Dimethoxycurcumin Acidifies Endolysosomes and Inhibits SARS-CoV-2 Entry
et al., Frontiers in Virology, doi:10.3389/fviro.2022.923018, Jun 2022
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
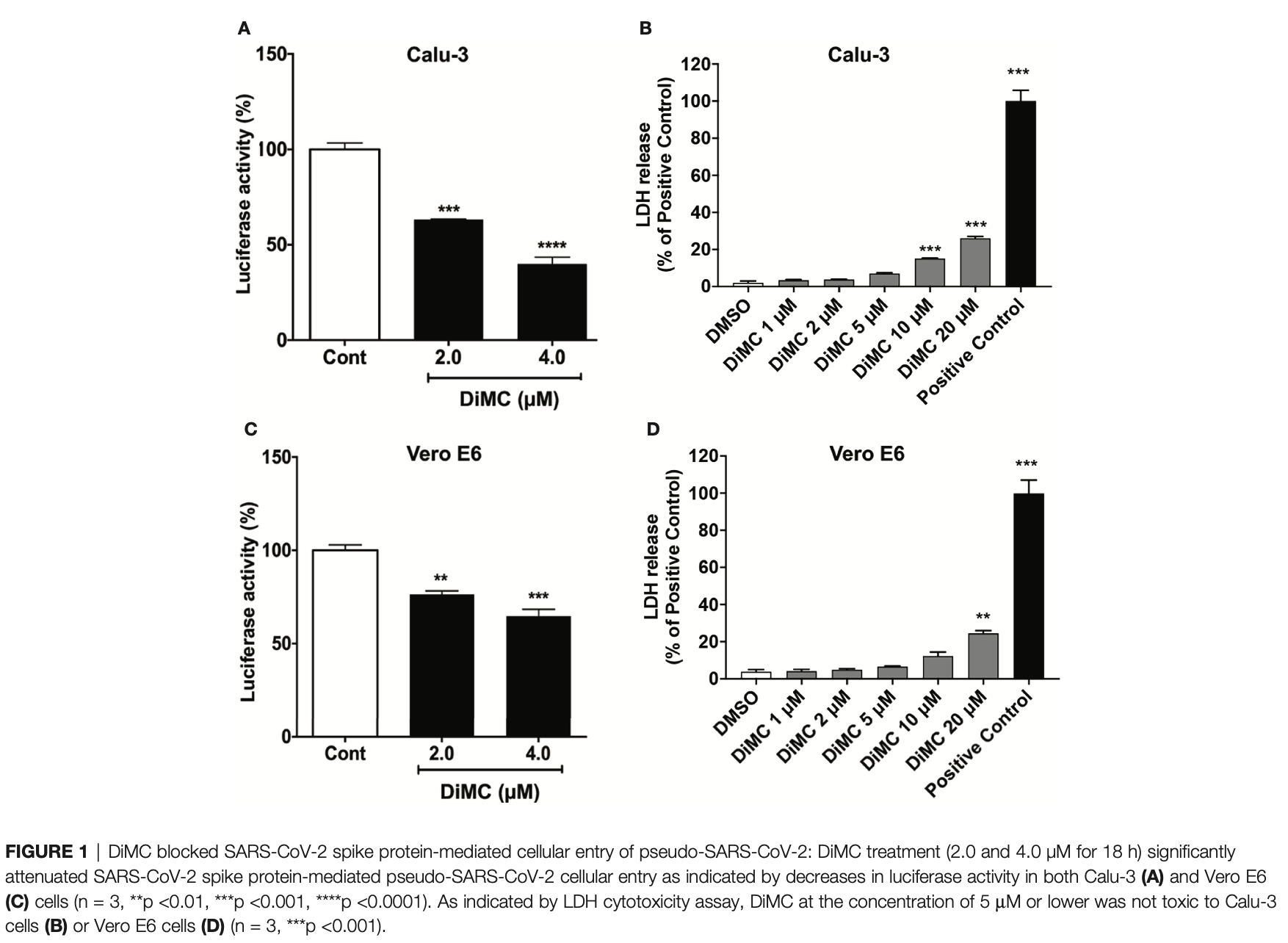
In vitro study showing that dimethoxycurcumin (DiMC), a methylated analog of curcumin, blocked the entry of SARS-CoV-2 pseudovirus into human lung Calu-3 cells and monkey kidney Vero E6 cells at 2-4μM concentrations. DiMC acidified endolysosomes, enhanced lysosome degradation, and promoted the degradation of the ACE2 receptor as well as internalized SARS-CoV-2 pseudovirus and S1 spike proteins. The lysosome acidifying agents ML-SA1 and NS1619 also blocked pseudovirus entry.
62 preclinical studies support the efficacy of curcumin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2 with curcumin or metabolites via binding to the spikeA,1,5,6,11,16,18,24,27 (and specifically the receptor binding domainB,2,4,14,17,20 ), MproC,4-6,11,13,15-17,19,20,22,25,27,28,30,48 , RNA-dependent RNA polymeraseD,4-6,17,26 , PLproE,6, ACE2F,2,18,19,21 , nucleocapsidG,12,29 , nsp10H,29, and helicaseI,36 proteins, and inhibition of spike-ACE2 interactionJ,3.
In vitro studies demonstrate inhibition of the spikeA,41 (and specifically the receptor binding domainB,51), MproC,23,41,48,50 , ACE2F,51, and TMPRSS2K,51 proteins, and inhibition of spike-ACE2 interactionJ,3,34 .
In vitro studies demonstrate efficacy in Calu-3L,49, A549M,41, A549-ATN,31, 293TO,7, HEK293-hACE2P,23,39 , 293T/hACE2/TMPRSS2Q,40, Vero E6R,1,13,17,27,39,41,43,45,47,49 , and SH-SY5YS,38 cells.
