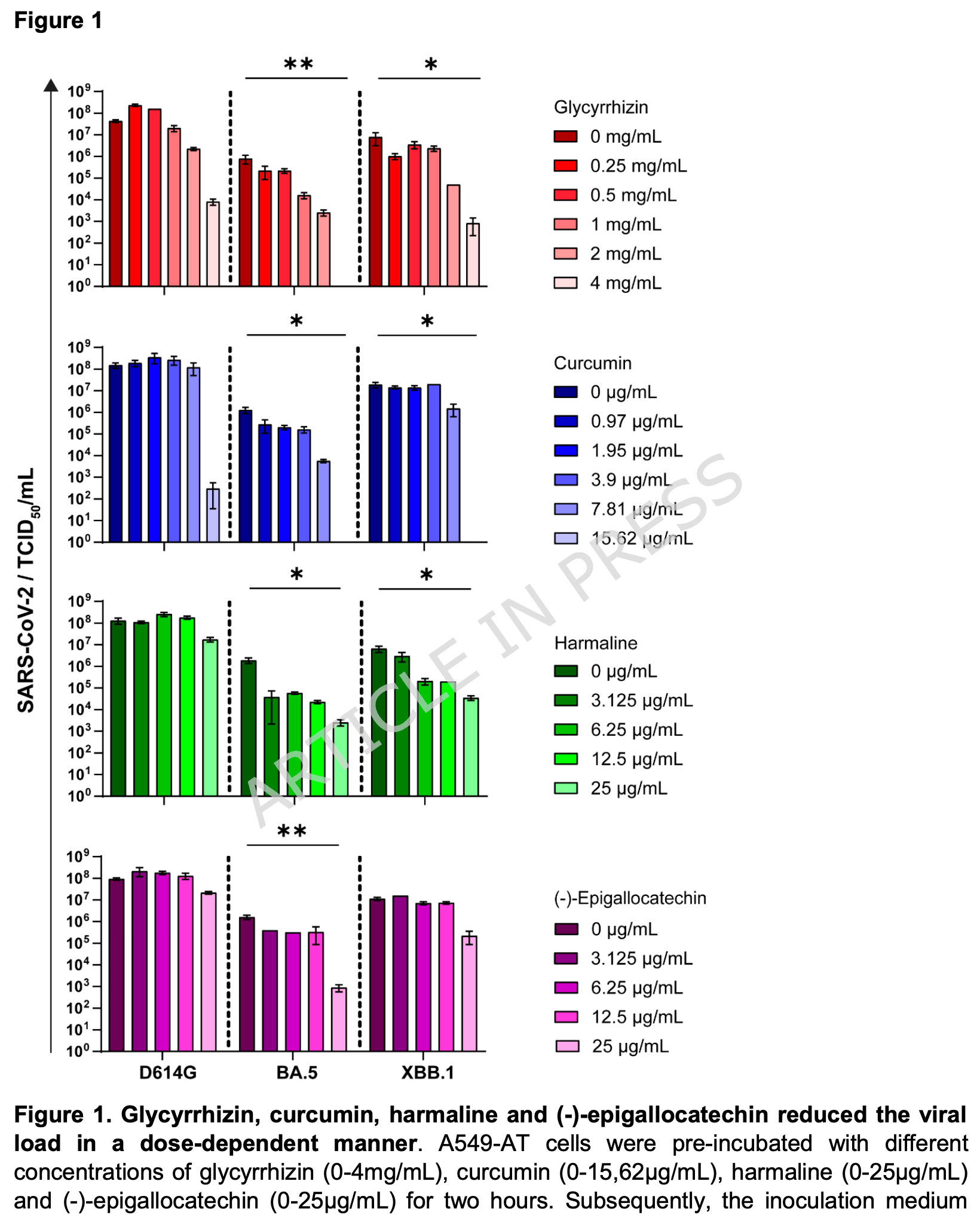
Dose-dependent antiviral effects of glycyrrhizin, curcumin, and harmaline against clinical SARS-CoV-2 isolates, including D614G, Omicron BA.5, and Omicron XBB.1
et al., BMC Complementary Medicine and Therapies, doi:10.1186/s12906-026-05253-1, Jan 2026
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study in A549-AT cells showing that curcumin and glycyrrhizin effectively inhibit SARS-CoV-2 D614G, Omicron BA.5, and Omicron XBB.1 variants, while harmaline effectively inhibits only the Omicron variants, all at subtoxic concentrations.
62 preclinical studies support the efficacy of curcumin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2 with curcumin or metabolites via binding to the spikeA,1,5,6,11,16,18,24,27 (and specifically the receptor binding domainB,2,4,14,17,20 ), MproC,4-6,11,13,15-17,19,20,22,25,27,28,30,48 , RNA-dependent RNA polymeraseD,4-6,17,26 , PLproE,6, ACE2F,2,18,19,21 , nucleocapsidG,12,29 , nsp10H,29, and helicaseI,36 proteins, and inhibition of spike-ACE2 interactionJ,3.
In vitro studies demonstrate inhibition of the spikeA,41 (and specifically the receptor binding domainB,51), MproC,23,41,48,50 , ACE2F,51, and TMPRSS2K,51 proteins, and inhibition of spike-ACE2 interactionJ,3,34 .
In vitro studies demonstrate efficacy in Calu-3L,49, A549M,41, A549-ATN,31, 293TO,7, HEK293-hACE2P,23,39 , 293T/hACE2/TMPRSS2Q,40, Vero E6R,1,13,17,27,39,41,43,45,47,49 , and SH-SY5YS,38 cells.
Curcumin decreases pro-inflammatory cytokines induced by SARS-CoV-2 in peripheral blood mononuclear cells47, alleviates SARS-CoV-2 spike protein-induced mitochondrial membrane damage and oxidative stress7, may limit COVID-19 induced cardiac damage by inhibiting the NF-κB signaling pathway which mediates the profibrotic effects of the SARS-CoV-2 spike protein on cardiac fibroblasts35, is predicted to inhibit the interaction between the SARS-CoV-2 spike protein receptor binding domain and the human ACE2 receptor for the delta and omicron variants14, lowers ACE2 and STAT3, curbing lung inflammation and ARDS in preclinical COVID-19 models32, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity42, has direct virucidal action by disrupting viral envelope integrity44, may inhibit viral replication and modulate inflammatory pathways like NF-κB via SIRT1 activation52, and can function as a photosensitizer in photodynamic therapy to generate reactive oxygen species that damage the virus44.
1.
Marzouk et al., Computational and Experimental Insights into the Antiviral Mechanism of Turmeric (Curcuma longa) against SARS-CoV-2 D614G, BIO Web of Conferences, doi:10.1051/bioconf/202519804002.
