Variations in Plasma Levels of Orally Administered Ivermectin Could Hamper Its Potential Drug Repositioning: Results of a Bioequivalence Study in Mexican Population
et al., Pharmaceuticals, doi:10.3390/ph18081193, Aug 2025
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
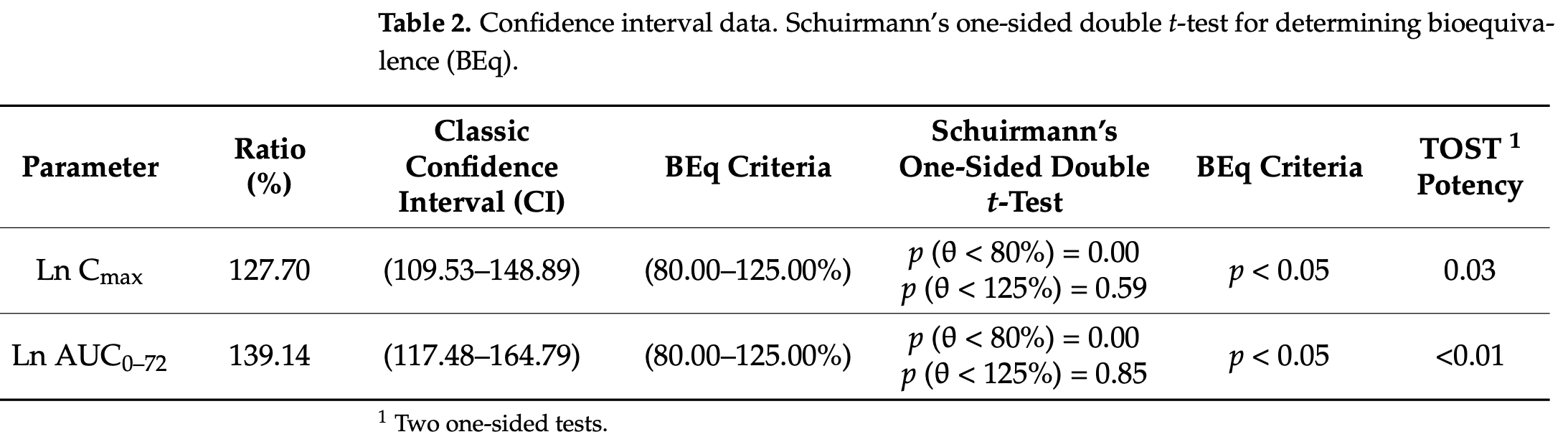
Bioequivalence study of 66 healthy Mexican volunteers comparing two oral ivermectin formulations, showing significant pharmacokinetic variability. The study found high inter- and intra-subject variability (>50% coefficient of variation) in maximum plasma concentration (Cmax) and area under the curve (AUC), with the test formulation producing slightly higher but highly variable drug exposure compared to the reference formulation. However analysis was done with fasting, and results may be different with the standard protocol for COVID-19 where administration is done with a high-fat meal, which significantly increases plasma concentration1.
74 preclinical studies support the efficacy of ivermectin for COVID-19:
Ivermectin, better known for antiparasitic activity, is a broad spectrum antiviral with activity against many viruses including H7N772, Dengue38,73,74 , HIV-174, Simian virus 4075, Zika38,76,77 , West Nile77, Yellow Fever78,79, Japanese encephalitis78, Chikungunya79, Semliki Forest virus79, Human papillomavirus58, Epstein-Barr58, BK Polyomavirus80, and Sindbis virus79.
Ivermectin inhibits importin-α/β-dependent nuclear import of viral proteins72,74,75,81 , shows spike-ACE2 disruption at 1nM with microfluidic diffusional sizing39, binds to glycan sites on the SARS-CoV-2 spike protein preventing interaction with blood and epithelial cells and inhibiting hemagglutination42,82, shows dose-dependent inhibition of wildtype and omicron variants37, exhibits dose-dependent inhibition of lung injury62,67, may inhibit SARS-CoV-2 via IMPase inhibition38, may inhibit SARS-CoV-2 induced formation of fibrin clots resistant to degradation10, inhibits SARS-CoV-2 3CLpro55, may inhibit SARS-CoV-2 RdRp activity29, may minimize viral myocarditis by inhibiting NF-κB/p65-mediated inflammation in macrophages61, may be beneficial for COVID-19 ARDS by blocking GSDMD and NET formation83, may interfere with SARS-CoV-2's immune evasion via ORF8 binding5, may inhibit SARS-CoV-2 by disrupting CD147 interaction84-87, may inhibit SARS-CoV-2 attachment to lipid rafts via spike NTD binding3, shows protection against inflammation, cytokine storm, and mortality in an LPS mouse model sharing key pathological features of severe COVID-1960,88, may be beneficial in severe COVID-19 by binding IGF1 to inhibit the promotion of inflammation, fibrosis, and cell proliferation that leads to lung damage9, may minimize SARS-CoV-2 induced cardiac damage41,49, may counter immune evasion by inhibiting NSP15-TBK1/KPNA1 interaction and restoring IRF3 activation89, may disrupt SARS-CoV-2 N and ORF6 protein nuclear transport and their suppression of host interferon responses2, reduces TAZ/YAP nuclear import, relieving SARS-CoV-2-driven suppression of IRF3 and NF-κB antiviral pathways36, increases Bifidobacteria which play a key role in the immune system90, has immunomodulatory52 and anti-inflammatory71,91 properties, and has an extensive and very positive safety profile92.
1.
Guzzo et al., Safety, Tolerability, and Pharmacokinetics of Escalating High Doses of Ivermectin in Healthy Adult Subjects, J. Clinical Pharmacology, doi:10.1177/009127002237994.
2.
Gayozo et al., Binding affinities analysis of ivermectin, nucleocapsid and ORF6 proteins of SARS-CoV-2 to human importins α isoforms: A computational approach, Biotecnia, doi:10.18633/biotecnia.v27.2485.
3.
Lefebvre et al., Characterization and Fluctuations of an Ivermectin Binding Site at the Lipid Raft Interface of the N-Terminal Domain (NTD) of the Spike Protein of SARS-CoV-2 Variants, Viruses, doi:10.3390/v16121836.
4.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
5.
Bagheri-Far et al., Non-spike protein inhibition of SARS-CoV-2 by natural products through the key mediator protein ORF8, Molecular Biology Research Communications, doi:10.22099/mbrc.2024.50245.2001.