Curcumin decreases pro-inflammatory cytokines induced by SARS-CoV-2 in peripheral blood mononuclear cells47, alleviates SARS-CoV-2 spike protein-induced mitochondrial membrane damage and oxidative stress7, may limit COVID-19 induced cardiac damage by inhibiting the NF-κB signaling pathway which mediates the profibrotic effects of the SARS-CoV-2 spike protein on cardiac fibroblasts35, is predicted to inhibit the interaction between the SARS-CoV-2 spike protein receptor binding domain and the human ACE2 receptor for the delta and omicron variants14, lowers ACE2 and STAT3, curbing lung inflammation and ARDS in preclinical COVID-19 models32, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity42, has direct virucidal action by disrupting viral envelope integrity44, may inhibit viral replication and modulate inflammatory pathways like NF-κB via SIRT1 activation52, and can function as a photosensitizer in photodynamic therapy to generate reactive oxygen species that damage the virus44.
1.
Marzouk et al., Computational and Experimental Insights into the Antiviral Mechanism of Turmeric (Curcuma longa) against SARS-CoV-2 D614G, BIO Web of Conferences, doi:10.1051/bioconf/202519804002.
2.
Wu et al., Utilizing natural compounds as ligands to disrupt the binding of SARS-CoV-2 receptor-binding domain to angiotensin-converting enzyme 2, impeding viral infection, Phytochemistry Letters, doi:10.1016/j.phytol.2025.102999.
3.
Najimi et al., Phytochemical Inhibitors of SARS‐CoV‐2 Entry: Targeting the ACE2‐RBD Interaction with l‐Tartaric Acid, l‐Ascorbic Acid, and Curcuma longa Extract, ChemistrySelect, doi:10.1002/slct.202406035.
4.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
5.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
6.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
7.
Zhang et al., Computational Discovery of Mitochondrial Dysfunction Biomarkers in Severe SARS-CoV-2 Infection: Facilitating Pytomedicine Screening, Phytomedicine, doi:10.1016/j.phymed.2024.155784.
8.
Öztürkkan et al., In Silico investigation of the effects of curcuminoids on the spike protein of the omicron variant of SARS-CoV-2, Baku State University Journal of Chemistry and Material Sciences, 1:2, bsuj.bsu.edu.az/uploads/pdf/ec4204d62f7802de54e6092bf7860029.pdf.
9.
Yunze et al., Therapeutic effect and potential mechanism of curcumin, an active ingredient in Tongnao Decoction, on COVID-19 combined with stroke: a network pharmacology study and GEO database mining, Research Square, doi:10.21203/rs.3.rs-4329762/v1.
10.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
11.
Boseila et al., Throat spray formulated with virucidal Pharmaceutical excipients as an effective early prophylactic or treatment strategy against pharyngitis post-exposure to SARS CoV-2, European Journal of Pharmaceutics and Biopharmaceutics, doi:10.1016/j.ejpb.2024.114279.
12.
Hidayah et al., Bioinformatics study of curcumin, demethoxycurcumin, bisdemethoxycurcumin and cyclocurcumin compounds in Curcuma longa as an antiviral agent via nucleocapsid on SARS-CoV-2 inhibition, International Conference on Organic and Applied Chemistry, doi:10.1063/5.0197724.
13.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
14.
Kant et al., Structure-based drug discovery to identify SARS-CoV2 spike protein–ACE2 interaction inhibitors, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2300060.
15.
Naderi Beni et al., In silico studies of anti-oxidative and hot temperament-based phytochemicals as natural inhibitors of SARS-CoV-2 Mpro, PLOS ONE, doi:10.1371/journal.pone.0295014.
16.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
17.
Eleraky et al., Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2, International Journal of Nanomedicine, doi:10.2147/IJN.S423251.
18.
Singh (B) et al., Computational studies to analyze effect of curcumin inhibition on coronavirus D614G mutated spike protein, The Seybold Report, doi:10.17605/OSF.IO/TKEXJ.
19.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
20.
Srivastava et al., Paradigm of Well-Orchestrated Pharmacokinetic Properties of Curcuminoids Relative to Conventional Drugs for the Inactivation of SARS-CoV-2 Receptors: An In Silico Approach, Stresses, doi:10.3390/stresses3030043.
21.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
22.
Winih Kinasih et al., Analisis in silico interaksi senyawa kurkuminoid terhadap enzim main protease 6LU7 dari SARS-CoV-2, Duta Pharma Journal, doi:10.47701/djp.v3i1.2904.
23.
Wu (B) et al., Potential Mechanism of Curcumin and Resveratrol against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-2780614/v1.
24.
Nag et al., Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2022.105552.
25.
Rampogu et al., Molecular Docking and Molecular Dynamics Simulations Discover Curcumin Analogue as a Plausible Dual Inhibitor for SARS-CoV-2, International Journal of Molecular Sciences, doi:10.3390/ijms23031771.
26.
Singh (C) et al., Potential of turmeric-derived compounds against RNA-dependent RNA polymerase of SARS-CoV-2: An in-silico approach, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2021.104965.
27.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
28.
Rajagopal et al., Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x.
29.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
30.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
31.
Grüneberg et al., Dose-dependent antiviral effects of glycyrrhizin, curcumin, and harmaline against clinical SARS-CoV-2 isolates, including D614G, Omicron BA.5, and Omicron XBB.1, BMC Complementary Medicine and Therapies, doi:10.1186/s12906-026-05253-1.
32.
Aktay et al., Oral Administration of Water-Soluble Curcumin Complex Prevents ARDS With the Potential for COVID-19 Treatment, Phytotherapy Research, doi:10.1002/ptr.70046.
33.
Olubiyi et al., Novel dietary herbal preparations with inhibitory activities against multiple SARS-CoV-2 targets: A multidisciplinary investigation into antiviral activities, Food Chemistry Advances, doi:10.1016/j.focha.2025.100969.
34.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
35.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
36.
Li et al., Thermal shift assay (TSA)-based drug screening strategy for rapid discovery of inhibitors against the Nsp13 helicase of SARS-CoV-2, Animals and Zoonoses, doi:10.1016/j.azn.2024.06.001.
37.
Kamble et al., Nanoparticulate curcumin spray imparts prophylactic and therapeutic properties against SARS-CoV-2, Emergent Materials, doi:10.1007/s42247-024-00754-6.
38.
Nicoliche et al., Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-024-61662-7.
39.