2.
Wu et al., Utilizing natural compounds as ligands to disrupt the binding of SARS-CoV-2 receptor-binding domain to angiotensin-converting enzyme 2, impeding viral infection, Phytochemistry Letters, doi:10.1016/j.phytol.2025.102999.
3.
Najimi et al., Phytochemical Inhibitors of SARS‐CoV‐2 Entry: Targeting the ACE2‐RBD Interaction with l‐Tartaric Acid, l‐Ascorbic Acid, and Curcuma longa Extract, ChemistrySelect, doi:10.1002/slct.202406035.
4.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
5.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
6.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
7.
Zhang et al., Computational Discovery of Mitochondrial Dysfunction Biomarkers in Severe SARS-CoV-2 Infection: Facilitating Pytomedicine Screening, Phytomedicine, doi:10.1016/j.phymed.2024.155784.
8.
Öztürkkan et al., In Silico investigation of the effects of curcuminoids on the spike protein of the omicron variant of SARS-CoV-2, Baku State University Journal of Chemistry and Material Sciences, 1:2, bsuj.bsu.edu.az/uploads/pdf/ec4204d62f7802de54e6092bf7860029.pdf.
9.
Yunze et al., Therapeutic effect and potential mechanism of curcumin, an active ingredient in Tongnao Decoction, on COVID-19 combined with stroke: a network pharmacology study and GEO database mining, Research Square, doi:10.21203/rs.3.rs-4329762/v1.
10.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
11.
Boseila et al., Throat spray formulated with virucidal Pharmaceutical excipients as an effective early prophylactic or treatment strategy against pharyngitis post-exposure to SARS CoV-2, European Journal of Pharmaceutics and Biopharmaceutics, doi:10.1016/j.ejpb.2024.114279.
12.
Hidayah et al., Bioinformatics study of curcumin, demethoxycurcumin, bisdemethoxycurcumin and cyclocurcumin compounds in Curcuma longa as an antiviral agent via nucleocapsid on SARS-CoV-2 inhibition, International Conference on Organic and Applied Chemistry, doi:10.1063/5.0197724.
13.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
14.
Kant et al., Structure-based drug discovery to identify SARS-CoV2 spike protein–ACE2 interaction inhibitors, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2300060.
15.
Naderi Beni et al., In silico studies of anti-oxidative and hot temperament-based phytochemicals as natural inhibitors of SARS-CoV-2 Mpro, PLOS ONE, doi:10.1371/journal.pone.0295014.
16.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
17.
Eleraky et al., Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2, International Journal of Nanomedicine, doi:10.2147/IJN.S423251.
18.
Singh (B) et al., Computational studies to analyze effect of curcumin inhibition on coronavirus D614G mutated spike protein, The Seybold Report, doi:10.17605/OSF.IO/TKEXJ.
19.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
20.
Srivastava et al., Paradigm of Well-Orchestrated Pharmacokinetic Properties of Curcuminoids Relative to Conventional Drugs for the Inactivation of SARS-CoV-2 Receptors: An In Silico Approach, Stresses, doi:10.3390/stresses3030043.
21.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
22.
Winih Kinasih et al., Analisis in silico interaksi senyawa kurkuminoid terhadap enzim main protease 6LU7 dari SARS-CoV-2, Duta Pharma Journal, doi:10.47701/djp.v3i1.2904.
23.
Wu (B) et al., Potential Mechanism of Curcumin and Resveratrol against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-2780614/v1.
24.
Nag et al., Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2022.105552.
25.
Rampogu et al., Molecular Docking and Molecular Dynamics Simulations Discover Curcumin Analogue as a Plausible Dual Inhibitor for SARS-CoV-2, International Journal of Molecular Sciences, doi:10.3390/ijms23031771.
26.
Singh (C) et al., Potential of turmeric-derived compounds against RNA-dependent RNA polymerase of SARS-CoV-2: An in-silico approach, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2021.104965.
27.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
28.
Rajagopal et al., Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x.
29.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
30.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
31.
Grüneberg et al., Dose-dependent antiviral effects of glycyrrhizin, curcumin, and harmaline against clinical SARS-CoV-2 isolates, including D614G, Omicron BA.5, and Omicron XBB.1, BMC Complementary Medicine and Therapies, doi:10.1186/s12906-026-05253-1.
32.
Aktay et al., Oral Administration of Water-Soluble Curcumin Complex Prevents ARDS With the Potential for COVID-19 Treatment, Phytotherapy Research, doi:10.1002/ptr.70046.
33.
Olubiyi et al., Novel dietary herbal preparations with inhibitory activities against multiple SARS-CoV-2 targets: A multidisciplinary investigation into antiviral activities, Food Chemistry Advances, doi:10.1016/j.focha.2025.100969.
34.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
35.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
36.
Li et al., Thermal shift assay (TSA)-based drug screening strategy for rapid discovery of inhibitors against the Nsp13 helicase of SARS-CoV-2, Animals and Zoonoses, doi:10.1016/j.azn.2024.06.001.
37.
Kamble et al., Nanoparticulate curcumin spray imparts prophylactic and therapeutic properties against SARS-CoV-2, Emergent Materials, doi:10.1007/s42247-024-00754-6.
38.
Nicoliche et al., Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-024-61662-7.
39.
Nittayananta et al., A novel film spray containing curcumin inhibits SARS-CoV-2 and influenza virus infection and enhances mucosal immunity, Virology Journal, doi:10.1186/s12985-023-02282-x.
40.
Septisetyani et al., Curcumin and turmeric extract inhibited SARS-CoV-2 pseudovirus cell entry and Spike mediated cell fusion, bioRxiv, doi:10.1101/2023.09.28.560070.
41.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
42.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
43.
Teshima et al., Antiviral activity of curcumin and its analogs selected by an artificial intelligence-supported activity prediction system in SARS-CoV-2-infected VeroE6 cells, Natural Product Research, doi:10.1080/14786419.2023.2194647.
44.
Zupin et al., Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer, Viruses, doi:10.3390/v14102132.
45.
Leka et al., In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds, Phytotherapy Research, doi:10.1002/ptr.7463.
46.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
47.
Marín-Palma et al., Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms, Molecules, doi:10.3390/molecules26226900.
48.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
49.
Bormann et al., Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro, Viruses, doi:10.3390/v13101914.
50.
Guijarro-Real et al., Potential In Vitro Inhibition of Selected Plant Extracts against SARS-CoV-2 Chymotripsin-Like Protease (3CLPro) Activity, Foods, doi:10.3390/foods10071503.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
h.