6.
de Oliveira Só et al., In Silico Comparative Analysis of Ivermectin and Nirmatrelvir Inhibitors Interacting with the SARS-CoV-2 Main Protease, Preprints, doi:10.20944/preprints202404.1825.v1.
7.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
8.
Oranu et al., Validation of the binding affinities and stabilities of ivermectin and moxidectin against SARS-CoV-2 receptors using molecular docking and molecular dynamics simulation, GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2024.26.1.0030.
9.
Zhao et al., Identification of the shared gene signatures between pulmonary fibrosis and pulmonary hypertension using bioinformatics analysis, Frontiers in Immunology, doi:10.3389/fimmu.2023.1197752.
10.
Vottero et al., Computational Prediction of the Interaction of Ivermectin with Fibrinogen, Molecular Sciences, doi:10.3390/ijms241411449.
11.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
12.
Umar et al., Inhibitory potentials of ivermectin, nafamostat, and camostat on spike protein and some nonstructural proteins of SARS-CoV-2: Virtual screening approach, Jurnal Teknologi Laboratorium, doi:10.29238/teknolabjournal.v11i1.344.
13.
Alvarado et al., Interaction of the New Inhibitor Paxlovid (PF-07321332) and Ivermectin With the Monomer of the Main Protease SARS-CoV-2: A Volumetric Study Based on Molecular Dynamics, Elastic Networks, Classical Thermodynamics and SPT, Computational Biology and Chemistry, doi:10.1016/j.compbiolchem.2022.107692.
14.
Aminpour et al., In Silico Analysis of the Multi-Targeted Mode of Action of Ivermectin and Related Compounds, Computation, doi:10.3390/computation10040051.
15.
Parvez et al., Insights from a computational analysis of the SARS-CoV-2 Omicron variant: Host–pathogen interaction, pathogenicity, and possible drug therapeutics, Immunity, Inflammation and Disease, doi:10.1002/iid3.639.
16.
Francés-Monerris et al., Microscopic interactions between ivermectin and key human and viral proteins involved in SARS-CoV-2 infection, Physical Chemistry Chemical Physics, doi:10.1039/D1CP02967C.
17.
González-Paz et al., Comparative study of the interaction of ivermectin with proteins of interest associated with SARS-CoV-2: A computational and biophysical approach, Biophysical Chemistry, doi:10.1016/j.bpc.2021.106677.
18.
González-Paz (B) et al., Structural Deformability Induced in Proteins of Potential Interest Associated with COVID-19 by binding of Homologues present in Ivermectin: Comparative Study Based in Elastic Networks Models, Journal of Molecular Liquids, doi:10.1016/j.molliq.2021.117284.
19.
Rana et al., A Computational Study of Ivermectin and Doxycycline Combination Drug Against SARS-CoV-2 Infection, Research Square, doi:10.21203/rs.3.rs-755838/v1.
20.
Muthusamy et al., Virtual Screening Reveals Potential Anti-Parasitic Drugs Inhibiting the Receptor Binding Domain of SARS-CoV-2 Spike protein, Journal of Virology & Antiviral Research, www.scitechnol.com/abstract/virtual-screening-reveals-potential-antiparasitic-drugs-inhibiting-the-receptor-binding-domain-of-sarscov2-spike-protein-16398.html.
21.
Qureshi et al., Mechanistic insights into the inhibitory activity of FDA approved ivermectin against SARS-CoV-2: old drug with new implications, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1906750.
22.
Schöning et al., Highly-transmissible Variants of SARS-CoV-2 May Be More Susceptible to Drug Therapy Than Wild Type Strains, Research Square, doi:10.21203/rs.3.rs-379291/v1.
23.
Bello et al., Elucidation of the inhibitory activity of ivermectin with host nuclear importin α and several SARS-CoV-2 targets, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1911857.
24.
Udofia et al., In silico studies of selected multi-drug targeting against 3CLpro and nsp12 RNA-dependent RNA-polymerase proteins of SARS-CoV-2 and SARS-CoV, Network Modeling Analysis in Health Informatics and Bioinformatics, doi:10.1007/s13721-021-00299-2.
25.
Choudhury et al., Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach, Future Medicine, doi:10.2217/fvl-2020-0342.
26.
Kern et al., Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing, Frontiers in Pharmacology, doi:10.3389/fphar.2021.625678.
27.
Saha et al., The Binding mechanism of ivermectin and levosalbutamol with spike protein of SARS-CoV-2, Structural Chemistry, doi:10.1007/s11224-021-01776-0.
28.
Eweas et al., Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2, Frontiers in Microbiology, doi:10.3389/fmicb.2020.592908.
29.
Parvez (B) et al., Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.09.098.
30.
Francés-Monerris (B) et al., Has Ivermectin Virus-Directed Effects against SARS-CoV-2? Rationalizing the Action of a Potential Multitarget Antiviral Agent, ChemRxiv, doi:10.26434/chemrxiv.12782258.v1.
31.
Kalhor et al., Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1824816.
32.
Swargiary, A., Ivermectin as a promising RNA-dependent RNA polymerase inhibitor and a therapeutic drug against SARS-CoV2: Evidence from in silico studies, Research Square, doi:10.21203/rs.3.rs-73308/v1.
33.
Maurya, D., A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID-19 Patients, American Chemical Society (ACS), doi:10.26434/chemrxiv.12630539.v1.
34.
Lehrer et al., Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, In Vivo, 34:5, 3023-3026, doi:10.21873/invivo.12134.
35.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
36.
Kofler et al., M-Motif, a potential non-conventional NLS in YAP/TAZ and other cellular and viral proteins that inhibits classic protein import, iScience, doi:10.1016/j.isci.2025.112105.
37.
Shahin et al., The selective effect of Ivermectin on different human coronaviruses; in-vitro study, Research Square, doi:10.21203/rs.3.rs-4180797/v1.
38.
Jitobaom et al., Identification of inositol monophosphatase as a broad‐spectrum antiviral target of ivermectin, Journal of Medical Virology, doi:10.1002/jmv.29552.
39.
Fauquet et al., Microfluidic Diffusion Sizing Applied to the Study of Natural Products and Extracts That Modulate the SARS-CoV-2 Spike RBD/ACE2 Interaction, Molecules, doi:10.3390/molecules28248072.
40.
García-Aguilar et al., In Vitro Analysis of SARS-CoV-2 Spike Protein and Ivermectin Interaction, International Journal of Molecular Sciences, doi:10.3390/ijms242216392.
41.
Liu et al., SARS-CoV-2 viral genes Nsp6, Nsp8, and M compromise cellular ATP levels to impair survival and function of human pluripotent stem cell-derived cardiomyocytes, Stem Cell Research & Therapy, doi:10.1186/s13287-023-03485-3.
