Moxidectin and ivermectin inhibit SARS-CoV-2 replication in Vero E6 cells but not in human primary airway epithelium cells
et al., Antimicrobial Agents and Chemotherapy, doi:10.1128/AAC.01543-21, Mar 2021 (preprint)
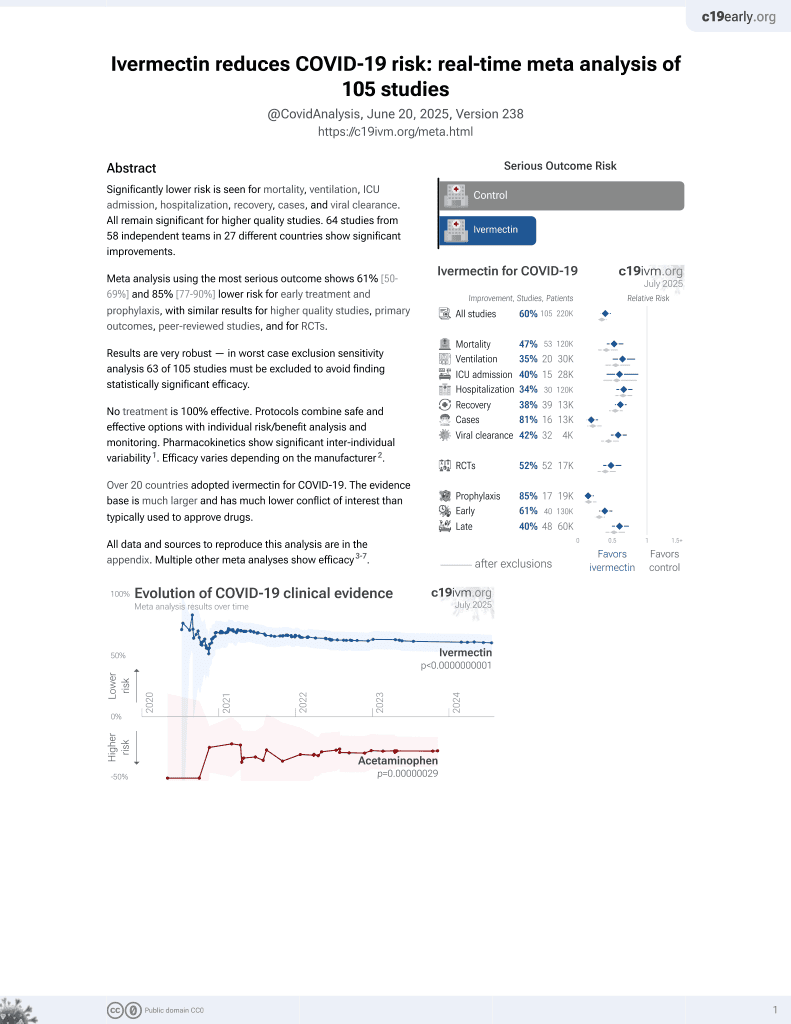
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing moxidectin and ivermectin exhibited antiviral activity in Vero E6 cells. Authors indicate that no statistically significant effect was seen in Calu-3/PBEC cells, however Figure 3 shows a dose dependent reduction with ivermectin and moxidectin, and the actual values are not provided. Calu-3 is one of many cell lines derived from human lung carcinomas1. Calu-3 cells resemble serous gland cells. They do not express 15-lipoxygenase, an enzyme specifically localized to the surface epithelium, but they do express secretory component, secretory leukocyte protease inhibitor, lysozyme, and lactoferrin, all markers of serous gland cells.1 note that the absence of systemic inflammation, circulatory factors, and other paracrine systemic influences is a potential limitation of the isolated cell system.
74 preclinical studies support the efficacy of ivermectin for COVID-19:
Ivermectin, better known for antiparasitic activity, is a broad spectrum antiviral with activity against many viruses including H7N772, Dengue38,73,74 , HIV-174, Simian virus 4075, Zika38,76,77 , West Nile77, Yellow Fever78,79, Japanese encephalitis78, Chikungunya79, Semliki Forest virus79, Human papillomavirus58, Epstein-Barr58, BK Polyomavirus80, and Sindbis virus79.
Ivermectin inhibits importin-α/β-dependent nuclear import of viral proteins72,74,75,81 , shows spike-ACE2 disruption at 1nM with microfluidic diffusional sizing39, binds to glycan sites on the SARS-CoV-2 spike protein preventing interaction with blood and epithelial cells and inhibiting hemagglutination42,82, shows dose-dependent inhibition of wildtype and omicron variants37, exhibits dose-dependent inhibition of lung injury62,67, may inhibit SARS-CoV-2 via IMPase inhibition38, may inhibit SARS-CoV-2 induced formation of fibrin clots resistant to degradation10, inhibits SARS-CoV-2 3CLpro55, may inhibit SARS-CoV-2 RdRp activity29, may minimize viral myocarditis by inhibiting NF-κB/p65-mediated inflammation in macrophages61, may be beneficial for COVID-19 ARDS by blocking GSDMD and NET formation83, may interfere with SARS-CoV-2's immune evasion via ORF8 binding5, may inhibit SARS-CoV-2 by disrupting CD147 interaction84-87, may inhibit SARS-CoV-2 attachment to lipid rafts via spike NTD binding3, shows protection against inflammation, cytokine storm, and mortality in an LPS mouse model sharing key pathological features of severe COVID-1960,88, may be beneficial in severe COVID-19 by binding IGF1 to inhibit the promotion of inflammation, fibrosis, and cell proliferation that leads to lung damage9, may minimize SARS-CoV-2 induced cardiac damage41,49, may counter immune evasion by inhibiting NSP15-TBK1/KPNA1 interaction and restoring IRF3 activation89, may disrupt SARS-CoV-2 N and ORF6 protein nuclear transport and their suppression of host interferon responses2, reduces TAZ/YAP nuclear import, relieving SARS-CoV-2-driven suppression of IRF3 and NF-κB antiviral pathways36, increases Bifidobacteria which play a key role in the immune system90, has immunomodulatory52 and anti-inflammatory71,91 properties, and has an extensive and very positive safety profile92.