Nittayananta et al., A novel film spray containing curcumin inhibits SARS-CoV-2 and influenza virus infection and enhances mucosal immunity, Virology Journal, doi:10.1186/s12985-023-02282-x.
40.
Septisetyani et al., Curcumin and turmeric extract inhibited SARS-CoV-2 pseudovirus cell entry and Spike mediated cell fusion, bioRxiv, doi:10.1101/2023.09.28.560070.
41.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
42.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
43.
Teshima et al., Antiviral activity of curcumin and its analogs selected by an artificial intelligence-supported activity prediction system in SARS-CoV-2-infected VeroE6 cells, Natural Product Research, doi:10.1080/14786419.2023.2194647.
44.
Zupin et al., Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer, Viruses, doi:10.3390/v14102132.
45.
Leka et al., In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds, Phytotherapy Research, doi:10.1002/ptr.7463.
46.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
47.
Marín-Palma et al., Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms, Molecules, doi:10.3390/molecules26226900.
48.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
49.
Bormann et al., Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro, Viruses, doi:10.3390/v13101914.
50.
Guijarro-Real et al., Potential In Vitro Inhibition of Selected Plant Extracts against SARS-CoV-2 Chymotripsin-Like Protease (3CLPro) Activity, Foods, doi:10.3390/foods10071503.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
h.
Non-structural protein 10 (nsp10) serves as an RNA chaperone and stabilizes conformations of nsp12 and nsp14 in the replicase-transcriptase complex, which synthesizes new viral RNAs. Nsp10 disruption may destabilize replicase-transcriptase complex activity.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
k.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
l.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
m.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
n.
A549-AT is a human lung carcinoma cell line stably transfected with ACE2 and TMPRSS2 receptors. Unlike the parental line, this overexpression ensures stable infection and enhanced viral entry, allowing for the evaluation of antiviral efficacy against various SARS-CoV-2 variants.
o.
293T is a human embryonic kidney cell line that can be engineered for high ACE2 expression and SARS-CoV-2 susceptibility. 293T cells are easily transfected and support high protein expression.
p.
HEK293-hACE2 is a human embryonic kidney cell line with high ACE2 expression and SARS-CoV-2 susceptibility. Cells have been transfected with a plasmid to express the human ACE2 (hACE2) protein.
q.
293T/hACE2/TMPRSS2 is a human embryonic kidney cell line engineered for high ACE2 and TMPRSS2 expression, which mimics key aspects of human infection. 293T/hACE2/TMPRSS2 cells are very susceptible to SARS-CoV-2 infection.
r.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
s.
SH-SY5Y is a human neuroblastoma cell line that exhibits neuronal phenotypes. It is commonly used as an in vitro model for studying neurotoxicity, neurodegenerative diseases, and neuronal differentiation.
Khan et al., 30 Jun 2022, Spain, peer-reviewed, 5 authors.
Contact: xuesong.chen@und.edu.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Dimethoxycurcumin Acidifies Endolysosomes and Inhibits SARS-CoV-2 Entry
Frontiers in Virology, doi:10.3389/fviro.2022.923018
The pandemic of coronavirus disease 2019 caused by infection by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) continues to take a huge toll on global health. Although improving, currently there are only limited therapies against SARS-CoV-2. Curcumin, a natural polyphenol, exerts antiviral effects against a wide variety of viruses and can inhibit SARS-CoV-2 entry. However, undesirable physicochemical and pharmacokinetic properties of curcumin limit its clinical application. Here, we determined the effects of dimethoxycurcumin (DiMC), a methylated analog of curcumin with improved bioavailability, on the entry of SARS-CoV-2. DiMC blocked entry of pseudo-SARS-CoV-2 into Calu-3 human non-small cell lung adenocarcinoma cells and Vero E6 green monkey kidney epithelial cells. Mechanistically, DiMC acidified lysosomes, enhanced lysosome degradation capabilities, and promoted lysosome degradation of angiotensin converting enzyme 2 (ACE2), a major receptor for SARS-CoV-2 entry, as well as pseudo-SARS-CoV-2 and the SARS-CoV-2 S1 protein. Furthermore, other lysosome acidifying agents, including the TRPML1 agonist ML-SA1 and the BK channel activator NS1619, also blocked the entry of pseudo-SARS-CoV-2. Thus, the anti-SARS-CoV-2 potential of DiMC and lysosome acidifying agents might be explored further as possible effective therapeutic strategies against COVID-19.
AUTHOR CONTRIBUTIONS NK and XC designed the research. NK performed all the experiments, analyzed the data, and drafted the manuscript. ZA analyzed data. AB performed immunoblotting. XC and JG wrote the manuscript.