42.
Boschi et al., SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects, bioRxiv, doi:10.1101/2022.11.24.517882.
43.
De Forni et al., Synergistic drug combinations designed to fully suppress SARS-CoV-2 in the lung of COVID-19 patients, PLoS ONE, doi:10.1371/journal.pone.0276751.
44.
Saha (B) et al., Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder, Pharmaceutics, doi:10.3390/pharmaceutics14071432.
45.
Jitobaom (B) et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8.
46.
Croci et al., Liposomal Systems as Nanocarriers for the Antiviral Agent Ivermectin, International Journal of Biomaterials, doi:10.1155/2016/8043983.
47.
Zheng et al., Red blood cell-hitchhiking mediated pulmonary delivery of ivermectin: Effects of nanoparticle properties, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121719.
48.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
49.
Liu (B) et al., Genome-wide analyses reveal the detrimental impacts of SARS-CoV-2 viral gene Orf9c on human pluripotent stem cell-derived cardiomyocytes, Stem Cell Reports, doi:10.1016/j.stemcr.2022.01.014.
50.
Segatori et al., Effect of Ivermectin and Atorvastatin on Nuclear Localization of Importin Alpha and Drug Target Expression Profiling in Host Cells from Nasopharyngeal Swabs of SARS-CoV-2- Positive Patients, Viruses, doi:10.3390/v13102084.
51.
Jitobaom (C) et al., Favipiravir and Ivermectin Showed in Vitro Synergistic Antiviral Activity against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-941811/v1.
52.
Munson et al., Niclosamide and ivermectin modulate caspase-1 activity and proinflammatory cytokine secretion in a monocytic cell line, British Society For Nanomedicine Early Career Researcher Summer Meeting, 2021, web.archive.org/web/20230401070026/https://michealmunson.github.io/COVID.pdf.
53.
Mountain Valley MD, Mountain Valley MD Receives Successful Results From BSL-4 COVID-19 Clearance Trial on Three Variants Tested With Ivectosol™, 5/18, www.globenewswire.com/en/news-release/2021/05/18/2231755/0/en/Mountain-Valley-MD-Receives-Successful-Results-From-BSL-4-COVID-19-Clearance-Trial-on-Three-Variants-Tested-With-Ivectosol.html.
54.
Yesilbag et al., Ivermectin also inhibits the replication of bovine respiratory viruses (BRSV, BPIV-3, BoHV-1, BCoV and BVDV) in vitro, Virus Research, doi:10.1016/j.virusres.2021.198384.
55.
Mody et al., Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents, Communications Biology, doi:10.1038/s42003-020-01577-x.
56.
Jeffreys et al., Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2022.106542.
57.
Surnar et al., Clinically Approved Antiviral Drug in an Orally Administrable Nanoparticle for COVID-19, ACS Pharmacol. Transl. Sci., doi:10.1021/acsptsci.0c00179.
58.
Li et al., Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment, J. Cellular Physiology, doi:10.1002/jcp.30055.
59.
Caly et al., The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research, doi:10.1016/j.antiviral.2020.104787.
60.
Zhang et al., Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflammation Research, doi:10.1007/s00011-008-8007-8.
61.
Gao et al., Ivermectin ameliorates acute myocarditis via the inhibition of importin-mediated nuclear translocation of NF-κB/p65, International Immunopharmacology, doi:10.1016/j.intimp.2024.112073.
62.
Abd-Elmawla et al., Suppression of NLRP3 inflammasome by ivermectin ameliorates bleomycin-induced pulmonary fibrosis, Journal of Zhejiang University-SCIENCE B, doi:10.1631/jzus.B2200385.
63.
Uematsu et al., Prophylactic administration of ivermectin attenuates SARS-CoV-2 induced disease in a Syrian Hamster Model, The Journal of Antibiotics, doi:10.1038/s41429-023-00623-0.
64.
Albariqi et al., Pharmacokinetics and Safety of Inhaled Ivermectin in Mice as a Potential COVID-19 Treatment, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121688.
65.
Errecalde et al., Safety and Pharmacokinetic Assessments of a Novel Ivermectin Nasal Spray Formulation in a Pig Model, Journal of Pharmaceutical Sciences, doi:10.1016/j.xphs.2021.01.017.
66.
Madrid et al., Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation, Heliyon, doi:10.1016/j.heliyon.2020.e05820.
67.
Ma et al., Ivermectin contributes to attenuating the severity of acute lung injury in mice, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2022.113706.
68.
de Melo et al., Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin, EMBO Mol. Med., doi:10.15252/emmm.202114122.
69.
Arévalo et al., Ivermectin reduces in vivo coronavirus infection in a mouse experimental model, Scientific Reports, doi:10.1038/s41598-021-86679-0.
70.
Chaccour et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Scientific Reports, doi:10.1038/s41598-020-74084-y.
71.
Yan et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflammation Research, doi:10.1007/s00011-011-0307-8.
72.
Götz et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Scientific Reports, doi:10.1038/srep23138.
73.
Tay et al., Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Research, doi:10.1016/j.antiviral.2013.06.002.
74.
Wagstaff et al., Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochemical Journal, doi:10.1042/BJ20120150.
75.
Wagstaff (B) et al., An AlphaScreen®-Based Assay for High-Throughput Screening for Specific Inhibitors of Nuclear Import, SLAS Discovery, doi:10.1177/1087057110390360.
76.
Barrows et al., A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection, Cell Host & Microbe, doi:10.1016/j.chom.2016.07.004.
77.
Yang et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Research, doi:10.1016/j.antiviral.2020.104760.
78.
Mastrangelo et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dks147.
79.
Varghese et al., Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses, Antiviral Research, doi:10.1016/j.antiviral.2015.12.012.
80.
Bennett et al., Role of a nuclear localization signal on the minor capsid Proteins VP2 and VP3 in BKPyV nuclear entry, Virology, doi:10.1016/j.virol.2014.10.013.
81.
Kosyna et al., The importin α/β-specific inhibitor Ivermectin affects HIF-dependent hypoxia response pathways, Biological Chemistry, doi:10.1515/hsz-2015-0171.
82.
Scheim et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
83.
Liu (C) et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
84.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
85.
Scheim (B), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
86.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
87.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
88.
DiNicolantonio et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
89.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
90.