2.
Gayozo et al., Binding affinities analysis of ivermectin, nucleocapsid and ORF6 proteins of SARS-CoV-2 to human importins α isoforms: A computational approach, Biotecnia, doi:10.18633/biotecnia.v27.2485.
3.
Lefebvre et al., Characterization and Fluctuations of an Ivermectin Binding Site at the Lipid Raft Interface of the N-Terminal Domain (NTD) of the Spike Protein of SARS-CoV-2 Variants, Viruses, doi:10.3390/v16121836.
4.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
5.
Bagheri-Far et al., Non-spike protein inhibition of SARS-CoV-2 by natural products through the key mediator protein ORF8, Molecular Biology Research Communications, doi:10.22099/mbrc.2024.50245.2001.
6.
de Oliveira Só et al., In Silico Comparative Analysis of Ivermectin and Nirmatrelvir Inhibitors Interacting with the SARS-CoV-2 Main Protease, Preprints, doi:10.20944/preprints202404.1825.v1.
7.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
8.
Oranu et al., Validation of the binding affinities and stabilities of ivermectin and moxidectin against SARS-CoV-2 receptors using molecular docking and molecular dynamics simulation, GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2024.26.1.0030.
9.
Zhao et al., Identification of the shared gene signatures between pulmonary fibrosis and pulmonary hypertension using bioinformatics analysis, Frontiers in Immunology, doi:10.3389/fimmu.2023.1197752.
10.
Vottero et al., Computational Prediction of the Interaction of Ivermectin with Fibrinogen, Molecular Sciences, doi:10.3390/ijms241411449.
11.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
12.
Umar et al., Inhibitory potentials of ivermectin, nafamostat, and camostat on spike protein and some nonstructural proteins of SARS-CoV-2: Virtual screening approach, Jurnal Teknologi Laboratorium, doi:10.29238/teknolabjournal.v11i1.344.
13.
Alvarado et al., Interaction of the New Inhibitor Paxlovid (PF-07321332) and Ivermectin With the Monomer of the Main Protease SARS-CoV-2: A Volumetric Study Based on Molecular Dynamics, Elastic Networks, Classical Thermodynamics and SPT, Computational Biology and Chemistry, doi:10.1016/j.compbiolchem.2022.107692.
14.
Aminpour et al., In Silico Analysis of the Multi-Targeted Mode of Action of Ivermectin and Related Compounds, Computation, doi:10.3390/computation10040051.
15.
Parvez et al., Insights from a computational analysis of the SARS-CoV-2 Omicron variant: Host–pathogen interaction, pathogenicity, and possible drug therapeutics, Immunity, Inflammation and Disease, doi:10.1002/iid3.639.
16.
Francés-Monerris et al., Microscopic interactions between ivermectin and key human and viral proteins involved in SARS-CoV-2 infection, Physical Chemistry Chemical Physics, doi:10.1039/D1CP02967C.
17.
González-Paz et al., Comparative study of the interaction of ivermectin with proteins of interest associated with SARS-CoV-2: A computational and biophysical approach, Biophysical Chemistry, doi:10.1016/j.bpc.2021.106677.
18.
González-Paz (B) et al., Structural Deformability Induced in Proteins of Potential Interest Associated with COVID-19 by binding of Homologues present in Ivermectin: Comparative Study Based in Elastic Networks Models, Journal of Molecular Liquids, doi:10.1016/j.molliq.2021.117284.
19.
Rana et al., A Computational Study of Ivermectin and Doxycycline Combination Drug Against SARS-CoV-2 Infection, Research Square, doi:10.21203/rs.3.rs-755838/v1.
20.
Muthusamy et al., Virtual Screening Reveals Potential Anti-Parasitic Drugs Inhibiting the Receptor Binding Domain of SARS-CoV-2 Spike protein, Journal of Virology & Antiviral Research, www.scitechnol.com/abstract/virtual-screening-reveals-potential-antiparasitic-drugs-inhibiting-the-receptor-binding-domain-of-sarscov2-spike-protein-16398.html.
21.
Qureshi et al., Mechanistic insights into the inhibitory activity of FDA approved ivermectin against SARS-CoV-2: old drug with new implications, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1906750.
22.
Schöning et al., Highly-transmissible Variants of SARS-CoV-2 May Be More Susceptible to Drug Therapy Than Wild Type Strains, Research Square, doi:10.21203/rs.3.rs-379291/v1.
23.
Bello et al., Elucidation of the inhibitory activity of ivermectin with host nuclear importin α and several SARS-CoV-2 targets, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1911857.
24.
Udofia et al., In silico studies of selected multi-drug targeting against 3CLpro and nsp12 RNA-dependent RNA-polymerase proteins of SARS-CoV-2 and SARS-CoV, Network Modeling Analysis in Health Informatics and Bioinformatics, doi:10.1007/s13721-021-00299-2.
25.
Choudhury et al., Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach, Future Medicine, doi:10.2217/fvl-2020-0342.
26.
Kern et al., Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing, Frontiers in Pharmacology, doi:10.3389/fphar.2021.625678.
27.
Saha et al., The Binding mechanism of ivermectin and levosalbutamol with spike protein of SARS-CoV-2, Structural Chemistry, doi:10.1007/s11224-021-01776-0.
28.
Eweas et al., Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2, Frontiers in Microbiology, doi:10.3389/fmicb.2020.592908.
29.
Parvez (B) et al., Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.09.098.
30.
Francés-Monerris (B) et al., Has Ivermectin Virus-Directed Effects against SARS-CoV-2? Rationalizing the Action of a Potential Multitarget Antiviral Agent, ChemRxiv, doi:10.26434/chemrxiv.12782258.v1.
31.
Kalhor et al., Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1824816.
32.