Non-structural protein 10 (nsp10) serves as an RNA chaperone and stabilizes conformations of nsp12 and nsp14 in the replicase-transcriptase complex, which synthesizes new viral RNAs. Nsp10 disruption may destabilize replicase-transcriptase complex activity.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
k.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
l.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
m.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
n.
A549-AT is a human lung carcinoma cell line stably transfected with ACE2 and TMPRSS2 receptors. Unlike the parental line, this overexpression ensures stable infection and enhanced viral entry, allowing for the evaluation of antiviral efficacy against various SARS-CoV-2 variants.
o.
293T is a human embryonic kidney cell line that can be engineered for high ACE2 expression and SARS-CoV-2 susceptibility. 293T cells are easily transfected and support high protein expression.
p.
HEK293-hACE2 is a human embryonic kidney cell line with high ACE2 expression and SARS-CoV-2 susceptibility. Cells have been transfected with a plasmid to express the human ACE2 (hACE2) protein.
q.
293T/hACE2/TMPRSS2 is a human embryonic kidney cell line engineered for high ACE2 and TMPRSS2 expression, which mimics key aspects of human infection. 293T/hACE2/TMPRSS2 cells are very susceptible to SARS-CoV-2 infection.
r.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
s.
SH-SY5Y is a human neuroblastoma cell line that exhibits neuronal phenotypes. It is commonly used as an in vitro model for studying neurotoxicity, neurodegenerative diseases, and neuronal differentiation.
Grüneberg et al., 22 Jan 2026, Germany, peer-reviewed, 11 authors.
Contact: adalbert.krawczyk@uk-essen.de.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Dose-dependent antiviral effects of glycyrrhizin, curcumin, and harmaline against clinical SARS-CoV-2 isolates, including D614G, Omicron BA.5, and Omicron XBB.1
BMC Complementary Medicine and Therapies, doi:10.1186/s12906-026-05253-1
Background SARS-CoV-2 remains a major global health challenge, as infection can lead to potential lifethreatening conditions such as COVID-19. Emerging variants of the virus are characterized by higher transmission rates and immune escape mutations, enabling them to evade vaccine-induced immunity. Existing treatment options, including monoclonal antibodies, are often variant-specific and not widely accessible, especially in low-and middle-income countries. Natural compounds derived from medicinal herbs and green tea have demonstrated antiviral activity against various viruses and may offer promising, variantindependent therapeutic potential.
Methods In this study, we examined the antiviral activity of four plant-derived compounds: glycyrrhizin, curcumin, harmaline, and (-)-epigallocatechin. The compounds were tested in vitro against SARS-CoV-2 D614G, Omicron BA.5, and Omicron XBB.1. The antiviral efficacy was assessed at subtoxic concentrations to evaluate potential therapeutic applicability.
Results All tested compounds showed effective neutralization of SARS-CoV-2 D614G, Omicron BA.5, and Omicron XBB.1 at subtoxic concentrations. In particular, glycyrrhizin, curcumin, and harmaline exhibited potent antiviral activity across all tested variants.
Conclusions Our findings support the potential of glycyrrhizin, curcumin, and harmaline as variantindependent treatment candidates for COVID-19. However, further clinical studies are necessary to validate their efficacy and safety in vivo.
Declarations Ethics approval and consent to participate The study was approved by the local ethics committee, Ethik-Kommission der Universität Duisburg-Essen (20-9374-BO), and was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent to participate was obtained from all participants.
Consent for publication All authors have read the manuscript in its entirety and consent to its submission and publication in the present form.
Competing interests The authors declare that they have no competing interests.
References
Al-Aly, Davis, Mccorkell, Soares, Wulf-Hanson et al., Long COVID science, research and policy, Nat Med
Ali, Banerjea, Curcumin inhibits HIV-1 by promoting Tat protein degradation, Sci Rep
Alves, Da Silva, De Leitao-Junior, De Balbino, Therapeutic Approaches for COVID-19: A Review of Antiviral Treatments, Immunotherapies, and Emerging Interventions, Adv Ther
Barouch, Covid-19 Vaccines -Immunity, Variants, Boosters, New Engl J Med
Bergwerk, Gonen, Lustig, Amit, Lipsitch et al., Covid-19 Breakthrough Infections in Vaccinated Health Care Workers, N Engl J Med
Bijelic, Hitl, Kladar, Phytochemicals in the Prevention and Treatment of SARS-CoV-2-Clinical Evidence, Antibiotics
Bormann, Alt, Schipper, Van De Sand, Vtk et al., Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro, Viruses-Basel
Bormann, Brochhagen, Alt, Otte, Thummler et al., Immune responses in COVID-19 patients during breakthrough infection with SARS-CoV-2 variants Delta, Omicron-BA.1 and Omicron-BA.5, Frontiers in immunology
Carvajal, Garcia-Castillo, Cuellar, Campillay-Veliz, Salazar-Ardiles et al., New insights into the pathogenesis of SARS-CoV-2 during and after the COVID-19 pandemic, Frontiers in immunology
Chen, Su, Fu, Wang, Lv et al., Harmine blocks herpes simplex virus infection through downregulating cellular NF-kappaB and MAPK pathways induced by oxidative stress, Antiviral research
Chenchula, Karunakaran, Sharma, Chavan, Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review, J Med Virol
Cherneha, Zydek, Brass, Korth, Jansen et al., Immunogenicity of the Monovalent Omicron XBB.1.5-Adapted BNT162b2 COVID-19 Vaccine in People Living with HIV (PLWH), Vaccines
Cinatl, Morgenstern, Bauer, Chandra, Rabenau et al., Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus, Lancet
Dahal, Clayton, Cabral, Cheng, Jahanshahi et al., On a path toward a broad-spectrum anti-viral: inhibition of HIV-1 and coronavirus replication by SR kinase inhibitor harmine, J Virol
Focosi, Mcconnell, Casadevall, Cappello, Valdiserra et al., Monoclonal antibody therapies against SARS-CoV-2, Lancet Infect Dis
Gmanyami, Lambert, Jarynowski, Belik, Amuasi, Excess mortality during the COVID-19 pandemic in low-and lower-middle-income countries: a systematic review and meta-analysis, BMC Public Health
Gomaa, Mohamed, Abd-Ellatief, Gomaa, Hammam, Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate COVID-19 infection: a randomized clinical trial, Inflammopharmacology
Harvey, Carabelli, Jackson, Gupta, Thomson et al., SARS-CoV-2 variants, spike mutations and immune escape, Nat Rev Microbiol
He, Zhou, Yu, Wang, Deng et al., Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms, Pharmacol Res