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher's Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abella, Jolkovsky, Biney, Uspal, Hyman et al., and Safety of Hydroxychloroquine vs Placebo for Pre-Exposure SARS-CoV-2 Prophylaxis Among Health Care Workers: A Randomized Clinical Trial, JAMA Intern Med, doi:10.1001/jamainternmed.2020.6319
Anderson, Vanslyke, Thulin, Jean, Thomas, Activation of the Furin Endoprotease Is a Multiple-Step Process: Requirements for Acidification and Internal Propeptide Cleavage, EMBO J, doi:10.1093/emboj/16.7.1508
Arabi, Gordon, Derde, Nichol, Murthy et al., Lopinavir-Ritonavir and Hydroxychloroquine for Critically Ill Patients With COVID-19: REMAP-CAP Randomized Controlled Trial, Intensive Care Med, doi:10.1007/s00134-021-06448-5
Badawi, Ali, ACE2 Nascence, Trafficking, and SARS-CoV-2 Pathogenesis: The Saga Continues, Hum Genomics, doi:10.1186/s40246-021-00304-9
Barnabas, Brown, Bershteyn, Karita, Johnston et al., Hydroxychloroquine as Postexposure Prophylaxis to Prevent Severe Acute Respiratory Syndrome Coronavirus 2 Infection : A Randomized Trial, Ann Intern Med, doi:10.7326/M20-6519
Bayati, Kumar, Francis, Mcpherson, SARS-CoV-2 Infects Cells After Viral Entry via Clathrin-Mediated Endocytosis, J Biol Chem, doi:10.1016/j.jbc.2021.100306
Boechat, Chora, Morais, Delgado, The Immune Response to SARS-CoV-2 and COVID-19 Immunopathology -Current Perspectives, Pulmonology, doi:10.1016/j.pulmoe.2021.03.008
Chen, Geiger, Janus Sword Actions of Chloroquine and Hydroxychloroquine Against COVID-19, Cell Signal, doi:10.1016/j.cellsig.2020.109706
Chia, Gasnereau, Lieu, Gleeson, Rab9-Dependent Retrograde Transport and Endosomal Sorting of the Endopeptidase Furin, J Cell Sci, doi:10.1242/jcs.083782
Datta, Miller, Halcrow, Khan, Colwell et al., SARS-CoV-2 S1 Protein Induces Endolysosome Dysfunction and Neuritic Dystrophy, Front Cell Neurosci, doi:10.3389/fncel.2021.777738
De Duve, The Lysosome Turns Fifty, Nat Cell Biol, doi:10.1038/ncb0905-847
Ghosh, Dellibovi-Ragheb, Kerviel, Pak, Qiu et al., Beta-Coronaviruses Use Lysosomes for Egress Instead of the Biosynthetic Secretory Pathway, Cell, doi:10.1016/j.cell.2020.10.039
Goc, Sumera, Rath, Niedzwiecki, Phenolic Compounds Disrupt Spike-Mediated Receptor-Binding and Entry of SARS-CoV-2 Pseudo-Virions, PloS One, doi:10.1371/journal.pone.0253489
Hamming, Timens, Bulthuis, Lely, Navis et al., Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis, J Pathol, doi:10.1002/path.1570
Hoffmann, Kleine-Weber, Pohlmann, A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells, Mol Cell, doi:10.1016/j.molcel.2020.04.022
Hoffmann, Kleine-Weber, Schroeder, Kruger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hoffmann, Mosbauer, Hofmann-Winkler, Kaul, Kleine-Weber et al., Chloroquine Does Not Inhibit Infection of Human Lung Cells With SARS-CoV-2, Nature, doi:10.1038/s41586-020-2575-3
Huotari, Helenius, Endosome Maturation, EMBO J, doi:10.1038/emboj.2011.286
Jena, Kanungo, Nayak, Chainy, Dandapat, Catechin and Curcumin Interact With S Protein of SARS-CoV2 and ACE2 of Human Cell Membrane: Insights From Computational Studies, Sci Rep, doi:10.1038/s41598-021-81462-7
Khan, Lakpa, Halcrow, Afghah, Miller et al., BK Channels Regulate Extracellular Tat-Mediated HIV-1 LTR Transactivation, Sci Rep, doi:10.1038/s41598-019-48777-y
Koch, Uckeley, Doldan, Stanifer, Boulant et al., TMPRSS2 Expression Dictates the Entry Route Used by SARS-CoV-2 to Infect Host Cells, EMBO J, doi:10.15252/embj.2021107821
Leon, Michelson, Olejnik, Chowdhary, Oh et al., A Virus-Specific Monocyte Inflammatory Phenotype is Induced by SARS-CoV-2 at the Immune-Epithelial Interface, Proc Natl Acad Sci, doi:10.1073/pnas.2116853118
Liu, Cao, Xu, Wang, Zhang et al., Hydroxychloroquine, a Less Toxic Derivative of Chloroquine, is Effective in Inhibiting SARS-CoV-2 Infection In Vitro, Cell Discovery, doi:10.1038/s41421-020-0156-0
Mcguire, Stransky, Cotter, Forgac, Regulation of V-ATPase Activity, Front Biosci, doi:10.2741/4506
Mindell, Lysosomal Acidification Mechanisms, Annu Rev Physiol, doi:10.1146/annurev-physiol-012110-142317
Mitja, Corbacho-Monne, Ubals, Alemany, Suner et al., A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021801
Moustapha, Peretout, Rainey, Sureau, Geze et al., Curcumin Induces Crosstalk Between Autophagy and Apoptosis Mediated by Calcium Release From the Endoplasmic Reticulum, Lysosomal Destabilization and Mitochondrial Events, Cell Death Discovery, doi:10.1038/cddiscovery.2015.17
Ohkuma, Poole, Cytoplasmic Vacuolation of Mouse Peritoneal Macrophages and the Uptake Into Lysosomes of Weakly Basic Substances, J Cell Biol, doi:10.1083/jcb.90.3.656
Ou, Liu, Lei, Li, Mi et al., Characterization of Spike Glycoprotein of SARS-CoV-2 on Virus Entry and its Immune Cross-Reactivity With SARS-CoV, Nat Commun, doi:10.1038/s41467-020-15562-9
Pae, Jeong, Kim, Kim, Song et al., Dimethoxycurcumin, A Synthetic Curcumin Analogue With Higher Metabolic Stability, Inhibits NO Production, Inducible NO Synthase Expression and NF-kappaB Activation in RAW264.7 Macrophages Activated With LPS, Mol Nutr Food Res, doi:10.1002/mnfr.200700333
Patel, Rajendran, Shah, Patel, Pakala et al., Virtual Screening of Curcumin and its Analogs Against the Spike Surface Glycoprotein of SARS-CoV-2 and SARS-CoV, J Biomol Struct Dyn, doi:10.1080/07391102.2020.1868338
Patwardhan, Checker, Sharma, Kohli, Priyadarsini et al., Dimethoxycurcumin, a Metabolically Stable Analogue of Curcumin, Exhibits Anti-Inflammatory Activities in Murine and Human Lymphocytes, Biochem Pharmacol, doi:10.1016/j.bcp.2011.06.024
Peng, Wu, Wang, Qi, Gao, Cell Entry by SARS-CoV-2, Trends Biochem Sci, doi:10.1016/j.tibs.2021.06.001
Prasad, Rao, Histone Deacetylase-Mediated Regulation of Endolysosomal pH, J Biol Chem, doi:10.1074/jbc.RA118.002025