Swargiary, A., Ivermectin as a promising RNA-dependent RNA polymerase inhibitor and a therapeutic drug against SARS-CoV2: Evidence from in silico studies, Research Square, doi:10.21203/rs.3.rs-73308/v1.
33.
Maurya, D., A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID-19 Patients, American Chemical Society (ACS), doi:10.26434/chemrxiv.12630539.v1.
34.
Lehrer et al., Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, In Vivo, 34:5, 3023-3026, doi:10.21873/invivo.12134.
35.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
36.
Kofler et al., M-Motif, a potential non-conventional NLS in YAP/TAZ and other cellular and viral proteins that inhibits classic protein import, iScience, doi:10.1016/j.isci.2025.112105.
37.
Shahin et al., The selective effect of Ivermectin on different human coronaviruses; in-vitro study, Research Square, doi:10.21203/rs.3.rs-4180797/v1.
38.
Jitobaom et al., Identification of inositol monophosphatase as a broad‐spectrum antiviral target of ivermectin, Journal of Medical Virology, doi:10.1002/jmv.29552.
39.
Fauquet et al., Microfluidic Diffusion Sizing Applied to the Study of Natural Products and Extracts That Modulate the SARS-CoV-2 Spike RBD/ACE2 Interaction, Molecules, doi:10.3390/molecules28248072.
40.
García-Aguilar et al., In Vitro Analysis of SARS-CoV-2 Spike Protein and Ivermectin Interaction, International Journal of Molecular Sciences, doi:10.3390/ijms242216392.
41.
Liu et al., SARS-CoV-2 viral genes Nsp6, Nsp8, and M compromise cellular ATP levels to impair survival and function of human pluripotent stem cell-derived cardiomyocytes, Stem Cell Research & Therapy, doi:10.1186/s13287-023-03485-3.
42.
Boschi et al., SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects, bioRxiv, doi:10.1101/2022.11.24.517882.
43.
De Forni et al., Synergistic drug combinations designed to fully suppress SARS-CoV-2 in the lung of COVID-19 patients, PLoS ONE, doi:10.1371/journal.pone.0276751.
44.
Saha (B) et al., Manipulation of Spray-Drying Conditions to Develop an Inhalable Ivermectin Dry Powder, Pharmaceutics, doi:10.3390/pharmaceutics14071432.
45.
Jitobaom (B) et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8.
46.
Croci et al., Liposomal Systems as Nanocarriers for the Antiviral Agent Ivermectin, International Journal of Biomaterials, doi:10.1155/2016/8043983.
47.
Zheng et al., Red blood cell-hitchhiking mediated pulmonary delivery of ivermectin: Effects of nanoparticle properties, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121719.
48.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
49.
Liu (B) et al., Genome-wide analyses reveal the detrimental impacts of SARS-CoV-2 viral gene Orf9c on human pluripotent stem cell-derived cardiomyocytes, Stem Cell Reports, doi:10.1016/j.stemcr.2022.01.014.
50.
Segatori et al., Effect of Ivermectin and Atorvastatin on Nuclear Localization of Importin Alpha and Drug Target Expression Profiling in Host Cells from Nasopharyngeal Swabs of SARS-CoV-2- Positive Patients, Viruses, doi:10.3390/v13102084.
51.
Jitobaom (C) et al., Favipiravir and Ivermectin Showed in Vitro Synergistic Antiviral Activity against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-941811/v1.
52.
Munson et al., Niclosamide and ivermectin modulate caspase-1 activity and proinflammatory cytokine secretion in a monocytic cell line, British Society For Nanomedicine Early Career Researcher Summer Meeting, 2021, web.archive.org/web/20230401070026/https://michealmunson.github.io/COVID.pdf.
53.
Mountain Valley MD, Mountain Valley MD Receives Successful Results From BSL-4 COVID-19 Clearance Trial on Three Variants Tested With Ivectosol™, 5/18, www.globenewswire.com/en/news-release/2021/05/18/2231755/0/en/Mountain-Valley-MD-Receives-Successful-Results-From-BSL-4-COVID-19-Clearance-Trial-on-Three-Variants-Tested-With-Ivectosol.html.
54.
Yesilbag et al., Ivermectin also inhibits the replication of bovine respiratory viruses (BRSV, BPIV-3, BoHV-1, BCoV and BVDV) in vitro, Virus Research, doi:10.1016/j.virusres.2021.198384.
55.
Mody et al., Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents, Communications Biology, doi:10.1038/s42003-020-01577-x.
56.
Jeffreys et al., Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2022.106542.
57.
Surnar et al., Clinically Approved Antiviral Drug in an Orally Administrable Nanoparticle for COVID-19, ACS Pharmacol. Transl. Sci., doi:10.1021/acsptsci.0c00179.
58.
Li et al., Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment, J. Cellular Physiology, doi:10.1002/jcp.30055.
59.
Caly et al., The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research, doi:10.1016/j.antiviral.2020.104787.
60.
Zhang et al., Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflammation Research, doi:10.1007/s00011-008-8007-8.
61.
Gao et al., Ivermectin ameliorates acute myocarditis via the inhibition of importin-mediated nuclear translocation of NF-κB/p65, International Immunopharmacology, doi:10.1016/j.intimp.2024.112073.
62.
Abd-Elmawla et al., Suppression of NLRP3 inflammasome by ivermectin ameliorates bleomycin-induced pulmonary fibrosis, Journal of Zhejiang University-SCIENCE B, doi:10.1631/jzus.B2200385.
63.
Uematsu et al., Prophylactic administration of ivermectin attenuates SARS-CoV-2 induced disease in a Syrian Hamster Model, The Journal of Antibiotics, doi:10.1038/s41429-023-00623-0.
64.
Albariqi et al., Pharmacokinetics and Safety of Inhaled Ivermectin in Mice as a Potential COVID-19 Treatment, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2022.121688.
65.