Hegazy, Mahmoud, Elshaier, Shama, Nasr et al., Antiviral activities of plant-derived indole and beta-carboline alkaloids against human and avian influenza viruses, Sci Rep
Heilingloh, Aufderhorst, Schipper, Dittmer, Witzke et al., Susceptibility of SARS-CoV-2 to UV Irradiation, Am J Infect Control
Hurst, Dickinson, Hsu, Epigallocatechin-3-Gallate (EGCG) Inhibits SARS-CoV-2 Infection in Primate Epithelial Cells: (A Short Communication), Microbiol Infect Dis
Huynh, Wang, Luan, In Silico Exploration of the Molecular Mechanism of Clinically Oriented Drugs for Possibly Inhibiting SARS-CoV-2's Main Protease, J Phys Chem Lett
Jassat, Reyes, Munblit, Caoili, Bozza et al., Long COVID in low-income and middle-income countries: the hidden public health crisis, Lancet
Jennings, Parks, Curcumin as an Antiviral Agent, Viruses
Lee, Kenward, Worrall, Vuckovic, Gentile et al., X-ray crystallographic characterization of the SARS-CoV-2 main protease polyprotein cleavage sites essential for viral processing and maturation, Nature communications
Li, Cheng, Wang, A review on traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology of the genus Peganum, J Ethnopharmacol
Majnooni, Fakhri, Bahrami, Naseri, Farzaei et al., Alkaloids as Potential Phytochemicals against SARS-CoV-2: Approaches to the Associated Pivotal Mechanisms, Evid Based Complement Alternat Med
Moradi, Karimi, Rafieian-Kopaei, Fotouhi, In vitro antiviral effects of Peganum harmala seed extract and its total alkaloids against Influenza virus, Microbial pathogenesis
Nag, Banerjee, Paul, Kundu, Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study, Comput Biol Med
Narwal, Armache, Edwards, Murakami, SARS-CoV-2 polyprotein substrate regulates the stepwise M(pro) cleavage reaction, J Biol Chem
Nazari, Rameshrad, Hosseinzadeh, Toxicological Effects of Glycyrrhiza glabra (Licorice): A Review, Phytother Res
Noske, Song, Fernandes, Chalk, Elmassoudi et al., An in-solution snapshot of SARS-COV-2 main protease maturation process and inhibition, Nature communications
Panneer, Kantamaneni, Akkayasamy, Susairaj, Panda et al., The Great Lockdown in the Wake of COVID-19 and Its Implications: Lessons for Low and Middle-Income Countries, International journal of environmental research and public health
Park, Jang, Park, Park, Park, Epigallocatechin Gallate (EGCG), a Green Tea Polyphenol, Reduces Coronavirus Replication in a Mouse Model, Viruses
Richart, Li, Mizushina, Chang, Chung et al., Synergic effect of curcumin and its structural analogue (Monoacetylcurcumin) on anti-influenza virus infection, J Food Drug Anal
Shafiee, Athar, Shahid, Ghafoor, Ayyan et al., Curcumin for the treatment of COVID-19 patients: A meta-analysis of randomized controlled trials, Phytother Res
Shin-Ya, Nakashio, Ohgitani, Suganami, Kawamoto et al., Effects of tea, catechins and catechin derivatives on Omicron subvariants of SARS-CoV-2, Sci Rep
Shoba, Joy, Joseph, Majeed, Rajendran et al., Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers, Planta medica
Stamm, Partheymuller, Mosor, Ritschl, Kritzinger et al., Determinants of COVID-19 vaccine fatigue, Nat Med
Steinmann, Buer, Pietschmann, Steinmann, Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea, Br J Pharmacol
Thimmulappa, Mudnakudu-Nagaraju, Shivamallu, Subramaniam, Radhakrishnan et al., Antiviral and immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19, Heliyon
Thümmler, Beckmann, Sehl, Soddemann, Brass et al., Fluoxetine and Sertraline Potently Neutralize the Replication of Distinct SARS-CoV-2 Variants, Viruses
Tuzun, Nasibova, Garaev, Sayin, Ataseven, Could Peganum harmala be effective in the treatment of COVID-19?, Bratisl Lek Listy
Van De Sand, Bormann, Alt, Schipper, Heilingloh et al., Glycyrrhizin Effectively Inhibits SARS-CoV-2 Replication by Inhibiting the Viral Main Protease, Viruses
Van De Sand, Bormann, Schmitz, Heilingloh, Witzke et al., Antiviral Active Compounds Derived from Natural Sources against Herpes Simplex Viruses, Viruses
Van Gelderen, Bijlsma, Van Dokkum, Savelkoul, Glycyrrhizic acid: the assessment of a no effect level, Hum Exp Toxicol
Vanblargan, Errico, Halfmann, Zost, Crowe et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies, Nat Med
Who, Tracking SARS-CoV-2 variants 2024
Widera, Wilhelm, Toptan, Raffel, Kowarz et al., Generation of a Sleeping Beauty Transposon-Based Cellular System for Rapid and Sensitive Screening for Compounds and Cellular Factors Limiting SARS-CoV-2 Replication, Frontiers in microbiology
Yang, Li, Huang, Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection, Nanoscale
Zendejas-Hernandez, Alcantara-Martinez, Vivar, Valenzuela, Espinoza et al., Nebulized glycyrrhizin/enoxolone drug modulates IL-17A in COVID-19 patients: a randomized clinical trial, Frontiers in immunology
Zhao, Deng, Bai, Guo, Kai et al., Promising natural products against SARS-CoV-2: Structure, function, and clinical trials, Phytother Res
DOI record:
{
"DOI": "10.1186/s12906-026-05253-1",
"ISSN": [
"2662-7671"
],
"URL": "http://dx.doi.org/10.1186/s12906-026-05253-1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>SARS-CoV-2 remains a major global health challenge, as infection can lead to potential life-threatening conditions such as COVID-19. Emerging variants of the virus are characterized by higher transmission rates and immune escape mutations, enabling them to evade vaccine-induced immunity. Existing treatment options, including monoclonal antibodies, are often variant-specific and not widely accessible, especially in low- and middle-income countries. Natural compounds derived from medicinal herbs and green tea have demonstrated antiviral activity against various viruses and may offer promising, variant-independent therapeutic potential.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In this study, we examined the antiviral activity of four plant-derived compounds: glycyrrhizin, curcumin, harmaline, and (-)-epigallocatechin. The compounds were tested in vitro against SARS-CoV-2 D614G, Omicron BA.5, and Omicron XBB.1. The antiviral efficacy was assessed at subtoxic concentrations to evaluate potential therapeutic applicability.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>All tested compounds showed effective neutralization of SARS-CoV-2 D614G, Omicron BA.5, and Omicron XBB.1 at subtoxic concentrations. In particular, glycyrrhizin, curcumin, and harmaline exhibited potent antiviral activity across all tested variants.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Our findings support the potential of glycyrrhizin, curcumin, and harmaline as variant-independent treatment candidates for COVID-19. However, further clinical studies are necessary to validate their efficacy and safety in vivo.</jats:p>\n </jats:sec>",