Hazan et al., Treatment with Ivermectin Increases the Population of Bifidobacterium in the Gut, ACG 2023, acg2023posters.eventscribe.net/posterspeakers.asp.
de la Puente et al., 13 Aug 2025, Mexico, peer-reviewed, 8 authors.
Contact: marcoantonio.loza@lasalle.mx (corresponding author), juande@lasallistas.org.mx, ei.flores@lasallistas.org.mx, mruiz@lasallistas.org.mx, arely.vergara@lasalle.mx, jreyes@inmegen.gob.mx, lizmedina@quimica.unam.mx.
Variations in Plasma Levels of Orally Administered Ivermectin Could Hamper Its Potential Drug Repositioning: Results of a Bioequivalence Study in Mexican Population
Pharmaceuticals, doi:10.3390/ph18081193
Background/Objectives: Despite its initial promise as a treatment for COVID-19 due to its antiviral properties, controlled randomized trials have demonstrated a lack of clinical efficacy at standard dosages. Although its overall clinical benefits remain contentious, a recent meta-analysis suggests that ivermectin may lower the risk of mechanical ventilation in COVID-19 patients. This study aims to assess the bioequivalence of different formulations of orally administered ivermectin within a Mexican population. Methods: A randomized, controlled bioequivalence study was conducted involving healthy volunteers who received two oral formulations of ivermectin. Plasma samples were collected at predetermined intervals for pharmacokinetic analysis. Results: The findings indicate significant variations in plasma concentration profiles among the evaluated formulations. Elevated inter-and intrasubject variations, independent of the formulation, highlighted implications for both clinical efficacy and safety. Conclusions: The potential repurposing of ivermectin for COVID-19 treatment raises concerns, particularly regarding the variability in plasma levels resulting from oral administration, which may impact its effectiveness. The study underscores the importance of pharmacokinetic properties in the repurposing of ivermectin as a therapeutic agent. Given the observed discrepancies in plasma levels, careful consideration of dosing and formulation is essential for optimizing clinical outcomes in potential new applications of ivermectin.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Aboulfotouh, Allam, El-Badry, El-Sayed, Role of self-emulsifying drug delivery systems in optimizing the oral delivery of hydrophilic macromolecules and reducing interindividual variability, Colloids Surf. B Biointerfaces, doi:10.1016/j.colsurfb.2018.03.034
Alaimo, Pulvirenti, Network-Based Drug Repositioning: Approaches, Resources, and Research Directions BT-Computational Methods for Drug Repurposing, doi:10.1007/978-1-4939-8955-3_6
Algorta, Krolewiecki, Pinto, Gold, Muñoz, Pharmacokinetic Characterization and Comparative Bioavailability of an Innovative Orodispersible Fixed-Dose Combination of Ivermectin and Albendazole: A Single Dose, Open Label, Sequence Randomized, Crossover Clinical Trial in Healthy Volunteers, Front. Pharmacol, doi:10.3389/fphar.2022.914886
Alshehri, Chhonker, Bala, Edi, Bjerum et al., Population pharmacokinetic model of ivermectin in mass drug administration against lymphatic filariasis, PLoS Negl. Trop. Dis, doi:10.1371/journal.pntd.0011319
Banerjee, Shankar, Prasad, Formulation development and systematic optimization of stabilized ziprasidone hydrochloride capsules devoid of any food effect, Pharm. Dev. Technol, doi:10.3109/10837450.2015.1055764
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
Chaccour, Casellas, Blanco-Di Matteo, Pineda, Fernandez-Montero et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial, eClinicalMedicine, doi:10.1016/j.eclinm.2020.100720
Chaccour, Hammann, Rabinovich, Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety, Malar. J, doi:10.1186/s12936-017-1801-4
Cofepris, Norma Oficial Mexicana
Constantinides, Wasan, Lipid Formulation Strategies for Enhancing Intestinal Transport and Absorption of P-Glycoprotein (P-gp) Substrate Drugs: In vitro/In vivo Case Studies, J. Pharm. Sci, doi:10.1002/jps.20780
Crump, Ivermectin: Enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations, J. Antibiot, doi:10.1038/ja.2017.11
Duthaler, Leisegang, Karlsson, Krähenbühl, Hammann, The effect of food on the pharmacokinetics of oral ivermectin, J. Antimicrob. Chemother, doi:10.1093/jac/dkz466
Duthaler, Suenderhauf, Karlsson, Hussner, Meyer Zu Schwabedissen et al., Population pharmacokinetics of oral ivermectin in venous plasma and dried blood spots in healthy volunteers, Br. J. Clin. Pharmacol, doi:10.1111/bcp.13840
Favela-Mendoza, Rangel-Villalobos, Fricke-Galindo, Ortega-Vázquez, Martínez-Cortés et al., Genetic variability among Mexican Mestizo and Amerindian populations based on three ABCB1 polymorphisms, Mol. Biol. Rep, doi:10.1007/s11033-018-4419-x
Fernandes, Van Oudtshoorn, Tam, González, Aurela et al., The bioequivalence study design recommendations for immediate-release solid oral dosage forms in the international pharmaceutical regulators programme participating regulators and organisations: Differences and commonalities, J. Pharm. Pharm. Sci, doi:10.3389/jpps.2024.12398
Gardon, Boussinesq, Kamgno, Gardon-Wendel, Demanga-Ngangue; Duke, Effects of standard and high doses of ivermectin on adult worms of Onchocerca volvulus: A randomised controlled trial, Lancet, doi:10.1016/S0140-6736(02)09456-4
Gera, Sampathi, Maddukuri, Dodoala, Junnuthula et al., Therapeutic Potential of Naringenin Nanosuspension: In Vitro and In Vivo Anti-Osteoporotic Studies, Pharmaceutics, doi:10.3390/pharmaceutics14071449
González Canga, Sahagún Prieto, Diez Liébana, Fernández Martínez, Sierra et al., The Pharmacokinetics and Interactions of Ivermectin in Humans-A Mini-review, AAPS J, doi:10.1208/s12248-007-9000-9
González Canga, Sahagún Prieto, José Diez Liébana, Martínez, Vega et al., The pharmacokinetics and metabolism of ivermectin in domestic animal species, Vet. J, doi:10.1016/j.tvjl.2007.07.011
Guzzo, Furtek, Porras, Chen, Tipping et al., Tolerability, and Pharmacokinetics of Escalating High Doses of Ivermectin in Healthy Adult Subjects, J. Clin. Pharmacol, doi:10.1177/009127002237994
Halder, Pradhan, Kar, Ghosh, Rath, Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer, Nanomed. Nanotechnol. Biol. Med, doi:10.1016/j.nano.2021.102494
Huang, Zhang, Teaching an old dog new tricks: Drug discovery by repositioning natural products and their derivatives, Drug Discov. Today, doi:10.1016/j.drudis.2022.02.007
Jourdan, Bureau, Rochais, Dallemagne, Drug repositioning: A brief overview, J. Pharm. Pharmacol, doi:10.1111/jphp.13273
Kim, Shim, Yee, Choi, Gwak, Effects of CYP3A4*22 polymorphism on trough concentration of tacrolimus in kidney transplantation: A systematic review and meta-analysis, Front. Pharmacol, doi:10.3389/fphar.2023.1201083