Rattis, Ramos, Celes, Curcumin as a Potential Treatment for COVID-19, Front Pharmacol, doi:10.3389/fphar.2021.675287
Schaefer, Jung, Hummer, Binding of SARS-CoV-2 Fusion Peptide to Host Endosome and Plasma Membrane, J Phys Chem B, doi:10.1021/acs.jpcb.1c04176
Shang, Wan, Luo, Ye, Geng et al., Cell Entry Mechanisms of SARS-CoV-2, Proc Natl Acad Sci, doi:10.1073/pnas.2003138117
Sharifi-Rad, Rayess, Rizk, Sadaka, Zgheib et al., Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications, Front Pharmacol, doi:10.3389/fphar.2020.01021
Shereen, Khan, Kazmi, Bashir, Siddique, COVID-19 Infection: Origin, Transmission, and Characteristics of Human Coronaviruses, J Adv Res, doi:10.1016/j.jare.2020.03.005
Simon, Aswini, Kumar, Mankadath, Curcumin and Its Synthetic Analogue Dimethoxycurcumin Differentially Modulates Antioxidant Status of Normal Human Peripheral Blood Mononuclear Cells, Free Radic Res, doi:10.1080/10715762.2018.1455002
Sivapalan, Ulrik, Lapperre, Bojesen, Eklof et al., Azithromycin and Hydroxychloroquine in Hospitalised Patients With Confirmed COVID-19: A Randomised Double-Blinded Placebo-Controlled Trial, Eur Respir J, doi:10.1183/13993003.00752-2021
Sohail, Guo, Yang, Li, Li et al., A Promising Anticancer Agent Dimethoxycurcumin: Aspects of Pharmacokinetics, Efficacy, Mechanism, and Nanoformulation for Drug Delivery, Front Pharmacol, doi:10.3389/fphar.2021.665387
Song, Malampati, Zeng, Durairajan, Yang et al., A Small Molecule Transcription Factor EB Activator Ameliorates Beta-Amyloid Precursor Protein and Tau Pathology in Alzheimer's Disease Models, Aging Cell, doi:10.1111/acel.13069
Song, Sun, Peluso, Zeng, Yu et al., A Novel Curcumin Analog Binds to and Activates TFEB In Vitro and In Vivo Independent of MTOR Inhibition, Autophagy, doi:10.1080/15548627.2016.1179404
Tamvakopoulos, Dimas, Sofianos, Hatziantoniou, Han et al., Metabolism and Anticancer Activity of the Curcumin Analogue, Dimethoxycurcumin, Clin Cancer Res, doi:10.1158/1078-0432.CCR-06-1839
Tang, Bidon, Jaimes, Whittaker, Daniel, Coronavirus Membrane Fusion Mechanism Offers a Potential Target for Antiviral Development, Antiviral Res, doi:10.1016/j.antiviral.2020.104792
Teymouri, Barati, Pirro, Sahebkar, Biological and Pharmacological Evaluation of Dimethoxycurcumin: A Metabolically Stable Curcumin Analogue With a Promising Therapeutic Potential, J Cell Physiol, doi:10.1002/jcp.25749
Turk, Dolenc, Lenarcic, Krizaj, Turk et al., Acidic pH as a Physiological Regulator of Human Cathepsin L Activity, Eur J Biochem, doi:10.1046/j.1432-1327.1999.00145.x
Walls, Xiong, Park, Tortorici, Snijder et al., Unexpected Receptor Functional Mimicry Elucidates Activation of Coronavirus Fusion, Cell, doi:10.1016/j.cell.2018.12.028
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus 2019-Ncov) In Vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wang, Horby, Hayden, Gao, A Novel Coronavirus Outbreak of Global Health Concern, Lancet, doi:10.1016/S0140-6736(20)30185-9
Wrapp, Wang, Corbett, Goldsmith, Hsieh et al., Cryo-EM Structure of the 2019-Ncov Spike in the Prefusion Conformation, Science, doi:10.1126/science.abb2507
Xia, Zhu, Liu, Lan, Xu et al., Fusion Mechanism of 2019-Ncov and Fusion Inhibitors Targeting HR1 Domain in Spike Protein, Cell Mol Immunol, doi:10.1038/s41423-020-0374-2
Yao, Ye, Zhang, Cui, Huang et al., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin Infect Dis, doi:10.1093/cid/ciaa237
Yoon, Kang, Lee, Kim, Kim et al., Stronger Proteasomal Inhibition and Higher CHOP Induction are Responsible for More Effective Induction of Paraptosis by Dimethoxycurcumin Than Curcumin, Cell Death Dis, doi:10.1038/cddis.2014.85
Zhang, Wang, Xu, Lu, Jiang et al., Curcumin Targets the TFEB-Lysosome Pathway for Induction of Autophagy, Oncotarget, doi:10.18632/oncotarget.12318
DOI record:
{
"DOI": "10.3389/fviro.2022.923018",
"ISSN": [
"2673-818X"
],
"URL": "http://dx.doi.org/10.3389/fviro.2022.923018",
"abstract": "<jats:p>The pandemic of coronavirus disease 2019 (COVID-19) caused by infection by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) continues to take a huge toll on global health. Although improving, currently there are only limited therapies against SARS-CoV-2. Curcumin, a natural polyphenol, exerts antiviral effects against a wide variety of viruses and can inhibit SARS-CoV-2 entry. However, undesirable physicochemical and pharmacokinetic properties of curcumin limit its clinical application. Here, we determined the effects of dimethoxycurcumin (DiMC), a methylated analog of curcumin with improved bioavailability, on the entry of SARS-CoV-2. DiMC blocked entry of pseudo-SARS-CoV-2 into Calu-3 human non-small cell lung adenocarcinoma cells and Vero E6 green monkey kidney epithelial cells. Mechanistically, DiMC acidified lysosomes, enhanced lysosome degradation capabilities, and promoted lysosome degradation of angiotensin converting enzyme 2 (ACE2), a major receptor for SARS-CoV-2 entry, as well as pseudo-SARS-CoV-2 and the SARS-CoV-2 S1 protein. Furthermore, other lysosome acidifying agents, including the TRPML1 agonist ML-SA1 and the BK channel activator NS1619, also blocked the entry of pseudo-SARS-CoV-2. Thus, the anti-SARS-CoV-2 potential of DiMC and lysosome acidifying agents might be explored further as possible effective therapeutic strategies against COVID-19.</jats:p>",
"alternative-id": [
"10.3389/fviro.2022.923018"
],
"author": [
{
"affiliation": [],
"family": "Khan",
"given": "Nabab",
"sequence": "first"
},
{
"affiliation": [],
"family": "Afghah",
"given": "Zahra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baral",
"given": "Aparajita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Geiger",
"given": "Jonathan D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Xuesong",
"sequence": "additional"
}
],
"container-title": "Frontiers in Virology",