"alternative-id": [
"5253"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "26 June 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "8 January 2026"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "22 January 2026"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The study was approved by the local ethics committee, Ethik-Kommission der Universität Duisburg-Essen (20-9374-BO), and was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent to participate was obtained from all participants."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "All authors have read the manuscript in its entirety and consent to its submission and publication in the present form."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Grüneberg",
"given": "Rabea",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zydek",
"given": "Isabel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elsner",
"given": "Carina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scheiermann",
"given": "Evelyn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dittmer",
"given": "Ulf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meyer",
"given": "Folker",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kraiselburd",
"given": "Ivana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rohn",
"given": "Hana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Witzke",
"given": "Oliver",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thümmler",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krawczyk",
"given": "Adalbert",
"sequence": "additional"
}
],
"container-title": "BMC Complementary Medicine and Therapies",
"container-title-short": "BMC Complement Med Ther",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2026,
1,
22
]
],
"date-time": "2026-01-22T18:17:21Z",
"timestamp": 1769105841000
},
"deposited": {
"date-parts": [
[
2026,
1,
22
]
],
"date-time": "2026-01-22T18:17:26Z",
"timestamp": 1769105846000
},
"funder": [
{
"DOI": "10.13039/100017590",
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/100017590",
"id-type": "DOI"
}
],
"name": "Universitätsklinikum Essen"
}
],
"indexed": {
"date-parts": [
[
2026,
1,
23
]
],
"date-time": "2026-01-23T09:24:14Z",
"timestamp": 1769160254465,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2026,
1,
22
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
22
]
],
"date-time": "2026-01-22T00:00:00Z",
"timestamp": 1769040000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
22
]
],
"date-time": "2026-01-22T00:00:00Z",
"timestamp": 1769040000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/article/10.1186/s12906-026-05253-1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2026,
1,
22
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
22
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.3390/ijerph19010610",
"doi-asserted-by": "publisher",
"key": "5253_CR1",
"unstructured": "Panneer S, Kantamaneni K, Akkayasamy VS, Susairaj AX, Panda PK, Acharya SS, et al. The great lockdown in the wake of COVID-19 and its implications: lessons for low and Middle-Income countries. Int J Environ Res Public Health. 2022;19(1):610. https://doi.org/10.3390/ijerph19010610."
},
{
"DOI": "10.1186/s12889-024-19154-w",
"author": "JM Gmanyami",
"doi-asserted-by": "crossref",
"first-page": "1643",
"issue": "1",
"journal-title": "BMC Public Health",
"key": "5253_CR2",
"unstructured": "Gmanyami JM, Quentin W, Lambert O, Jarynowski A, Belik V, Amuasi JH. Excess mortality during the COVID-19 pandemic in low-and lower-middle-income countries: a systematic review and meta-analysis. BMC Public Health. 2024;24(1):1643.",
"volume": "24",
"year": "2024"
},
{
"DOI": "10.1016/S0140-6736(23)01685-9",
"author": "W Jassat",
"doi-asserted-by": "crossref",
"first-page": "1115",
"issue": "10408",
"journal-title": "Lancet",
"key": "5253_CR3",
"unstructured": "Jassat W, Reyes LF, Munblit D, Caoili J, Bozza F, Hashmi M, et al. Long COVID in low-income and middle-income countries: the hidden public health crisis. Lancet. 2023;402(10408):1115–7.",
"volume": "402",
"year": "2023"
},
{
"DOI": "10.1038/s41591-024-03173-6",
"author": "Z Al-Aly",
"doi-asserted-by": "crossref",
"first-page": "2148",
"issue": "8",
"journal-title": "Nat Med",
"key": "5253_CR4",
"unstructured": "Al-Aly Z, Davis H, McCorkell L, Soares L, Wulf-Hanson S, Iwasaki A, et al. Long COVID science, research and policy. Nat Med. 2024;30(8):2148–64.",
"volume": "30",
"year": "2024"
},
{
"DOI": "10.1002/jmv.27697",
"author": "S Chenchula",
"doi-asserted-by": "crossref",
"first-page": "2969",
"issue": "7",
"journal-title": "J Med Virol",
"key": "5253_CR5",
"unstructured": "Chenchula S, Karunakaran P, Sharma S, Chavan M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J Med Virol. 2022;94(7):2969–76.",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2024.1363572",
"author": "JJ Carvajal",
"doi-asserted-by": "crossref",
"first-page": "1363572",
"journal-title": "Front Immunol",
"key": "5253_CR6",
"unstructured": "Carvajal JJ, Garcia-Castillo V, Cuellar SV, Campillay-Veliz CP, Salazar-Ardiles C, Avellaneda AM, et al. New insights into the pathogenesis of SARS-CoV-2 during and after the COVID-19 pandemic. Front Immunol. 2024;15:1363572.",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1056/NEJMra2206573",
"author": "DH Barouch",
"doi-asserted-by": "crossref",
"first-page": "1011",
"issue": "11",
"journal-title": "New Engl J Med",
"key": "5253_CR7",
"unstructured": "Barouch DH. Covid-19 Vaccines - Immunity, Variants, boosters. New Engl J Med. 2022;387(11):1011–20.",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1038/s41591-023-02282-y",
"author": "TA Stamm",
"doi-asserted-by": "crossref",
"first-page": "1164",
"issue": "5",
"journal-title": "Nat Med",
"key": "5253_CR8",
"unstructured": "Stamm TA, Partheymuller J, Mosor E, Ritschl V, Kritzinger S, Alunno A, et al. Determinants of COVID-19 vaccine fatigue. Nat Med. 2023;29(5):1164–71.",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2023.1150667",
"author": "M Bormann",
"doi-asserted-by": "crossref",
"first-page": "1150667",
"journal-title": "Front Immunol",
"key": "5253_CR9",
"unstructured": "Bormann M, Brochhagen L, Alt M, Otte M, Thummler L, van de Sand L, et al. Immune responses in COVID-19 patients during breakthrough infection with SARS-CoV-2 variants Delta, Omicron-BA.1 and Omicron-BA.5. Front Immunol. 2023;14:1150667.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2109072",
"author": "M Bergwerk",
"doi-asserted-by": "crossref",
"first-page": "1474",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "5253_CR10",