Lifschitz, Virkel, Sallovitz, Sutra, Galtier et al., Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle, Vet. Parasitol, doi:10.1016/S0304-4017(99)00175-2
Low, Yip, Lal, Repositioning Ivermectin for COVID-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication, Biochim. Biophys. Acta (BBA) Mol. Basis Dis, doi:10.1016/j.bbadis.2021.166294
Maji, Mahajan, Sriram, Medtiya, Vasave et al., Solid self emulsifying drug delivery system: Superior mode for oral delivery of hydrophobic cargos, J. Control. Release, doi:10.1016/j.jconrel.2021.08.013
Marcolino, Meira, Guimarães, Motta, Chagas et al., Systematic review and meta-analysis of ivermectin for treatment of COVID-19: Evidence beyond the hype, BMC Infect. Dis, doi:10.1186/s12879-022-07589-8
Mittal, Mittal, Repurposing old molecules for new indications: Defining pillars of success from lessons in the past, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2021.174569
Modi, Wang, Hu, Gupta, Effect of food on the pharmacokinetics of osmotic controlled-release methylphenidate HCl in healthy subjects, Biopharm. Drug Dispos, doi:10.1002/1099-081X(200001)21:1%3C23::AID-BDD212%3E3.0.CO;2-V
Murteira, Millier, Ghezaiel, Lamure, Drug Reformulations and Repositioning in the Pharmaceutical Industry and Their Impact on Market Access: Regulatory Implications, J. Mark. Access Health Policy, doi:10.3402/jmahp.v2.22813
Muñoz, Ballester, Antonijoan, Gich, Rodríguez et al., Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers, PLoS Negl. Trop. Dis, doi:10.1371/journal.pntd.0006020
Naggie, Boulware, Lindsell, Stewart, Slandzicki et al., Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients with COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2023.1650
Nguyen, Duong, Maeng, Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability, Pharmaceutics, doi:10.3390/pharmaceutics13071103
O' Sullivan, Blake, Berntgen, Salmonson, Welink, on behalf of the Pharmacokinetics Working Party. Overview of the European Medicines Agency's Development of Product-Specific Bioequivalence Guidelines, Clin. Pharmacol. Ther, doi:10.1002/cpt.957
Ozeki, Nagahama, Fujita, Suzuki, Sugino et al., Influence of CYP3A4/5 and ABC transporter polymorphisms on lenvatinib plasma trough concentrations in Japanese patients with thyroid cancer, Sci. Rep, doi:10.1038/s41598-019-41820-y
Ponce, Green, Strassle, Nápoles, Positive and negative aspects of the COVID-19 pandemic among a diverse sample of US adults: An exploratory mixed-methods analysis of online survey data, BMC Public Health, doi:10.1186/s12889-023-17491-w
Raman, Polli, Prediction of positive food effect: Bioavailability enhancement of BCS class II drugs, Int. J. Pharm, doi:10.1016/j.ijpharm.2016.04.013
Rangaraj, Sampathi, Junnuthula, Kolimi, Mandati et al., Fast-Fed Variability: Insights into Drug Delivery, Molecular Manifestations, and Regulatory Aspects, Pharmaceutics, doi:10.3390/pharmaceutics14091807
Reis, Silva, Silva, Thabane, Milagres et al., Effect of Early Treatment with Ivermectin among Patients with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2115869
Reyes-Hernández, Lares-Asseff, Sosa-Macias, Vega, Albores et al., A Comparative Study of CYP3A4 Polymorphisms in Mexican Amerindian and Mestizo Populations, Pharmacology, doi:10.1159/000109983
Seo, Jin, Yoo, Pharmacokinetic considerations for enhancing drug repurposing opportunities of anthelmintics: Niclosamide as a case study, Biomed. Pharmacother, doi:10.1016/j.biopha.2024.116394
Song, Shi, Zhang, Ivermectin for treatment of COVID-19: A systematic review and meta-analysis, Heliyon, doi:10.1016/j.heliyon.2024.e27647
Talevi, Bellera, Challenges and opportunities with drug repurposing: Finding strategies to find alternative uses of therapeutics, Expert Opin. Drug Discov, doi:10.1080/17460441.2020.1704729
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem. J, doi:10.1042/BJ20120150
Wang, Aldahdooh, Hu, Yang, Vähä-Koskela et al., DrugRepo: A novel approach to repurposing drugs based on chemical and genomic features, Sci. Rep, doi:10.1038/s41598-022-24980-2
Wang, Chen, Chen, Wang, Influence of ABCB1 Gene Polymorphism on Rivaroxaban Blood Concentration and Hemorrhagic Events in Patients With Atrial Fibrillation, Front. Pharmacol, doi:10.3389/fphar.2021.639854
DOI record:
{
"DOI": "10.3390/ph18081193",
"ISSN": [
"1424-8247"
],
"URL": "http://dx.doi.org/10.3390/ph18081193",
"abstract": "<jats:p>Background/Objectives: Despite its initial promise as a treatment for COVID-19 due to its antiviral properties, controlled randomized trials have demonstrated a lack of clinical efficacy at standard dosages. Although its overall clinical benefits remain contentious, a recent meta-analysis suggests that ivermectin may lower the risk of mechanical ventilation in COVID-19 patients. This study aims to assess the bioequivalence of different formulations of orally administered ivermectin within a Mexican population. Methods: A randomized, controlled bioequivalence study was conducted involving healthy volunteers who received two oral formulations of ivermectin. Plasma samples were collected at predetermined intervals for pharmacokinetic analysis. Results: The findings indicate significant variations in plasma concentration profiles among the evaluated formulations. Elevated inter- and intrasubject variations, independent of the formulation, highlighted implications for both clinical efficacy and safety. Conclusions: The potential repurposing of ivermectin for COVID-19 treatment raises concerns, particularly regarding the variability in plasma levels resulting from oral administration, which may impact its effectiveness. The study underscores the importance of pharmacokinetic properties in the repurposing of ivermectin as a therapeutic agent. Given the observed discrepancies in plasma levels, careful consideration of dosing and formulation is essential for optimizing clinical outcomes in potential new applications of ivermectin.</jats:p>",
"alternative-id": [
"ph18081193"
],
"author": [
{
"affiliation": [
{
"name": "Chemical Sciences School, Universidad La Salle-México, Benjamín Franklin 45, Mexico City 06140, Mexico"
}
],
"family": "de la Puente",
"given": "Ernesto",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Chemical Sciences School, Universidad La Salle-México, Benjamín Franklin 45, Mexico City 06140, Mexico"
}
],
"family": "Ramos-Mundo",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Chemical Sciences School, Universidad La Salle-México, Benjamín Franklin 45, Mexico City 06140, Mexico"
}
],
"family": "Flores-Pérez",