Errecalde et al., Safety and Pharmacokinetic Assessments of a Novel Ivermectin Nasal Spray Formulation in a Pig Model, Journal of Pharmaceutical Sciences, doi:10.1016/j.xphs.2021.01.017.
66.
Madrid et al., Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation, Heliyon, doi:10.1016/j.heliyon.2020.e05820.
67.
Ma et al., Ivermectin contributes to attenuating the severity of acute lung injury in mice, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2022.113706.
68.
de Melo et al., Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin, EMBO Mol. Med., doi:10.15252/emmm.202114122.
69.
Arévalo et al., Ivermectin reduces in vivo coronavirus infection in a mouse experimental model, Scientific Reports, doi:10.1038/s41598-021-86679-0.
70.
Chaccour et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Scientific Reports, doi:10.1038/s41598-020-74084-y.
71.
Yan et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflammation Research, doi:10.1007/s00011-011-0307-8.
72.
Götz et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Scientific Reports, doi:10.1038/srep23138.
73.
Tay et al., Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Research, doi:10.1016/j.antiviral.2013.06.002.
74.
Wagstaff et al., Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochemical Journal, doi:10.1042/BJ20120150.
75.
Wagstaff (B) et al., An AlphaScreen®-Based Assay for High-Throughput Screening for Specific Inhibitors of Nuclear Import, SLAS Discovery, doi:10.1177/1087057110390360.
76.
Barrows et al., A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection, Cell Host & Microbe, doi:10.1016/j.chom.2016.07.004.
77.
Yang et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Research, doi:10.1016/j.antiviral.2020.104760.
78.
Mastrangelo et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dks147.
79.
Varghese et al., Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses, Antiviral Research, doi:10.1016/j.antiviral.2015.12.012.
80.
Bennett et al., Role of a nuclear localization signal on the minor capsid Proteins VP2 and VP3 in BKPyV nuclear entry, Virology, doi:10.1016/j.virol.2014.10.013.
81.
Kosyna et al., The importin α/β-specific inhibitor Ivermectin affects HIF-dependent hypoxia response pathways, Biological Chemistry, doi:10.1515/hsz-2015-0171.
82.
Scheim et al., Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19, International Journal of Molecular Sciences, doi:10.3390/ijms242317039.
83.
Liu (C) et al., Crosstalk between neutrophil extracellular traps and immune regulation: insights into pathobiology and therapeutic implications of transfusion-related acute lung injury, Frontiers in Immunology, doi:10.3389/fimmu.2023.1324021.
84.
Shouman et al., SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147, Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3.
85.
Scheim (B), D., Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion, SSRN, doi:10.2139/ssrn.3636557.
86.
Scheim (C), D., From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate and Then Clot Blood Cells, Center for Open Science, doi:10.31219/osf.io/sgdj2.
87.
Behl et al., CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target, Science of The Total Environment, doi:10.1016/j.scitotenv.2021.152072.
88.
DiNicolantonio et al., Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart, doi:10.1136/openhrt-2020-001350.
89.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
90.
Hazan et al., Treatment with Ivermectin Increases the Population of Bifidobacterium in the Gut, ACG 2023, acg2023posters.eventscribe.net/posterspeakers.asp.
Dinesh Kumar et al., 17 Mar 2021, peer-reviewed, 14 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Moxidectin and Ivermectin Inhibit SARS-CoV-2 Replication in Vero E6 Cells but Not in Human Primary Bronchial Epithelial Cells
Antiviral therapies are urgently needed to treat and limit the development of severe COVID-19 disease. Ivermectin, a broad-spectrum anti-parasitic agent, has been shown to have anti-SARS-CoV-2 activity in Vero cells at a concentration of 5 mM. These limited in vitro results triggered the investigation of ivermectin as a treatment option to alleviate COVID-19 disease. However, in April 2021, the World Health Organization stated the following: "The current evidence on the use of ivermectin to treat COVID-19 patients is inconclusive." It is speculated that the in vivo concentration of ivermectin is too low to exert a strong antiviral effect. Here, we performed a head-to-head comparison of the antiviral activity of ivermectin and the structurally related, but metabolically more stable moxidectin in multiple in vitro models of SARS-CoV-2 infection, including physiologically relevant human respiratory epithelial cells. Both moxidectin and ivermectin exhibited antiviral activity in Vero E6 cells. Subsequent experiments revealed that these compounds predominantly act on the steps following virus cell entry. Surprisingly, however, in human-airway-derived cell models, both moxidectin and ivermectin failed to inhibit SARS-CoV-2 infection, even at concentrations of 10 mM. These disappointing results call for a word of caution in the interpretation of anti-SARS-CoV-2 activity of drugs solely based on their activity in Vero cells. Altogether, these findings suggest that even using a high-dose regimen of ivermectin, or switching to another drug in the same class, is unlikely to be useful for treatment of SARS-CoV-2 in humans. KEYWORDS moxidectin, ivermectin, antiviral, SARS-CoV-2, ALI, in vitro W ithin less than 1.5 years, the pandemic SARS coronavirus 2 (SARS-CoV-2) has infected over 153 million individuals and resulted in over 3.2 million deaths worldwide (1-3). The social and economic burden of this still-ongoing pandemic is staggering, and, besides vaccine development, it is of utmost importance to develop therapeutic interventions to reduce disease symptoms. To date, multiple compounds have been shown to exert SARS-CoV-2 antiviral activity in vitro and several compounds have reached clinical trials (4, 5). Remdesivir and hydroxychloroquine were thought to be effective early in the pandemic, but after a careful evaluation in an interim solidarity trial, the WHO released a conditional yet strong recommendation against the usage of
% cytotoxicity ¼ ðcompound-treated LDH activity 2 spontaneous LDH activityÞ ðmaximum LDH activity 2 spontaneous LDH activityÞ Â 100 Live/dead staining and flow cytometry. PBECs cultured under ALI conditions were treated with 10 mM moxidectin, ivermectin, or an equivalent volume of EtOH at the basolateral side for 48 h at 37°C. Subsequently, cells were harvested by trypsinization and stained with fixable viability dye eFluor780 (Thermo Fisher Scientific) for 20 min at 4°C. Next, cells were washed with fluorescence-activated cell sorter (FACS) buffer (1X phosphate-buffered saline, 2% FBS, 1% EDTA), centrifuged, and fixed with 4% paraformaldehyde for 10 min at 4°C. After fixation, cells were washed, centrifuged, and resuspended in FACS buffer. Flow cytometry analyses were performed using the LSR-2 flow cytometer (BD Biosciences, San Jose, CA, USA) and data was further analyzed using Kaluza analysis software, version 2.1 (Beckman Coulter, Fullerton, CA, USA). Antiviral assays in Vero E6 and Calu-3. Vero E6 cells were seeded at a density of 1.3 Â 10 5 cells/well in 12-well plates. The next day, the medium was replaced with 0.25 ml of DMEM (2% FBS) containing the virus inoculum (MOI 1), in the presence of either increasing concentrations of compounds or the equivalent volume of EtOH. Following 2 h adsorption at 37°C, the virus inoculum was removed, after which the cells were washed twice and fresh DMEM (10% FBS) containing either the compounds or EtOH was added. At 8 hpi, cell..