"container-title-short": "Front. Virol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
6,
30
]
],
"date-time": "2022-06-30T09:21:52Z",
"timestamp": 1656580912000
},
"deposited": {
"date-parts": [
[
2022,
6,
30
]
],
"date-time": "2022-06-30T09:21:56Z",
"timestamp": 1656580916000
},
"funder": [
{
"DOI": "10.13039/100000057",
"award": [
"P30GM100329, U54GM115458"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000057",
"id-type": "DOI"
}
],
"name": "National Institute of General Medical Sciences"
},
{
"DOI": "10.13039/100000025",
"award": [
"R01MH100972, R01MH105329, R01MH119000"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000025",
"id-type": "DOI"
}
],
"name": "National Institute of Mental Health"
},
{
"DOI": "10.13039/100000065",
"award": [
"2R01NS065957"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000065",
"id-type": "DOI"
}
],
"name": "National Institute of Neurological Disorders and Stroke"
},
{
"DOI": "10.13039/100000026",
"award": [
"2R01DA032444"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000026",
"id-type": "DOI"
}
],
"name": "National Institute on Drug Abuse"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
15
]
],
"date-time": "2024-09-15T21:55:52Z",
"timestamp": 1726437352299
},
"is-referenced-by-count": 4,
"issued": {
"date-parts": [
[
2022,
6,
30
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
30
]
],
"date-time": "2022-06-30T00:00:00Z",
"timestamp": 1656547200000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fviro.2022.923018/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
6,
30
]
]
},
"published-online": {
"date-parts": [
[
2022,
6,
30
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1016/j.jare.2020.03.005",
"article-title": "COVID-19 Infection: Origin, Transmission, and Characteristics of Human Coronaviruses",
"author": "Shereen",
"doi-asserted-by": "publisher",
"journal-title": "J Adv Res",
"key": "B1",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30185-9",
"article-title": "A Novel Coronavirus Outbreak of Global Health Concern",
"author": "Wang",
"doi-asserted-by": "publisher",
"journal-title": "Lancet",
"key": "B2",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-15562-9",
"article-title": "Characterization of Spike Glycoprotein of SARS-CoV-2 on Virus Entry and its Immune Cross-Reactivity With SARS-CoV",
"author": "Ou",
"doi-asserted-by": "publisher",
"first-page": "1620",
"journal-title": "Nat Commun",
"key": "B3",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2003138117",
"article-title": "Cell Entry Mechanisms of SARS-CoV-2",
"author": "Shang",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci USA",
"key": "B4",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1016/j.jbc.2021.100306",
"article-title": "SARS-CoV-2 Infects Cells After Viral Entry via Clathrin-Mediated Endocytosis",
"author": "Bayati",
"doi-asserted-by": "publisher",
"first-page": "100306",
"journal-title": "J Biol Chem",
"key": "B5",
"volume": "296",
"year": "2021"
},
{
"DOI": "10.1016/j.tibs.2021.06.001",
"article-title": "Cell Entry by SARS-CoV-2",
"author": "Peng",
"doi-asserted-by": "publisher",
"journal-title": "Trends Biochem Sci",
"key": "B6",
"volume": "46",
"year": "2021"
},
{
"DOI": "10.1038/s41423-020-0374-2",
"article-title": "Fusion Mechanism of 2019-Ncov and Fusion Inhibitors Targeting HR1 Domain in Spike Protein",
"author": "Xia",
"doi-asserted-by": "publisher",
"journal-title": "Cell Mol Immunol",
"key": "B7",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1021/acs.jpcb.1c04176",
"article-title": "Binding of SARS-CoV-2 Fusion Peptide to Host Endosome and Plasma Membrane",
"author": "Schaefer",
"doi-asserted-by": "publisher",
"journal-title": "J Phys Chem B",
"key": "B8",
"volume": "125",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.675287",
"article-title": "Curcumin as a Potential Treatment for COVID-19",
"author": "Rattis",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "B9",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-81462-7",
"article-title": "Catechin and Curcumin Interact With S Protein of SARS-CoV2 and ACE2 of Human Cell Membrane: Insights From Computational Studies",
"author": "Jena",
"doi-asserted-by": "publisher",
"first-page": "2043",
"journal-title": "Sci Rep",
"key": "B10",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1080/07391102.2020.1868338",
"article-title": "Virtual Screening of Curcumin and its Analogs Against the Spike Surface Glycoprotein of SARS-CoV-2 and SARS-CoV",
"author": "Patel",
"doi-asserted-by": "publisher",
"journal-title": "J Biomol Struct Dyn",
"key": "B11",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0253489",
"article-title": "Phenolic Compounds Disrupt Spike-Mediated Receptor-Binding and Entry of SARS-CoV-2 Pseudo-Virions",
"author": "Goc",
"doi-asserted-by": "publisher",
"journal-title": "PloS One",
"key": "B12",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1002/jcp.25749",
"article-title": "Biological and Pharmacological Evaluation of Dimethoxycurcumin: A Metabolically Stable Curcumin Analogue With a Promising Therapeutic Potential",
"author": "Teymouri",
"doi-asserted-by": "publisher",
"journal-title": "J Cell Physiol",
"key": "B13",
"volume": "233",
"year": "2018"
},
{
"DOI": "10.3389/fphar.2020.01021",
"article-title": "Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications",
"author": "Sharifi-Rad",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "B14",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2021.665387",
"article-title": "A Promising Anticancer Agent Dimethoxycurcumin: Aspects of Pharmacokinetics, Efficacy, Mechanism, and Nanoformulation for Drug Delivery",
"author": "Sohail",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "B15",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1080/10715762.2018.1455002",