"given": "Elena I.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Research Group on Development and Innovation in Health and Nutrition Promotion and Education, Universidad La Salle-México, Benjamín Franklin 45, Mexico City 06140, Mexico"
}
],
"family": "Vergara-Castañeda",
"given": "Arely",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6498-3286",
"affiliation": [
{
"name": "Instituto Nacional de Medicina Genómica, Periférico Sur 4809, Mexico City 14610, Mexico"
}
],
"authenticated-orcid": false,
"family": "Reyes-Grajeda",
"given": "Juan Pablo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Facultad de Química, National Autonomous University of Mexico (UNAM), Mexico City 04510, Mexico"
}
],
"family": "Medina-Reyes",
"given": "Liz J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Chemical Sciences School, Universidad La Salle-México, Benjamín Franklin 45, Mexico City 06140, Mexico"
}
],
"family": "Ruiz-Olmedo",
"given": "María Isabel",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-8449-0806",
"affiliation": [
{
"name": "Design, Isolation, and Synthesis of Bioactive Molecules Research Group, Universidad La Salle-México, Benjamín Franklin 45, Mexico City 06140, Mexico"
}
],
"authenticated-orcid": false,
"family": "Loza-Mejía",
"given": "Marco A.",
"sequence": "additional"
}
],
"container-title": "Pharmaceuticals",
"container-title-short": "Pharmaceuticals",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
8,
13
]
],
"date-time": "2025-08-13T09:43:01Z",
"timestamp": 1755078181000
},
"deposited": {
"date-parts": [
[
2025,
8,
13
]
],
"date-time": "2025-08-13T09:48:50Z",
"timestamp": 1755078530000
},
"indexed": {
"date-parts": [
[
2025,
8,
15
]
],
"date-time": "2025-08-15T02:35:59Z",
"timestamp": 1755225359640,
"version": "3.43.0"
},
"is-referenced-by-count": 0,
"issue": "8",
"issued": {
"date-parts": [
[
2025,
8,
13
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2025,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
8,
13
]
],
"date-time": "2025-08-13T00:00:00Z",
"timestamp": 1755043200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1424-8247/18/8/1193/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1193",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2025,
8,
13
]
]
},
"published-online": {
"date-parts": [
[
2025,
8,
13
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1007/978-1-4939-8955-3",
"doi-asserted-by": "crossref",
"key": "ref_1",
"unstructured": "Vanhaelen, Q. (2019). Network-Based Drug Repositioning: Approaches, Resources, and Research Directions BT-Computational Methods for Drug Repurposing. Computational Methods for Drug Repurposing, Springer."
},
{
"DOI": "10.1016/j.drudis.2022.02.007",
"article-title": "Teaching an old dog new tricks: Drug discovery by repositioning natural products and their derivatives",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "1936",
"journal-title": "Drug Discov. Today",
"key": "ref_2",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1038/s41598-022-24980-2",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Wang, Y., Aldahdooh, J., Hu, Y., Yang, H., Vähä-Koskela, M., Tang, J., and Tanoli, Z. (2022). DrugRepo: A novel approach to repurposing drugs based on chemical and genomic features. Sci. Rep., 12."
},
{
"DOI": "10.1186/s12889-023-17491-w",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "Ponce, S.A., Green, A., Strassle, P.D., and Nápoles, A.M. (2024). Positive and negative aspects of the COVID-19 pandemic among a diverse sample of US adults: An exploratory mixed-methods analysis of online survey data. BMC Public Health, 24."
},
{
"DOI": "10.1186/s12879-022-07589-8",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Marcolino, M.S., Meira, K.C., Guimarães, N.S., Motta, P.P., Chagas, V.S., Kelles, S.M.B., de Sá, L.C., Valacio, R.A., and Ziegelmann, P.K. (2022). Systematic review and meta-analysis of ivermectin for treatment of COVID-19: Evidence beyond the hype. BMC Infect. Dis., 22."
},
{
"DOI": "10.1016/j.bbadis.2021.166294",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Low, Z.Y., Yip, A.J.W., and Lal, S.K. (2022). Repositioning Ivermectin for COVID-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication. Biochim. Biophys. Acta (BBA) Mol. Basis Dis., 1868."
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"first-page": "104787",
"journal-title": "Antiviral Res.",
"key": "ref_7",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2020.100720",
"article-title": "The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial",
"author": "Chaccour",
"doi-asserted-by": "crossref",
"first-page": "100720",
"journal-title": "eClinicalMedicine",
"key": "ref_8",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2115869",
"article-title": "Effect of Early Treatment with Ivermectin among Patients with COVID-19",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "1721",
"journal-title": "N. Engl. J. Med.",
"key": "ref_9",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jama.2023.1650",
"article-title": "Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients with COVID-19: A Randomized Clinical Trial",
"author": "Naggie",
"doi-asserted-by": "crossref",
"first-page": "888",
"journal-title": "JAMA",
"key": "ref_10",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1016/j.heliyon.2024.e27647",
"article-title": "Ivermectin for treatment of COVID-19: A systematic review and meta-analysis",
"author": "Song",
"doi-asserted-by": "crossref",
"first-page": "e27647",
"journal-title": "Heliyon",
"key": "ref_11",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1038/ja.2017.11",
"article-title": "Ivermectin: Enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations",
"author": "Crump",
"doi-asserted-by": "crossref",
"first-page": "495",
"journal-title": "J. Antibiot.",
"key": "ref_12",
"volume": "70",
"year": "2017"
},
{
"DOI": "10.1080/17460441.2020.1704729",
"article-title": "Challenges and opportunities with drug repurposing: Finding strategies to find alternative uses of therapeutics",
"author": "Talevi",
"doi-asserted-by": "crossref",
"first-page": "397",
"journal-title": "Expert Opin. Drug Discov.",
"key": "ref_13",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1111/jphp.13273",
"article-title": "Drug repositioning: A brief overview",
"author": "Jourdan",
"doi-asserted-by": "crossref",
"first-page": "1145",
"journal-title": "J. Pharm. Pharmacol.",
"key": "ref_14",
"volume": "72",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2024.116394",
"doi-asserted-by": "crossref",
"key": "ref_15",
"unstructured": "Seo, J.I., Jin, G., and Yoo, H.H. (2024). Pharmacokinetic considerations for enhancing drug repurposing opportunities of anthelmintics: Niclosamide as a case study. Biomed. Pharmacother., 173."