References
Ahmed, Karim, Ross, Hossain, Clemens et al., A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.191
Bmt, Kumar, Bouma, Troost, Van De Pol et al., Resveratrol and pterostilbene inhibit SARS-CoV-2 replication in air liquid interface cultured human primary bronchial epithelial cells, Viruses, doi:10.3390/v13071335
Bray, Rayner, Noel, Jans, Wagstaff, Ivermectin and COVID-19: a report in antiviral research, widespread interest, an FDA warning, two letters to the editor and the authors' responses, Antiviral Res, doi:10.1016/j.antiviral.2020.104805
Caly, Druce, Catton, Jans, Wagstaff, The FDAapproved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
Cao, Coyle, Xiong, Wang, Heflich et al., Invited review: human air-liquid-interface organotypic tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives, Vitro Cell Dev Biol Anim, doi:10.1007/s11626-020-00517-7
Chaccour, Casellas, Blanco-Di Matteo, Pineda, Fernandez-Montero et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial, EClinicalMedicine, doi:10.1016/j.eclinm.2020.100720
Chan, He, Wang, He, Pandemic COVID-19: current status and challenges of antiviral therapies, Genes Dis, doi:10.1016/j.gendis.2020.07.001
Chowdhury, Shahbaz, Karim, A Randomized Trial of Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin therapy on COVID-19 patients
Chu, Chan, Yuen, Shuai, Yuan et al., Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study, Lancet Microbe, doi:10.1016/S2666-5247(20)30004-5
Cobb, Boeckh, Moxidectin: a review of chemistry, pharmacokinetics and use in horses, Parasit Vectors, doi:10.1186/1756-3305-2-S2-S5
De Melo, Benincasa, Cruz, Maricato, Porcionatto, 3D culture models to study SARS-CoV-2 infectivity and antiviral candidates: From spheroids to bioprinting, Biomed J, doi:10.1016/j.bj.2020.11.009
Gonzalez Canga, Prieto, Diez Liebana, Martinez, Vega et al., The pharmacokinetics and metabolism of ivermectin in domestic animal species, Vet J, doi:10.1016/j.tvjl.2007.07.011
Gonzalez, Gámez, Enciso, Maldonado, Palacios et al., Efficacy and safety of ivermectin and hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial, doi:10.1101/2021.02.18.21252037
Gotz, Magar, Dornfeld, Giese, Pohlmann et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Sci Rep, doi:10.1038/srep25428
Hashim, Maulood, Rasheed, Fatak, Kabah et al., Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, doi:10.1101/2020.10.26.20219345
Heijink, Kies, Kauffman, Postma, Van Oosterhout et al., Down-regulation of E-cadherin in human bronchial epithelial cells leads to epidermal growth factor receptor-dependent Th2 cell-promoting activity, J Immunol, doi:10.4049/jimmunol.178.12.7678
Heijink, Postma, Noordhoek, Broekema, Kapus, House dust mite-promoted epithelial-to-mesenchymal transition in human bronchial epithelium, Am J Respir Cell Mol Biol, doi:10.1165/rcmb.2008-0449OC
Jia, Look, Shi, Hickey, Pewe et al., ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia, J Virol, doi:10.1128/JVI.79.23.14614-14621.2005
Jonsdottir, Dijkman, Coronaviruses and the human airway: a universal system for virus-host interaction studies, Virol J, doi:10.1186/s12985-016-0479-5
Ketkar, Yang, Wormser, Wang, Lack of efficacy of ivermectin for prevention of a lethal Zika virus infection in a murine system, Diagn Microbiol Infect Dis, doi:10.1016/j.diagmicrobio.2019.03.012
Lam, Bordin, Waman, Scholes, Ashford et al., SARS-CoV-2 spike protein predicted to form complexes with host receptor protein orthologues from a broad range of mammals, Sci Rep, doi:10.1038/s41598-020-71936-5
Leist, Schafer, Martinez, Cell and animal models of SARS-CoV-2 pathogenesis and immunity, Dis Model Mech, doi:10.1242/dmm.046581
Lopez-Medina, Lopez, Hurtado, Davalos, Ramirez et al., Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.3071
Lundberg, Pinkham, Baer, Amaya, Narayanan et al., Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication, Antiviral Res, doi:10.1016/j.antiviral.2013.10.004
Makhani, Khatib, Corbeil, Kariyawasam, Raheel et al., 2018 in review: five hot topics in tropical medicine, Trop Dis Travel Med Vaccines, doi:10.1186/s40794-019-0082-z
Mastrangelo, Pezzullo, Burghgraeve, Kaptein, Pastorino et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, J Antimicrob Chemother, doi:10.1093/jac/dks147
Matsuyama, Nao, Shirato, Kawase, Saito et al., Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2002589117
Mcaloose, Laverack, Wang, Killian, Caserta et al., From people to Panthera: natural SARS-CoV-2 infection in tigers and lions at the Bronx Zoo, mBio, doi:10.1128/mBio.02220-20
Milton, Hamley, Walker, Basanez, Moxidectin: an oral treatment for human onchocerciasis, Expert Rev Anti Infect Ther, doi:10.1080/14787210.2020.1792772:1&hx2013;15
Mohan, Tiwari, Suri, Mittal, Patel et al., Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial, Res Square Preprint, doi:10.21203/rs.3.rs-191648/v1
Momekov, Momekova, Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens, Biotechnol Biotechnol Equip, doi:10.1080/13102818.2020.1775118
Niaee, Gheibi, Namdar, Allami, Zolghadr et al., Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial, Res Square Preprint, doi:10.21203/rs.3.rs-109670/v1
Nolan, Lok, Macrocyclic lactones in the treatment and control of parasitism in small companion animals, Curr Pharm Biotechnol, doi:10.2174/138920112800399167