"article-title": "Curcumin and Its Synthetic Analogue Dimethoxycurcumin Differentially Modulates Antioxidant Status of Normal Human Peripheral Blood Mononuclear Cells",
"author": "Simon",
"doi-asserted-by": "publisher",
"journal-title": "Free Radic Res",
"key": "B16",
"volume": "52",
"year": "2018"
},
{
"DOI": "10.1016/j.bcp.2011.06.024",
"article-title": "Dimethoxycurcumin, a Metabolically Stable Analogue of Curcumin, Exhibits Anti-Inflammatory Activities in Murine and Human Lymphocytes",
"author": "Patwardhan",
"doi-asserted-by": "publisher",
"journal-title": "Biochem Pharmacol",
"key": "B17",
"volume": "82",
"year": "2011"
},
{
"DOI": "10.1158/1078-0432.CCR-06-1839",
"article-title": "Metabolism and Anticancer Activity of the Curcumin Analogue, Dimethoxycurcumin",
"author": "Tamvakopoulos",
"doi-asserted-by": "publisher",
"journal-title": "Clin Cancer Res",
"key": "B18",
"volume": "13",
"year": "2007"
},
{
"DOI": "10.1038/cddis.2014.85",
"article-title": "Stronger Proteasomal Inhibition and Higher CHOP Induction are Responsible for More Effective Induction of Paraptosis by Dimethoxycurcumin Than Curcumin",
"author": "Yoon",
"doi-asserted-by": "publisher",
"journal-title": "Cell Death Dis",
"key": "B19",
"volume": "5",
"year": "2014"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"journal-title": "Cell",
"key": "B20",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2575-3",
"article-title": "Chloroquine Does Not Inhibit Infection of Human Lung Cells With SARS-CoV-2",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B21",
"volume": "585",
"year": "2020"
},
{
"DOI": "10.1186/s40246-021-00304-9",
"article-title": "ACE2 Nascence, Trafficking, and SARS-CoV-2 Pathogenesis: The Saga Continues",
"author": "Badawi",
"doi-asserted-by": "publisher",
"first-page": "8",
"journal-title": "Hum Genomics",
"key": "B22",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1038/cddiscovery.2015.17",
"article-title": "Curcumin Induces Crosstalk Between Autophagy and Apoptosis Mediated by Calcium Release From the Endoplasmic Reticulum, Lysosomal Destabilization and Mitochondrial Events",
"author": "Moustapha",
"doi-asserted-by": "publisher",
"first-page": "15017",
"journal-title": "Cell Death Discovery",
"key": "B23",
"volume": "1",
"year": "2015"
},
{
"DOI": "10.18632/oncotarget.12318",
"article-title": "Curcumin Targets the TFEB-Lysosome Pathway for Induction of Autophagy",
"author": "Zhang",
"doi-asserted-by": "publisher",
"journal-title": "Oncotarget",
"key": "B24",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1080/15548627.2016.1179404",
"article-title": "A Novel Curcumin Analog Binds to and Activates TFEB In Vitro and In Vivo Independent of MTOR Inhibition",
"author": "Song",
"doi-asserted-by": "publisher",
"journal-title": "Autophagy",
"key": "B25",
"volume": "12",
"year": "2016"
},
{
"DOI": "10.3389/fncel.2021.777738",
"article-title": "SARS-CoV-2 S1 Protein Induces Endolysosome Dysfunction and Neuritic Dystrophy",
"author": "Datta",
"doi-asserted-by": "publisher",
"journal-title": "Front Cell Neurosci",
"key": "B26",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1038/s41598-019-48777-y",
"article-title": "BK Channels Regulate Extracellular Tat-Mediated HIV-1 LTR Transactivation",
"author": "Khan",
"doi-asserted-by": "publisher",
"first-page": "12285",
"journal-title": "Sci Rep",
"key": "B27",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1038/emboj.2011.286",
"article-title": "Endosome Maturation",
"author": "Huotari",
"doi-asserted-by": "publisher",
"journal-title": "EMBO J",
"key": "B28",
"volume": "30",
"year": "2011"
},
{
"DOI": "10.1146/annurev-physiol-012110-142317",
"article-title": "Lysosomal Acidification Mechanisms",
"author": "Mindell",
"doi-asserted-by": "publisher",
"first-page": "69",
"journal-title": "Annu Rev Physiol",
"key": "B29",
"volume": "74",
"year": "2012"
},
{
"DOI": "10.2741/4506",
"article-title": "Regulation of V-ATPase Activity",
"author": "Mcguire",
"doi-asserted-by": "publisher",
"journal-title": "Front Biosci (Landmark Ed)",
"key": "B30",
"volume": "22",
"year": "2017"
},
{
"DOI": "10.1074/jbc.RA118.002025",
"article-title": "Histone Deacetylase-Mediated Regulation of Endolysosomal pH",
"author": "Prasad",
"doi-asserted-by": "publisher",
"journal-title": "J Biol Chem",
"key": "B31",
"volume": "293",
"year": "2018"
},
{
"DOI": "10.1038/ncb0905-847",
"article-title": "The Lysosome Turns Fifty",
"author": "De Duve",
"doi-asserted-by": "publisher",
"journal-title": "Nat Cell Biol",
"key": "B32",
"volume": "7",
"year": "2005"
},
{
"DOI": "10.15252/embj.2021107821",
"article-title": "TMPRSS2 Expression Dictates the Entry Route Used by SARS-CoV-2 to Infect Host Cells",
"author": "Koch",
"doi-asserted-by": "publisher",
"first-page": "e107821",
"journal-title": "EMBO J",
"key": "B33",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.10.039",
"article-title": "Beta-Coronaviruses Use Lysosomes for Egress Instead of the Biosynthetic Secretory Pathway",
"author": "Ghosh",
"doi-asserted-by": "publisher",
"first-page": "1520",
"journal-title": "Cell",
"key": "B34",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1126/science.abb2507",
"article-title": "Cryo-EM Structure of the 2019-Ncov Spike in the Prefusion Conformation",
"author": "Wrapp",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B35",
"volume": "367",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2018.12.028",
"article-title": "Unexpected Receptor Functional Mimicry Elucidates Activation of Coronavirus Fusion",
"author": "Walls",
"doi-asserted-by": "publisher",
"first-page": "1026",
"journal-title": "Cell",
"key": "B36",
"volume": "176",
"year": "2019"
},
{
"DOI": "10.1002/path.1570",
"article-title": "Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis",
"author": "Hamming",
"doi-asserted-by": "publisher",
"journal-title": "J Pathol",
