.843",
"doi-asserted-by": "publisher",
"key": "3113_CR55",
"unstructured": "Vishvakarma P, Sharma S. LIPOSOMES: AN OVERVIEW. Journal of Drug Delivery and Therapeutics. 2014. https://doi.org/10.22270/jddt.v0i0.843."
},
{
"DOI": "10.1016/S0076-6879(03)72013-8",
"doi-asserted-by": "publisher",
"key": "3113_CR56",
"unstructured": "Nir S, Nieva JL. Uptake of Liposomes by Cells: Experimental procedures and modeling. methods in enzymology. 372: Academic Press; 2003;235–48. https://doi.org/10.1016/S0076-6879(03)72013-8."
},
{
"DOI": "10.1074/jbc.M111764200",
"author": "A Lakkaraju",
"doi-asserted-by": "publisher",
"first-page": "15085",
"issue": "17",
"journal-title": "J Biol Chem",
"key": "3113_CR57",
"unstructured": "Lakkaraju A, Rahman Y-E, Dubinsky JM. Low-density lipoprotein receptor-related protein mediates the endocytosis of anionic liposomes in neurons* 210. J Biol Chem. 2002;277(17):15085–92.",
"volume": "277",
"year": "2002"
},
{
"DOI": "10.1016/j.nano.2020.102300",
"author": "D Montizaan",
"doi-asserted-by": "publisher",
"first-page": "102300",
"journal-title": "Nanomedicine: Nanotechnol Biol Med",
"key": "3113_CR58",
"unstructured": "Montizaan D, Yang K, Reker-Smit C, Salvati A. Comparison of the uptake mechanisms of zwitterionic and negatively charged liposomes by HeLa cells. Nanomedicine: Nanotechnol Biol Med. 2020;30:102300.",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1007/s11095-013-1171-8",
"author": "AU Andar",
"doi-asserted-by": "publisher",
"first-page": "401",
"issue": "2",
"journal-title": "Pharm Res",
"key": "3113_CR59",
"unstructured": "Andar AU, Hood RR, Vreeland WN, DeVoe DL, Swaan PW. Microfluidic preparation of liposomes to determine particle size influence on cellular uptake mechanisms. Pharm Res. 2014;31(2):401–13.",
"volume": "31",
"year": "2014"
},
{
"DOI": "10.1016/j.chemphyslip.2019.01.004",
"author": "K Sakai-Kato",
"doi-asserted-by": "publisher",
"first-page": "104726",
"journal-title": "Chem Phys Lipids",
"key": "3113_CR60",
"unstructured": "Sakai-Kato K, Yoshida K, Izutsu K. Effect of surface charge on the size-dependent cellular internalization of liposomes. Chem Phys Lipids. 2019;224:104726.",
"volume": "224",
"year": "2019"
},
{
"DOI": "10.3109/08982104.2013.863208",
"author": "S Zhaorigetu",
"doi-asserted-by": "publisher",
"first-page": "182",
"issue": "3",
"journal-title": "J Liposome Res",
"key": "3113_CR61",
"unstructured": "Zhaorigetu S, Rodriguez-Aguayo C, Sood AK, Lopez-Berestein G, Walton BL. Delivery of negatively charged liposomes into the atherosclerotic plaque of apolipoprotein E-deficient mouse aortic tissue. J Liposome Res. 2014;24(3):182–90.",
"volume": "24",
"year": "2014"
},
{
"DOI": "10.1039/C7NR06437C",
"author": "L Digiacomo",
"doi-asserted-by": "publisher",
"first-page": "17254",
"issue": "44",
"journal-title": "Nanoscale",
"key": "3113_CR62",
"unstructured": "Digiacomo L, Cardarelli F, Pozzi D, Palchetti S, Digman M, Gratton E, et al. An apolipoprotein-enriched biomolecular corona switches the cellular uptake mechanism and trafficking pathway of lipid nanoparticles. Nanoscale. 2017;9(44):17254–62.",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.1002/tox.23440",
"author": "Y Feng",
"doi-asserted-by": "publisher",
"first-page": "754",
"issue": "4",
"journal-title": "Environ Toxicol",
"key": "3113_CR63",
"unstructured": "Feng Y, Wang J, Cai B, Bai X, Zhu Y. Ivermectin accelerates autophagic death of glioma cells by inhibiting glycolysis through blocking GLUT4 mediated JAK/STAT signaling pathway activation. Environ Toxicol. 2022;37(4):754–64.",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.1016/j.onano.2022.100108",
"author": "ME Saddiqi",
"doi-asserted-by": "publisher",
"first-page": "100108",
"journal-title": "OpenNano",
"key": "3113_CR64",
"unstructured": "Saddiqi ME, Abdul Kadir A, Abdullah FFJ, Abu Bakar Zakaria MZ, Banke IS. Preparation, characterization and in vitro cytotoxicity evaluation of free and liposome-encapsulated tylosin. OpenNano. 2022;8:100108.",
"volume": "8",
"year": "2022"
}
],
"reference-count": 64,
"references-count": 64,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1208/s12249-025-03113-8"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Enhancing Intracellular Uptake of Ivermectin through Liposomal Encapsulation",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "26"
}