Ogando, Dalebout, Zevenhoven-Dobbe, Limpens, Van Der Meer et al., SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology, J Gen Virol, doi:10.1099/jgv.0.001453
Okumus, Demirtürk, Çetinkaya, Güner, Avcı et al., Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients, Res Square Preprint, doi:10.21203/rs.3.rs-224203/v1
Opoku, Bakajika, Kanza, Howard, Mambandu et al., Single dose moxidectin versus ivermectin for Onchocerca volvulus infection in Ghana, Liberia, and the Democratic Republic of the Congo: a randomized, controlled, doubleblind phase 3 trial, Lancet, doi:10.1016/S0140-6736(17)32844-1
Oreshkova, Molenaar, Vreman, Harders, Munnink et al., SARS-CoV-2 infection in farmed minks, the Netherlands, Euro Surveill, doi:10.2807/1560-7917.ES.2020.25.23.2001005
Pan, Peto, Henao-Restrepo, Preziosi, Sathiyamoorthy et al., Repurposed antiviral drugs for COVID-19 -interim WHO solidarity trial results, N Engl J Med, doi:10.1056/NEJMoa2023184
Patterson, Elia, Grassi, Giordano, Desario et al., Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy, Nat Commun, doi:10.1038/s41467-020-20097-0
Pena-Silva, Duffull, Steer, Jaramillo-Rincon, Gwee et al., Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID-19, Br J Clin Pharmacol, doi:10.1111/bcp.14476
Podder, Chowdhury, Sina, Haque, Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-center, openlabel, randomised controlled study, IMC J Medical Science
Prichard, Geary, Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance, Int J Parasitol Drugs Drug Resist, doi:10.1016/j.ijpddr.2019.06.002
Prichard, Menez, Lespine, Moxidectin and the avermectins: consanguinity but not identity, Int J Parasitol Drugs Drug Resist, doi:10.1016/j.ijpddr.2012.04.001
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen Study, Chest, doi:10.1016/j.chest.2020.10.009
Rochwerg, Agarwal, Siemieniuk, Agoritsas, Lamontagne et al., A living WHO guideline on drugs for COVID-19, BMJ, doi:10.1136/bmj.m3379
Roy, Pattadar, Raj, Agarwal, Biswas et al., Ivermectin as a potential treatment for mild to moderate COVID-19 -A double blind randomized placebo-controlled trial, doi:10.1101/2021.01.05.21249310
Schmith, Zhou, Lohmer, The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19, Clin Pharmacol Ther, doi:10.1002/cpt.1889
Sharun, Tiwari, Natesan, Dhama, SARS-CoV-2 infection in farmed minks, associated zoonotic concerns, and importance of the One Health approach during the ongoing COVID-19 pandemic, Vet Q, doi:10.1080/01652176.2020.1867776:1-14
Shi, Wen, Zhong, Yang, Wang et al., Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2, Science, doi:10.1126/science.abb7015
Sims, Burkett, Yount, Pickles, SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium, Virus Res, doi:10.1016/j.virusres.2007.03.013
Sohrabi, Alsafi, Neill, Khan, Kerwan et al., World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19), Int J Surg, doi:10.1016/j.ijsu.2020.02.034
Soto-Becerra, Culquichicón, Hurtado-Roca, Araujo-Castillo, Real-world effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: results of a target trial emulation using observational data from a nationwide healthcare system in Peru, doi:10.1101/2020.10.06.20208066
Tay, Fraser, Chan, Moreland, Rathore et al., Nuclear localization of dengue virus (DENV) 1-4 nonstructural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Res, doi:10.1016/j.antiviral.2013.06.002
Varghese, Kaukinen, Glasker, Bespalov, Hanski et al., Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses, Antiviral Res, doi:10.1016/j.antiviral.2015.12.012
Vieira Braga, Kar, Berg, Carpaij, Polanski et al., A cellular census of human lungs identifies novel cell states in health and in asthma, Nat Med, doi:10.1038/s41591-019-0468-5
Wagstaff, Rawlinson, Hearps, Jans, An AlphaScreen (R)-based assay for high-throughput screening for specific inhibitors of nuclear import, J Biomol Screen, doi:10.1177/1087057110390360
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J, doi:10.1042/BJ20120150
Who, Coronavirus disease (COVID-19) pandemic
Who, Therapeutics and COVID-19: living guidelines
Yamasmith, Avirutnan, Mairiang, Tanrumluk, Suputtamongkol et al., Efficacy and safety of ivermectin against dengue infection: a phase III, randomized, double-blind, placebo-controlled trial
Zhang, Xie, Hashimoto, Current status of potential therapeutic candidates for the COVID-19 crisis, Brain Behav Immun, doi:10.1016/j.bbi.2020.04.046
Zhou, Yang, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2012-7
DOI record:
{
"DOI": "10.1128/aac.01543-21",
"ISSN": [
"0066-4804",
"1098-6596"
],
"URL": "http://dx.doi.org/10.1128/aac.01543-21",
"abstract": "<jats:p>Antiviral therapies are urgently needed to treat and limit the development of severe COVID-19 disease. Ivermectin, a broad-spectrum anti-parasitic agent, has been shown to have anti-SARS-CoV-2 activity in Vero cells at a concentration of 5 μM.</jats:p>",
"alternative-id": [
"10.1128/AAC.01543-21"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-08-04"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-10-08"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-01-18"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Biomedical Sciences of Cells & Systems, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands"
},
{
"name": "Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands"