"key": "B37",
"volume": "203",
"year": "2004"
},
{
"DOI": "10.1016/j.molcel.2020.04.022",
"article-title": "A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "779",
"journal-title": "Mol Cell",
"key": "B38",
"volume": "78",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104792",
"article-title": "Coronavirus Membrane Fusion Mechanism Offers a Potential Target for Antiviral Development",
"author": "Tang",
"doi-asserted-by": "publisher",
"first-page": "104792",
"journal-title": "Antiviral Res",
"key": "B39",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1093/emboj/16.7.1508",
"article-title": "Activation of the Furin Endoprotease Is a Multiple-Step Process: Requirements for Acidification and Internal Propeptide Cleavage",
"author": "Anderson",
"doi-asserted-by": "publisher",
"journal-title": "EMBO J",
"key": "B40",
"volume": "16",
"year": "1997"
},
{
"DOI": "10.1242/jcs.083782",
"article-title": "Rab9-Dependent Retrograde Transport and Endosomal Sorting of the Endopeptidase Furin",
"author": "Chia",
"doi-asserted-by": "publisher",
"journal-title": "J Cell Sci",
"key": "B41",
"volume": "124",
"year": "2011"
},
{
"DOI": "10.1046/j.1432-1327.1999.00145.x",
"article-title": "Acidic pH as a Physiological Regulator of Human Cathepsin L Activity",
"author": "Turk",
"doi-asserted-by": "publisher",
"journal-title": "Eur J Biochem",
"key": "B42",
"volume": "259",
"year": "1999"
},
{
"DOI": "10.1083/jcb.90.3.656",
"article-title": "Cytoplasmic Vacuolation of Mouse Peritoneal Macrophages and the Uptake Into Lysosomes of Weakly Basic Substances",
"author": "Ohkuma",
"doi-asserted-by": "publisher",
"journal-title": "J Cell Biol",
"key": "B43",
"volume": "90",
"year": "1981"
},
{
"DOI": "10.1016/j.cellsig.2020.109706",
"article-title": "Janus Sword Actions of Chloroquine and Hydroxychloroquine Against COVID-19",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "109706",
"journal-title": "Cell Signal",
"key": "B44",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"article-title": "Hydroxychloroquine, a Less Toxic Derivative of Chloroquine, is Effective in Inhibiting SARS-CoV-2 Infection In Vitro",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "16",
"journal-title": "Cell Discovery",
"key": "B45",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus 2019-Ncov) In Vitro",
"author": "Wang",
"doi-asserted-by": "publisher",
"journal-title": "Cell Res",
"key": "B46",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa237",
"article-title": "In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)",
"author": "Yao",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "B47",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.6319",
"article-title": "Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-Exposure SARS-CoV-2 Prophylaxis Among Health Care Workers: A Randomized Clinical Trial",
"author": "Abella",
"doi-asserted-by": "publisher",
"first-page": "195",
"journal-title": "JAMA Intern Med",
"key": "B48",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021801",
"article-title": "A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of Covid-19",
"author": "Mitja",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B49",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1183/13993003.00752-2021",
"article-title": "Azithromycin and Hydroxychloroquine in Hospitalised Patients With Confirmed COVID-19: A Randomised Double-Blinded Placebo-Controlled Trial",
"author": "Sivapalan",
"doi-asserted-by": "publisher",
"first-page": "2100752",
"journal-title": "Eur Respir J",
"key": "B50",
"volume": "59",
"year": "2022"
},
{
"DOI": "10.1007/s00134-021-06448-5",
"article-title": "Lopinavir-Ritonavir and Hydroxychloroquine for Critically Ill Patients With COVID-19: REMAP-CAP Randomized Controlled Trial",
"author": "Arabi",
"doi-asserted-by": "publisher",
"journal-title": "Intensive Care Med",
"key": "B51",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.7326/M20-6519",
"article-title": "Hydroxychloroquine as Postexposure Prophylaxis to Prevent Severe Acute Respiratory Syndrome Coronavirus 2 Infection : A Randomized Trial",
"author": "Barnabas",
"doi-asserted-by": "publisher",
"journal-title": "Ann Intern Med",
"key": "B52",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1002/mnfr.200700333",
"article-title": "Dimethoxycurcumin, A Synthetic Curcumin Analogue With Higher Metabolic Stability, Inhibits NO Production, Inducible NO Synthase Expression and NF-kappaB Activation in RAW264.7 Macrophages Activated With LPS",
"author": "Pae",
"doi-asserted-by": "publisher",
"journal-title": "Mol Nutr Food Res",
"key": "B53",
"volume": "52",
"year": "2008"
},
{
"DOI": "10.1016/j.pulmoe.2021.03.008",
"article-title": "The Immune Response to SARS-CoV-2 and COVID-19 Immunopathology - Current Perspectives",
"author": "Boechat",
"doi-asserted-by": "publisher",
"journal-title": "Pulmonology",
"key": "B54",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1073/pnas.2116853118",
"article-title": "A Virus-Specific Monocyte Inflammatory Phenotype is Induced by SARS-CoV-2 at the Immune-Epithelial Interface",
"author": "Leon",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci USA",
"key": "B55",
"volume": "119",
"year": "2022"
},
{
"DOI": "10.1111/acel.13069",
"article-title": "A Small Molecule Transcription Factor EB Activator Ameliorates Beta-Amyloid Precursor Protein and Tau Pathology in Alzheimer's Disease Models",
"author": "Song",
"doi-asserted-by": "publisher",
"journal-title": "Aging Cell",
"key": "B56",
"volume": "19",
"year": "2020"
}
],
"reference-count": 56,
"references-count": 56,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fviro.2022.923018/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Dimethoxycurcumin Acidifies Endolysosomes and Inhibits SARS-CoV-2 Entry",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "2"
}