"unstructured": "Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–84.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(22)00311-5",
"author": "D Focosi",
"doi-asserted-by": "crossref",
"first-page": "e311",
"issue": "11",
"journal-title": "Lancet Infect Dis",
"key": "5253_CR11",
"unstructured": "Focosi D, McConnell S, Casadevall A, Cappello E, Valdiserra G, Tuccori M. Monoclonal antibody therapies against SARS-CoV-2. Lancet Infect Dis. 2022;22(11):e311–26.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1038/s41579-021-00573-0",
"author": "WT Harvey",
"doi-asserted-by": "crossref",
"first-page": "409",
"issue": "7",
"journal-title": "Nat Rev Microbiol",
"key": "5253_CR12",
"unstructured": "Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, et al. SARS-CoV-2 variants, Spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–24.",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01678-y",
"author": "LA VanBlargan",
"doi-asserted-by": "crossref",
"first-page": "490",
"issue": "3",
"journal-title": "Nat Med",
"key": "5253_CR13",
"unstructured": "VanBlargan LA, Errico JM, Halfmann PJ, Zost SJ, Crowe JE Jr., Purcell LA, et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022;28(3):490–5.",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1007/s12325-025-03218-3",
"author": "MCS Alves",
"doi-asserted-by": "crossref",
"first-page": "3045",
"issue": "7",
"journal-title": "Adv Ther",
"key": "5253_CR14",
"unstructured": "Alves MCS, da Silva RCC, de Leitao-Junior SSP, de Balbino VQ. Therapeutic approaches for COVID-19: A review of antiviral Treatments, Immunotherapies, and emerging interventions. Adv Ther. 2025;42(7):3045–58.",
"volume": "42",
"year": "2025"
},
{
"DOI": "10.1002/ptr.7580",
"author": "Y Zhao",
"doi-asserted-by": "crossref",
"first-page": "3833",
"issue": "10",
"journal-title": "Phytother Res",
"key": "5253_CR15",
"unstructured": "Zhao Y, Deng S, Bai Y, Guo J, Kai G, Huang X, et al. Promising natural products against SARS-CoV-2: Structure, function, and clinical trials. Phytother Res. 2022;36(10):3833–58.",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.3390/ijerph19010610",
"doi-asserted-by": "publisher",
"key": "5253_CR16",
"unstructured": "Bijelic K, Hitl M, Kladar N. Phytochemicals in the prevention and treatment of SARS-CoV-2-Clinical evidence. Antibiot (Basel). 2022;11(11):1614. https://doi.org/10.3390/ijerph19010610."
},
{
"DOI": "10.1016/j.phrs.2020.105224",
"author": "YQ He",
"doi-asserted-by": "crossref",
"first-page": "105224",
"journal-title": "Pharmacol Res",
"key": "5253_CR17",
"unstructured": "He YQ, Zhou CC, Yu LY, Wang L, Deng JL, Tao YL, et al. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol Res. 2021;163:105224.",
"volume": "163",
"year": "2021"
},
{
"DOI": "10.3390/v13071386",
"doi-asserted-by": "publisher",
"key": "5253_CR18",
"unstructured": "van de Sand L, Bormann M, Schmitz Y, Heilingloh CS, Witzke O, Krawczyk A, et al. Antiviral active compounds derived from natural sources against herpes simplex viruses. Viruses. 2021;13(7):1386. https://doi.org/10.3390/v13071386."
},
{
"DOI": "10.1016/S0140-6736(03)13615-X",
"author": "J Cinatl",
"doi-asserted-by": "crossref",
"first-page": "2045",
"issue": "9374",
"journal-title": "Lancet",
"key": "5253_CR19",
"unstructured": "Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361(9374):2045–6.",
"volume": "361",
"year": "2003"
},
{
"DOI": "10.3390/v12111242",
"doi-asserted-by": "publisher",
"key": "5253_CR20",
"unstructured": "Jennings MR, Parks RJ. Curcumin as an antiviral agent. Viruses. 2020;12(11):1242 https://doi.org/10.3390/v12111242."
},
{
"DOI": "10.1038/srep27539",
"author": "A Ali",
"doi-asserted-by": "crossref",
"first-page": "27539",
"journal-title": "Sci Rep",
"key": "5253_CR21",
"unstructured": "Ali A, Banerjea AC. Curcumin inhibits HIV-1 by promoting Tat protein degradation. Sci Rep. 2016;6:27539.",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1016/j.jfda.2017.12.006",
"author": "SM Richart",
"doi-asserted-by": "crossref",
"first-page": "1015",
"issue": "3",
"journal-title": "J Food Drug Anal",
"key": "5253_CR22",
"unstructured": "Richart SM, Li YL, Mizushina Y, Chang YY, Chung TY, Chen GH, et al. Synergic effect of Curcumin and its structural analogue (Monoacetylcurcumin) on anti-influenza virus infection. J Food Drug Anal. 2018;26(3):1015–23.",
"volume": "26",
"year": "2018"
},
{
"DOI": "10.1039/C5NR07918G",
"author": "XX Yang",
"doi-asserted-by": "crossref",
"first-page": "3040",
"issue": "5",
"journal-title": "Nanoscale",
"key": "5253_CR23",
"unstructured": "Yang XX, Li CM, Huang CZ. Curcumin modified silver nanoparticles for highly efficient Inhibition of respiratory syncytial virus infection. Nanoscale. 2016;8(5):3040–8.",
"volume": "8",
"year": "2016"
},
{
"DOI": "10.1038/s41598-023-27954-0",
"author": "A Hegazy",
"doi-asserted-by": "crossref",
"first-page": "1612",
"issue": "1",
"journal-title": "Sci Rep",
"key": "5253_CR24",
"unstructured": "Hegazy A, Mahmoud SH, Elshaier Y, Shama NMA, Nasr NF, Ali MA, et al. Antiviral activities of plant-derived Indole and beta-carboline alkaloids against human and avian influenza viruses. Sci Rep. 2023;13(1):1612.",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1016/j.antiviral.2015.09.003",
"author": "D Chen",
"doi-asserted-by": "crossref",
"first-page": "27",
"journal-title": "Antiviral Res",
"key": "5253_CR25",
"unstructured": "Chen D, Su A, Fu Y, Wang X, Lv X, Xu W, et al. Harmine blocks herpes simplex virus infection through downregulating cellular NF-kappaB and MAPK pathways induced by oxidative stress. Antiviral Res. 2015;123:27–38.",
"volume": "123",
"year": "2015"
},
{
"DOI": "10.1111/bph.12009",
"author": "J Steinmann",
"doi-asserted-by": "crossref",
"first-page": "1059",
"issue": "5",
"journal-title": "Br J Pharmacol",
"key": "5253_CR26",
"unstructured": "Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol. 2013;168(5):1059–73.",
"volume": "168",
"year": "2013"
},
{
"DOI": "10.3390/v13040609",
"author": "L van de Sand",
"doi-asserted-by": "crossref",
"first-page": "609",
"issue": "4",
"journal-title": "Viruses",
"key": "5253_CR27",
"unstructured": "van de Sand L, Bormann M, Alt M, Schipper L, Heilingloh CS, Steinmann E, et al. Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses. 2021;13(4):609.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/v13101914",
"doi-asserted-by": "publisher",
"key": "5253_CR28",
"unstructured": "Bormann M, Alt M, Schipper L, van de Sand L, Le-Trilling VTK, Rink L, et al. Turmeric root and its bioactive ingredient Curcumin effectively neutralize SARS-CoV-2 in vitro. Viruses-Basel. 2021;13(10):1914. https://doi.org/10.3390/v13101914."