},
{
"DOI": "10.1208/s12248-007-9000-9",
"article-title": "The Pharmacokinetics and Interactions of Ivermectin in Humans—A Mini-review",
"doi-asserted-by": "crossref",
"first-page": "42",
"journal-title": "AAPS J.",
"key": "ref_16",
"volume": "10",
"year": "2008"
},
{
"DOI": "10.1016/j.tvjl.2007.07.011",
"article-title": "The pharmacokinetics and metabolism of ivermectin in domestic animal species",
"author": "Vega",
"doi-asserted-by": "crossref",
"first-page": "25",
"journal-title": "Vet. J.",
"key": "ref_17",
"volume": "179",
"year": "2009"
},
{
"DOI": "10.1111/bcp.13840",
"article-title": "Population pharmacokinetics of oral ivermectin in venous plasma and dried blood spots in healthy volunteers",
"author": "Duthaler",
"doi-asserted-by": "crossref",
"first-page": "626",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "ref_18",
"volume": "85",
"year": "2019"
},
{
"article-title": "The effect of food on the pharmacokinetics of oral ivermectin",
"author": "Duthaler",
"first-page": "438",
"journal-title": "J. Antimicrob. Chemother.",
"key": "ref_19",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1042/BJ20120150",
"article-title": "Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus",
"author": "Wagstaff",
"doi-asserted-by": "crossref",
"first-page": "851",
"journal-title": "Biochem. J.",
"key": "ref_20",
"volume": "443",
"year": "2012"
},
{
"DOI": "10.3389/jpps.2024.12398",
"article-title": "The bioequivalence study design recommendations for immediate-release solid oral dosage forms in the international pharmaceutical regulators programme participating regulators and organisations: Differences and commonalities",
"author": "Fernandes",
"doi-asserted-by": "crossref",
"first-page": "12398",
"journal-title": "J. Pharm. Pharm. Sci.",
"key": "ref_21",
"volume": "27",
"year": "2024"
},
{
"DOI": "10.1371/journal.pntd.0006020",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "Muñoz, J., Ballester, M.R., Antonijoan, R.M., Gich, I., Rodríguez, M., Colli, E., Gold, S., and Krolewiecki, A.J. (2018). Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers. PLoS Negl. Trop. Dis., 12."
},
{
"DOI": "10.1177/009127002237994",
"article-title": "Safety, Tolerability, and Pharmacokinetics of Escalating High Doses of Ivermectin in Healthy Adult Subjects",
"author": "Guzzo",
"doi-asserted-by": "crossref",
"first-page": "1122",
"journal-title": "J. Clin. Pharmacol.",
"key": "ref_23",
"volume": "42",
"year": "2002"
},
{
"DOI": "10.3389/fphar.2022.914886",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "Algorta, J., Krolewiecki, A., Pinto, F., Gold, S., and Muñoz, J. (2022). Pharmacokinetic Characterization and Comparative Bioavailability of an Innovative Orodispersible Fixed-Dose Combination of Ivermectin and Albendazole: A Single Dose, Open Label, Sequence Randomized, Crossover Clinical Trial in Healthy Volunteers. Front. Pharmacol., 13."
},
{
"DOI": "10.1186/s12936-017-1801-4",
"article-title": "Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety",
"author": "Chaccour",
"doi-asserted-by": "crossref",
"first-page": "161",
"journal-title": "Malar. J.",
"key": "ref_25",
"volume": "16",
"year": "2017"
},
{
"DOI": "10.1016/j.ijpharm.2016.04.013",
"article-title": "Prediction of positive food effect: Bioavailability enhancement of BCS class II drugs",
"author": "Raman",
"doi-asserted-by": "crossref",
"first-page": "110",
"journal-title": "Int. J. Pharm.",
"key": "ref_26",
"volume": "506",
"year": "2016"
},
{
"DOI": "10.3389/fphar.2023.1201083",
"doi-asserted-by": "crossref",
"key": "ref_27",
"unstructured": "Kim, J.S., Shim, S., Yee, J., Choi, K.H., and Gwak, H.S. (2023). Effects of CYP3A4*22 polymorphism on trough concentration of tacrolimus in kidney transplantation: A systematic review and meta-analysis. Front. Pharmacol., 14."
},
{
"DOI": "10.1038/s41598-019-41820-y",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Ozeki, T., Nagahama, M., Fujita, K., Suzuki, A., Sugino, K., Ito, K., and Miura, M. (2019). Influence of CYP3A4/5 and ABC transporter polymorphisms on lenvatinib plasma trough concentrations in Japanese patients with thyroid cancer. Sci. Rep., 9."
},
{
"DOI": "10.3389/fphar.2021.639854",
"doi-asserted-by": "crossref",
"key": "ref_29",
"unstructured": "Wang, Y., Chen, M., Chen, H., and Wang, F. (2021). Influence of ABCB1 Gene Polymorphism on Rivaroxaban Blood Concentration and Hemorrhagic Events in Patients With Atrial Fibrillation. Front. Pharmacol., 12."
},
{
"article-title": "A Comparative Study of CYP3A4 Polymorphisms in Mexican Amerindian and Mestizo Populations",
"author": "Vega",
"first-page": "97",
"journal-title": "Pharmacology",
"key": "ref_30",
"volume": "81",
"year": "2007"
},
{
"DOI": "10.1007/s11033-018-4419-x",
"article-title": "Genetic variability among Mexican Mestizo and Amerindian populations based on three ABCB1 polymorphisms",
"doi-asserted-by": "crossref",
"first-page": "2525",
"journal-title": "Mol. Biol. Rep.",
"key": "ref_31",
"volume": "45",
"year": "2018"
},
{
"DOI": "10.3390/pharmaceutics13071103",
"doi-asserted-by": "crossref",
"key": "ref_32",
"unstructured": "Nguyen, T.-T.-L., Duong, V.-A., and Maeng, H.-J. (2021). Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics, 13."