}
],
"family": "Dinesh Kumar",
"given": "Nilima",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands"
}
],
"family": "ter Ellen",
"given": "Bram M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands"
}
],
"family": "Bouma",
"given": "Ellen M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands"
}
],
"family": "Troost",
"given": "Berit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands"
}
],
"family": "van de Pol",
"given": "Denise P. I.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands"
}
],
"family": "van der Ende-Metselaar",
"given": "Heidi H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatrics, Beatrix Children’s Hospital, University Medical Center Groningen, University of Groningen, GRIAC Research Institute, Groningen, The Netherlands"
}
],
"family": "van Gosliga",
"given": "Djoke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology and Medical Biology, University Medical Center Groningen, University of Groningen, GRIAC Research Institute, Groningen, The Netherlands"
}
],
"family": "Apperloo",
"given": "Leonie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Diseases, University Medical Center Groningen, University of Groningen, GRIAC Research Institute, Groningen, The Netherlands"
}
],
"family": "Carpaij",
"given": "Orestes A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Diseases, University Medical Center Groningen, University of Groningen, GRIAC Research Institute, Groningen, The Netherlands"
}
],
"family": "van den Berge",
"given": "Maarten",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology and Medical Biology, University Medical Center Groningen, University of Groningen, GRIAC Research Institute, Groningen, The Netherlands"
}
],
"family": "Nawijn",
"given": "Martijn C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine/Infectious Diseases, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands"
}
],
"family": "Stienstra",
"given": "Ymkje",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands"
}
],
"family": "Rodenhuis-Zybert",
"given": "Izabela A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6951-1448",
"affiliation": [
{
"name": "Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands"
}
],
"authenticated-orcid": true,
"family": "Smit",
"given": "Jolanda M.",
"sequence": "additional"
}
],
"container-title": [
"Antimicrobial Agents and Chemotherapy"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.asm.org"
]
},
"created": {
"date-parts": [
[
2021,
10,
11
]
],
"date-time": "2021-10-11T17:20:05Z",
"timestamp": 1633972805000
},
"deposited": {
"date-parts": [
[
2022,
1,
18
]
],
"date-time": "2022-01-18T15:47:58Z",
"timestamp": 1642520878000
},
"indexed": {
"date-parts": [
[
2022,
1,
19
]
],
"date-time": "2022-01-19T18:14:28Z",
"timestamp": 1642616068584
},
"is-referenced-by-count": 4,
"issn-type": [
{
"type": "print",
"value": "0066-4804"
},
{
"type": "electronic",
"value": "1098-6596"
}
],
"issue": "1",
"issued": {
"date-parts": [
[
2022,
1,
18
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
1,
18
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://doi.org/10.1128/ASMCopyrightv2",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
18
]
],
"date-time": "2022-01-18T00:00:00Z",
"timestamp": 1642464000000
}
},
{
"URL": "https://journals.asm.org/non-commercial-tdm-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
18
]
],
"date-time": "2022-01-18T00:00:00Z",
"timestamp": 1642464000000
}
}
],
"link": [
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/AAC.01543-21",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/AAC.01543-21",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "235",
"original-title": [],
"prefix": "10.1128",
"published": {
"date-parts": [
[
2022,
1,
18
]
]
},
"published-print": {
"date-parts": [
[
2022,
1,
18
]
]
},
"publisher": "American Society for Microbiology",
"reference": [
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_2_2"
},
{
"DOI": "10.1016/j.ijsu.2020.02.034",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_3_2"
},
{
"key": "e_1_3_3_4_2",
"unstructured": "WHO. 2021. Coronavirus disease (COVID-19) pandemic. https://covid19.who.int/. Accessed 29 April 2021."
},
{
"DOI": "10.1016/j.gendis.2020.07.001",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_5_2"
},
{
"DOI": "10.1016/j.bbi.2020.04.046",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_6_2"
},
{
"DOI": "10.1056/NEJMoa2023184",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_7_2"
},
{
"DOI": "10.1136/bmj.m3379",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_8_2"
},
{
"key": "e_1_3_3_9_2",
"unstructured": "NIH. 2021. Therapeutic management of adults with COVID-19. https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/. Accessed 29 April 2021."
},
{
"DOI": "10.1016/j.ijpddr.2012.04.001",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_10_2"
},
{
"key": "e_1_3_3_11_2",
"unstructured": "EMA. 2017. Moxidectin-containing veterinary medicines used in cattle sheep and horses https://www.ema.europa.eu/en/medicines/veterinary/referrals/moxidectin-containing-veterinary-medicines-used-cattle-sheep-horses. Accessed 24 February 2021."
},
{
"key": "e_1_3_3_12_2",
"unstructured": "EMA. 2009. Ivermectin. https://www.ema.europa.eu/en/medicines/veterinary/referrals/ivermectin. Accessed 24 February 2021."