},
{
"DOI": "10.33425/2639-9458.1116",
"doi-asserted-by": "publisher",
"key": "5253_CR29",
"unstructured": "Hurst BL, Dickinson D, Hsu S. Epigallocatechin-3-Gallate (EGCG) inhibits SARS-CoV-2 infection in primate epithelial cells: (A short Communication). Microbiol Infect Dis. 2021;5(2):10.33425/2639-9458.1116. https://doi.org/10.33425/2639-9458.1116."
},
{
"DOI": "10.1128/jvi.00396-23",
"author": "S Dahal",
"doi-asserted-by": "crossref",
"first-page": "e0039623",
"issue": "10",
"journal-title": "J Virol",
"key": "5253_CR30",
"unstructured": "Dahal S, Clayton K, Cabral T, Cheng R, Jahanshahi S, Ahmed C, et al. On a path toward a broad-spectrum anti-viral: Inhibition of HIV-1 and coronavirus replication by SR kinase inhibitor Harmine. J Virol. 2023;97(10):e0039623.",
"volume": "97",
"year": "2023"
},
{
"DOI": "10.3389/fmicb.2021.701198",
"author": "M Widera",
"doi-asserted-by": "crossref",
"first-page": "701198",
"journal-title": "Front Microbiol",
"key": "5253_CR31",
"unstructured": "Widera M, Wilhelm A, Toptan T, Raffel JM, Kowarz E, Roesmann F, et al. Generation of a sleeping beauty Transposon-Based cellular system for rapid and sensitive screening for compounds and cellular factors limiting SARS-CoV-2 replication. Front Microbiol. 2021;12:701198.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3390/vaccines12070785",
"doi-asserted-by": "publisher",
"key": "5253_CR32",
"unstructured": "Cherneha M, Zydek I, Brass P, Korth J, Jansen S, Esser S, et al. Immunogenicity of the monovalent Omicron XBB.1.5-Adapted BNT162b2 COVID-19 vaccine in people living with HIV (PLWH). Vaccines. 2024;12(7):785. https://doi.org/10.3390/vaccines12070785."
},
{
"DOI": "10.1016/j.ajic.2020.07.031",
"doi-asserted-by": "publisher",
"key": "5253_CR33",
"unstructured": "Heilingloh CS, Aufderhorst UW, Schipper L, Dittmer U, Witzke O, Yang D, et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am J Infect Control. 2020:1273–75. https://doi.org/10.1016/j.ajic.2020.07.031."
},
{
"key": "5253_CR34",
"unstructured": "WHO, Tracking. SARS-CoV-2 variants 2024. Available from: https://www.who.int/activities/tracking-SARS-CoV-2-variants."
},
{
"DOI": "10.3390/v16040545",
"doi-asserted-by": "publisher",
"key": "5253_CR35",
"unstructured": "Thümmler L, Beckmann N, Sehl C, Soddemann M, Brass P, Bormann M, et al. Fluoxetine and Sertraline potently neutralize the replication of distinct SARS-CoV-2 variants. Viruses. 2024;16(4):545. https://doi.org/10.3390/v16040545."
},
{
"DOI": "10.3389/fimmu.2023.1282280",
"author": "U Zendejas-Hernandez",
"doi-asserted-by": "crossref",
"first-page": "1282280",
"journal-title": "Front Immunol",
"key": "5253_CR36",
"unstructured": "Zendejas-Hernandez U, Alcantara-Martinez N, Vivar DT, Valenzuela F, Sosa Espinoza A, Cervera Ceballos EE. Nebulized glycyrrhizin/enoxolone drug modulates IL-17A in COVID-19 patients: a randomized clinical trial. Front Immunol. 2023;14:1282280.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1007/s10787-022-00939-7",
"author": "AA Gomaa",
"doi-asserted-by": "crossref",
"first-page": "477",
"issue": "2",
"journal-title": "Inflammopharmacology",
"key": "5253_CR37",
"unstructured": "Gomaa AA, Mohamed HS, Abd-Ellatief RB, Gomaa MA, Hammam DS. Advancing combination treatment with glycyrrhizin and Boswellic acids for hospitalized patients with moderate COVID-19 infection: a randomized clinical trial. Inflammopharmacology. 2022;30(2):477–86.",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.1002/ptr.5893",
"author": "S Nazari",
"doi-asserted-by": "crossref",
"first-page": "1635",
"issue": "11",
"journal-title": "Phytother Res",
"key": "5253_CR38",
"unstructured": "Nazari S, Rameshrad M, Hosseinzadeh H. Toxicological effects of glycyrrhiza glabra (Licorice): A review. Phytother Res. 2017;31(11):1635–50.",
"volume": "31",
"year": "2017"
},
{
"DOI": "10.1191/096032700682694251",
"author": "CE van Gelderen",
"doi-asserted-by": "crossref",
"first-page": "434",
"issue": "8",
"journal-title": "Hum Exp Toxicol",
"key": "5253_CR39",
"unstructured": "van Gelderen CE, Bijlsma JA, van Dokkum W, Savelkoul TJ. Glycyrrhizic acid: the assessment of a no effect level. Hum Exp Toxicol. 2000;19(8):434–9.",
"volume": "19",
"year": "2000"
},
{
"DOI": "10.1038/s41467-022-32854-4",
"author": "J Lee",
"doi-asserted-by": "crossref",
"first-page": "5196",
"issue": "1",
"journal-title": "Nat Commun",
"key": "5253_CR40",
"unstructured": "Lee J, Kenward C, Worrall LJ, Vuckovic M, Gentile F, Ton A-T, et al. X-ray crystallographic characterization of the SARS-CoV-2 main protease polyprotein cleavage sites essential for viral processing and maturation. Nat Commun. 2022;13(1):5196.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.jbc.2023.104697",
"author": "M Narwal",
"doi-asserted-by": "crossref",
"first-page": "104697",
"issue": "5",
"journal-title": "J Biol Chem",
"key": "5253_CR41",
"unstructured": "Narwal M, Armache JP, Edwards TJ, Murakami KS. SARS-CoV-2 polyprotein substrate regulates the Stepwise M(pro) cleavage reaction. J Biol Chem. 2023;299(5):104697.",
"volume": "299",
"year": "2023"
},
{
"DOI": "10.1038/s41467-023-37035-5",
"author": "GD Noske",