},
{
"DOI": "10.1002/jps.20780",
"article-title": "Lipid Formulation Strategies for Enhancing Intestinal Transport and Absorption of P-Glycoprotein (P-gp) Substrate Drugs: In vitro/In vivo Case Studies",
"author": "Constantinides",
"doi-asserted-by": "crossref",
"first-page": "235",
"journal-title": "J. Pharm. Sci.",
"key": "ref_33",
"volume": "96",
"year": "2007"
},
{
"DOI": "10.1016/j.jconrel.2021.08.013",
"article-title": "Solid self emulsifying drug delivery system: Superior mode for oral delivery of hydrophobic cargos",
"author": "Maji",
"doi-asserted-by": "crossref",
"first-page": "646",
"journal-title": "J. Control. Release",
"key": "ref_34",
"volume": "337",
"year": "2021"
},
{
"DOI": "10.1016/j.nano.2021.102494",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Halder, J., Pradhan, D., Kar, B., Ghosh, G., and Rath, G. (2022). Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer. Nanomed. Nanotechnol. Biol. Med., 40."
},
{
"DOI": "10.1002/cpt.957",
"article-title": "Overview of the European Medicines Agency’s Development of Product-Specific Bioequivalence Guidelines",
"author": "Blake",
"doi-asserted-by": "crossref",
"first-page": "539",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "ref_36",
"volume": "104",
"year": "2018"
},
{
"DOI": "10.1371/journal.pntd.0011319",
"doi-asserted-by": "crossref",
"key": "ref_37",
"unstructured": "Alshehri, A., Chhonker, Y.S., Bala, V., Edi, C., Bjerum, C.M., Koudou, B.G., John, L.N., Mitjà, O., Marks, M., and King, C.L. (2023). Population pharmacokinetic model of ivermectin in mass drug administration against lymphatic filariasis. PLoS Negl. Trop. Dis., 17."
},
{
"DOI": "10.1002/1099-081X(200001)21:1<23::AID-BDD212>3.0.CO;2-V",
"article-title": "Effect of food on the pharmacokinetics of osmotic controlled-release methylphenidate HCl in healthy subjects",
"author": "Modi",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "Biopharm. Drug Dispos.",
"key": "ref_38",
"volume": "21",
"year": "2000"
},
{
"article-title": "Formulation development and systematic optimization of stabilized ziprasidone hydrochloride capsules devoid of any food effect",
"author": "Banerjee",
"first-page": "775",
"journal-title": "Pharm. Dev. Technol.",
"key": "ref_39",
"volume": "21",
"year": "2016"
},
{
"DOI": "10.3390/pharmaceutics14071449",
"doi-asserted-by": "crossref",
"key": "ref_40",
"unstructured": "Gera, S., Sampathi, S., Maddukuri, S., Dodoala, S., Junnuthula, V., and Dyawanapelly, S. (2022). Therapeutic Potential of Naringenin Nanosuspension: In Vitro and In Vivo Anti-Osteoporotic Studies. Pharmaceutics, 14."
},
{
"DOI": "10.1016/j.colsurfb.2018.03.034",
"article-title": "Role of self-emulsifying drug delivery systems in optimizing the oral delivery of hydrophilic macromolecules and reducing interindividual variability",
"author": "AboulFotouh",
"doi-asserted-by": "crossref",
"first-page": "82",
"journal-title": "Colloids Surf. B Biointerfaces",
"key": "ref_41",
"volume": "167",
"year": "2018"
},
{
"DOI": "10.3390/pharmaceutics14091807",
"doi-asserted-by": "crossref",
"key": "ref_42",
"unstructured": "Rangaraj, N., Sampathi, S., Junnuthula, V., Kolimi, P., Mandati, P., Narala, S., Nyavanandi, D., and Dyawanapelly, S. (2022). Fast-Fed Variability: Insights into Drug Delivery, Molecular Manifestations, and Regulatory Aspects. Pharmaceutics, 14."
},
{
"DOI": "10.3402/jmahp.v2.22813",
"article-title": "Drug Reformulations and Repositioning in the Pharmaceutical Industry and Their Impact on Market Access: Regulatory Implications",
"author": "Murteira",
"doi-asserted-by": "crossref",
"first-page": "22813",
"journal-title": "J. Mark. Access Health Policy",
"key": "ref_43",
"volume": "2",
"year": "2014"
},
{
"DOI": "10.1016/j.ejphar.2021.174569",
"article-title": "Repurposing old molecules for new indications: Defining pillars of success from lessons in the past",
"author": "Mittal",
"doi-asserted-by": "crossref",
"first-page": "174569",
"journal-title": "Eur. J. Pharmacol.",
"key": "ref_44",
"volume": "912",
"year": "2021"
},
{
"DOI": "10.1016/S0304-4017(99)00175-2",
"article-title": "Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle",
"author": "Lifschitz",
"doi-asserted-by": "crossref",
"first-page": "327",
"journal-title": "Vet. Parasitol.",
"key": "ref_45",
"volume": "87",
"year": "2000"
},
{
"DOI": "10.1016/S0140-6736(02)09456-4",
"article-title": "Effects of standard and high doses of ivermectin on adult worms of Onchocerca volvulus: A randomised controlled trial",
"author": "Gardon",
"doi-asserted-by": "crossref",
"first-page": "203",
"journal-title": "Lancet",
"key": "ref_46",
"volume": "360",
"year": "2002"
},
{
"key": "ref_47",
"unstructured": "(2021, March 15). COFEPRIS. Norma Oficial Mexicana NOM-177-SSA1-2013; Que Establece las Pruebas y Procedimientos Para Demostrar Que un Medicamento es Intercambiable. Requisitos a Que Deben Sujetarse los Terceros Autorizados Que Realicen las Pruebas de Intercambiabilidad. Requisitos Para Realizar los Estudios de Biocomparabilidad. Requisitos a Que Deben Sujetarse los Terceros Autorizados, Centros de Investigación o Instituciones Hospitalarias Que Realicen las Pruebas de Biocomparabilidad; Diario Oficial de la Federación (DOF). Published on 20 September 2013. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5314833&fecha=20/09/2013#gsc.tab=0."
}
],
"reference-count": 47,
"references-count": 47,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1424-8247/18/8/1193"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Variations in Plasma Levels of Orally Administered Ivermectin Could Hamper Its Potential Drug Repositioning: Results of a Bioequivalence Study in Mexican Population",
"type": "journal-article",
"volume": "18"
}