},
{
"DOI": "10.1186/s40794-019-0082-z",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_13_2"
},
{
"DOI": "10.1042/BJ20120150",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_14_2"
},
{
"DOI": "10.1016/j.antiviral.2013.06.002",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_15_2"
},
{
"DOI": "10.1016/j.antiviral.2013.10.004",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_16_2"
},
{
"DOI": "10.1038/srep25428",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_17_2"
},
{
"DOI": "10.1016/j.antiviral.2015.12.012",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_18_2"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_19_2"
},
{
"DOI": "10.1016/j.diagmicrobio.2019.03.012",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_20_2"
},
{
"author": "Yamasmith E",
"key": "e_1_3_3_21_2",
"unstructured": "Yamasmith E, Avirutnan P, Mairiang D, Tanrumluk S, Suputtamongkol Y, A-Hamad Saleh-Arong F, Angkasekwinai N, Wongsawa E, Fongsri U. 2018. Efficacy and safety of ivermectin against dengue infection: a phase III, randomized, double-blind, placebo-controlled trial, p In (ed), Internal Medicine and One Health, Chonburi",
"volume-title": "Efficacy and safety of ivermectin against dengue infection: a phase III, randomized, double-blind, placebo-controlled trial",
"year": "2018"
},
{
"DOI": "10.1080/13102818.2020.1775118",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_22_2"
},
{
"DOI": "10.1016/j.antiviral.2020.104805",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_23_2"
},
{
"DOI": "10.1002/cpt.1889",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_24_2"
},
{
"DOI": "10.1111/bcp.14476",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_25_2"
},
{
"key": "e_1_3_3_26_2",
"unstructured": "clinicaltrials.gov. 2021. Ivermectin and COVID-19. https://clinicaltrials.gov/ct2/results?recrs=&cond=covid-19&term=ivermectin+and+covid-19&cntry=&state=&city=&dist=. Accessed 29 April 2021."
},
{
"DOI": "10.1016/j.eclinm.2020.100720",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_27_2"
},
{
"DOI": "10.1001/jama.2021.3071",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_28_2"
},
{
"key": "e_1_3_3_29_2",
"unstructured": "WHO. 2021. Therapeutics and COVID-19: living guidelines. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1. Accessed 29 April 2021."
},
{
"DOI": "10.1101/2021.02.18.21252037",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_30_2"
},
{
"DOI": "10.1186/1756-3305-2-S2-S5",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_31_2"
},
{
"DOI": "10.1016/j.ijpddr.2019.06.002",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_32_2"
},
{
"DOI": "10.1080/14787210.2020.1792772:1–15",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_33_2"
},
{
"DOI": "10.1016/S0140-6736(17)32844-1",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_34_2"
},
{
"DOI": "10.1242/dmm.046581",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_35_2"
},
{
"DOI": "10.1073/pnas.2002589117",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_36_2"
},
{
"DOI": "10.1099/jgv.0.001453",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_37_2"
},
{
"DOI": "10.1016/S2666-5247(20)30004-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_38_2"
},
{
"DOI": "10.1186/s12985-016-0479-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_39_2"
},
{
"DOI": "10.1007/s11626-020-00517-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_40_2"
},
{
"DOI": "10.1128/JVI.79.23.14614-14621.2005",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_41_2"
},
{
"DOI": "10.1016/j.virusres.2007.03.013",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_42_2"
},
{
"DOI": "10.3390/v13071335",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_43_2"
},
{
"DOI": "10.1177/1087057110390360",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_44_2"
},
{
"DOI": "10.1093/jac/dks147",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_45_2"
},
{
"DOI": "10.21203/rs.3.rs-224203/v1",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_46_2"
},
{
"article-title": "Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-center, open-label, randomised controlled study",
"author": "Podder CS",
"first-page": "1",
"journal-title": "IMC J Medical Science",
"key": "e_1_3_3_47_2",
"unstructured": "Podder CS, Chowdhury N, Sina MI, Haque WMMU. 2020. Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-center, open-label, randomised controlled study. IMC J Medical Science 14:1–8.",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1101/2020.10.26.20219345",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_48_2"
},
{
"author": "Chowdhury A",
"key": "e_1_3_3_49_2",
"unstructured": "Chowdhury A, Shahbaz M, Karim MR. 2020. A Randomized Trial of Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin therapy on COVID-19 patients. Preprint Research Square.",
"volume-title": "A Randomized Trial of Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin therapy on COVID-19 patients.",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.10.009",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_50_2"
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_51_2"
},
{
"DOI": "10.1101/2020.10.06.20208066",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_52_2"
},
{
"DOI": "10.1101/2021.01.05.21249310",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_53_2"
},
{
"DOI": "10.21203/rs.3.rs-109670/v1",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_54_2"
},
{
"DOI": "10.21203/rs.3.rs-191648/v1",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_55_2"
},
{
"DOI": "10.1038/s41598-020-71936-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_56_2"
},
{
"DOI": "10.1038/s41467-020-20097-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_57_2"
},
{
"DOI": "10.1080/01652176.2020.1867776:1-14",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_58_2"
},
{
"DOI": "10.1126/science.abb7015",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_59_2"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.23.2001005",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_60_2"
},
{
"DOI": "10.1128/mBio.02220-20",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_61_2"
},
{
"DOI": "10.1016/j.tvjl.2007.07.011",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_62_2"
},
{
"DOI": "10.2174/138920112800399167",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_63_2"
},
{
"DOI": "10.1016/j.bj.2020.11.009",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_64_2"
},
{
"DOI": "10.1038/s41591-019-0468-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_65_2"
},
{
"DOI": "10.4049/jimmunol.178.12.7678",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_66_2"
},
{
"DOI": "10.1165/rcmb.2008-0449OC",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_67_2"
},
{
"key": "e_1_3_3_68_2",
"unstructured": "Stemcell Technologies. 2021. Air-Liquid Interface Culture for Respiratory Research on https://www.stemcell.com/air-liquid-interface-culture-respiratory-research-lp.html. Accessed 24 February 2021."
}
],
"reference-count": 67,
"references-count": 67,
"relation": {},
"score": 1,
"short-container-title": [
"Antimicrob Agents Chemother"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": [
"Moxidectin and Ivermectin Inhibit SARS-CoV-2 Replication in Vero E6 Cells but Not in Human Primary Bronchial Epithelial Cells"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1128/asmj-crossmark-policy-page",
"volume": "66"
}