"doi-asserted-by": "crossref",
"first-page": "1545",
"issue": "1",
"journal-title": "Nat Commun",
"key": "5253_CR42",
"unstructured": "Noske GD, Song Y, Fernandes RS, Chalk R, Elmassoudi H, Koekemoer L, et al. An in-solution snapshot of SARS-COV-2 main protease maturation process and Inhibition. Nat Commun. 2023;14(1):1545.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/j.heliyon.2021.e06350",
"author": "RK Thimmulappa",
"doi-asserted-by": "crossref",
"first-page": "e06350",
"issue": "2",
"journal-title": "Heliyon",
"key": "5253_CR43",
"unstructured": "Thimmulappa RK, Mudnakudu-Nagaraju KK, Shivamallu C, Subramaniam KJT, Radhakrishnan A, Bhojraj S, et al. Antiviral and Immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19. Heliyon. 2021;7(2):e06350.",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1021/acs.jpclett.0c00994",
"author": "T Huynh",
"doi-asserted-by": "crossref",
"first-page": "4413",
"issue": "11",
"journal-title": "J Phys Chem Lett",
"key": "5253_CR44",
"unstructured": "Huynh T, Wang H, Luan B. In Silico exploration of the molecular mechanism of clinically oriented drugs for possibly inhibiting SARS-CoV-2’s main protease. J Phys Chem Lett. 2020;11(11):4413–20.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.compbiomed.2022.105552",
"author": "A Nag",
"doi-asserted-by": "crossref",
"first-page": "105552",
"journal-title": "Comput Biol Med",
"key": "5253_CR45",
"unstructured": "Nag A, Banerjee R, Paul S, Kundu R. Curcumin inhibits Spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in Silico study. Comput Biol Med. 2022;146:105552.",
"volume": "146",
"year": "2022"
},
{
"DOI": "10.1055/s-2006-957450",
"author": "G Shoba",
"doi-asserted-by": "crossref",
"first-page": "353",
"issue": "4",
"journal-title": "Planta Med",
"key": "5253_CR46",
"unstructured": "Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of Piperine on the pharmacokinetics of Curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–6.",
"volume": "64",
"year": "1998"
},
{
"DOI": "10.1002/ptr.7724",
"author": "A Shafiee",
"doi-asserted-by": "crossref",
"first-page": "1167",
"issue": "3",
"journal-title": "Phytother Res",
"key": "5253_CR47",
"unstructured": "Shafiee A, Athar MMT, Shahid A, Ghafoor MS, Ayyan M, Zahid A, et al. Curcumin for the treatment of COVID-19 patients: A meta-analysis of randomized controlled trials. Phytother Res. 2023;37(3):1167–75.",
"volume": "37",
"year": "2023"
},
{
"DOI": "10.1016/j.jep.2017.03.049",
"author": "S Li",
"doi-asserted-by": "crossref",
"first-page": "127",
"journal-title": "J Ethnopharmacol",
"key": "5253_CR48",
"unstructured": "Li S, Cheng X, Wang C. A review on traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology of the genus peganum. J Ethnopharmacol. 2017;203:127–62.",
"volume": "203",
"year": "2017"
},
{
"DOI": "10.1016/j.micpath.2017.06.014",
"author": "MT Moradi",
"doi-asserted-by": "crossref",
"first-page": "42",
"journal-title": "Microb Pathog",
"key": "5253_CR49",
"unstructured": "Moradi MT, Karimi A, Rafieian-Kopaei M, Fotouhi F. In vitro antiviral effects of peganum Harmala seed extract and its total alkaloids against influenza virus. Microb Pathog. 2017;110:42–9.",
"volume": "110",
"year": "2017"
},
{
"author": "B Tuzun",
"first-page": "670",
"issue": "9",
"journal-title": "Bratisl Lek Listy",
"key": "5253_CR50",
"unstructured": "Tuzun B, Nasibova T, Garaev E, Sayin K, Ataseven H. Could peganum Harmala be effective in the treatment of COVID-19? Bratisl Lek Listy. 2021;122(9):670–9.",
"volume": "122",
"year": "2021"
},
{
"DOI": "10.1155/2021/6632623",
"author": "MB Majnooni",
"doi-asserted-by": "crossref",
"first-page": "6632623",
"journal-title": "Evid Based Complement Alternat Med",
"key": "5253_CR51",
"unstructured": "Majnooni MB, Fakhri S, Bahrami G, Naseri M, Farzaei MH, Echeverria J. Alkaloids as potential phytochemicals against SARS-CoV-2: approaches to the associated pivotal mechanisms. Evid Based Complement Alternat Med. 2021;2021:6632623.",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.3390/v13122533",
"doi-asserted-by": "publisher",
"key": "5253_CR52",
"unstructured": "Park R, Jang M, Park YI, Park Y, Jung W, Park J, et al. Epigallocatechin gallate (EGCG), a green tea Polyphenol, reduces coronavirus replication in a mouse model. Viruses. 2021;13(12):2533. https://doi.org/10.3390/v13122533."
},
{
"DOI": "10.1038/s41598-023-43563-3",
"author": "M Shin-Ya",
"doi-asserted-by": "crossref",
"first-page": "16577",
"issue": "1",
"journal-title": "Sci Rep",
"key": "5253_CR53",
"unstructured": "Shin-Ya M, Nakashio M, Ohgitani E, Suganami A, Kawamoto M, Ichitani M, et al. Effects of tea, Catechins and Catechin derivatives on Omicron subvariants of SARS-CoV-2. Sci Rep. 2023;13(1):16577.",
"volume": "13",
"year": "2023"
}
],
"reference-count": 53,
"references-count": 53,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1186/s12906-026-05253-1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Dose-dependent antiviral effects of glycyrrhizin, curcumin, and harmaline against clinical SARS-CoV-2 isolates, including D614G, Omicron BA.5, and Omicron XBB.1",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy"
}
