The Interaction of Vitamin D and Corticosteroids: A Mortality Analysis of 26,508 Veterans Who Tested Positive for SARS-CoV-2
et al., International Journal of Environmental Research and Public Health, doi:10.3390/ijerph19010447, Dec 2021
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
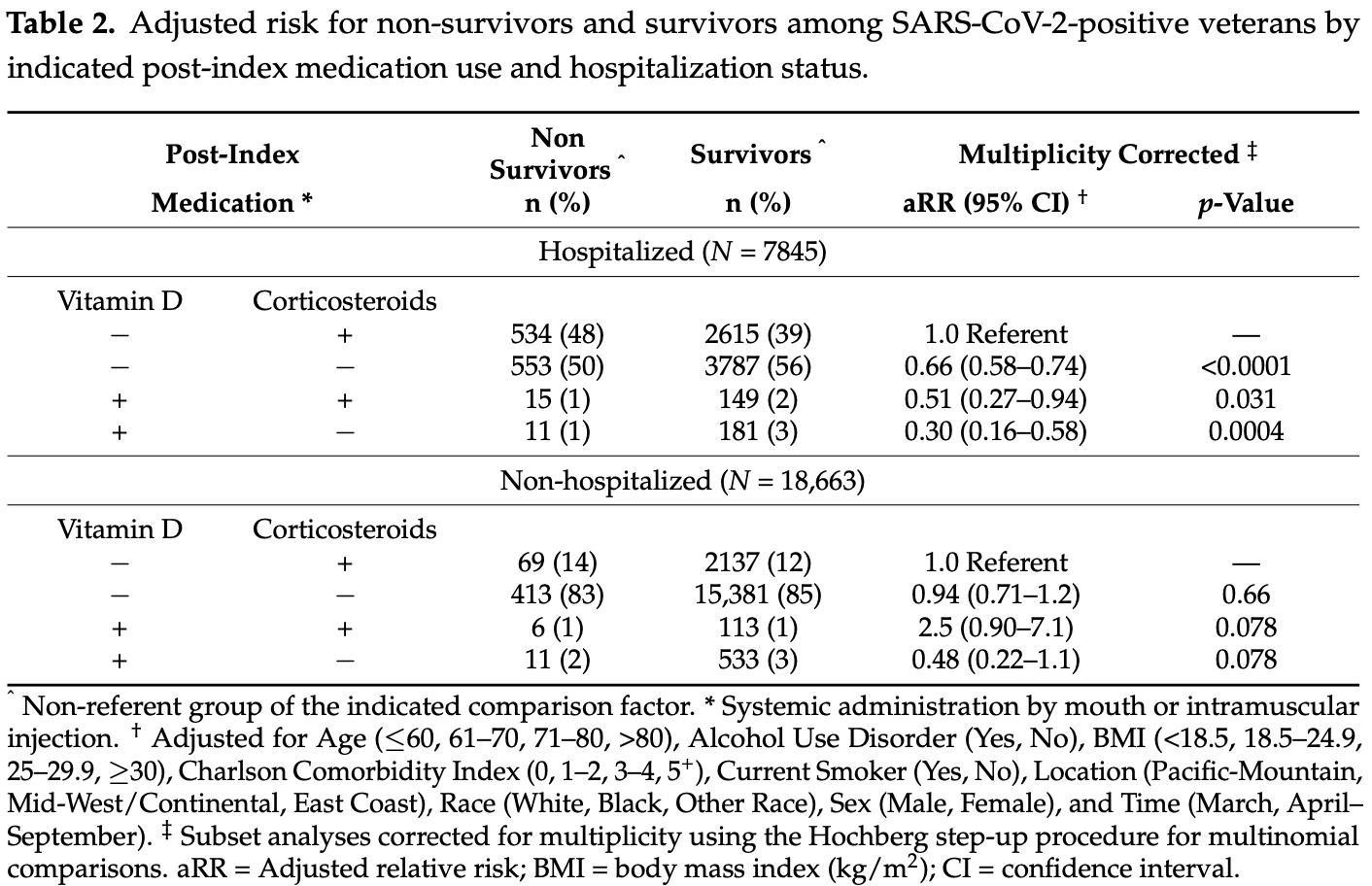
Retrospective 26,508 COVID+ veterans in USA, showing lower mortality with vitamin D use after testing positive (defined as being administered ≥7 days or half of the survival time within 2 weeks after testing), with statistical significance for hospitalized patients.
This is the 64th of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 48.9% lower, RR 0.51, p = 0.10, treatment 11 of 544 (2.0%), control 413 of 15,794 (2.6%), adjusted per study, non-hospitalized patients, vitamin D + no corticosteroids vs. no vitamin D + no corticosteroids.
|
|
risk of death, 54.5% lower, RR 0.45, p = 0.02, treatment 11 of 192 (5.7%), control 553 of 4,340 (12.7%), NNT 14, adjusted per study, hospitalized patients, vitamin D + no corticosteroids vs. no vitamin D + no corticosteroids.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Efird et al., 31 Dec 2021, retrospective, USA, peer-reviewed, 10 authors, study period 1 March, 2020 - 10 September, 2020, dosage varies.
The Interaction of Vitamin D and Corticosteroids: A Mortality Analysis of 26,508 Veterans Who Tested Positive for SARS-CoV-2
International Journal of Environmental Research and Public Health, doi:10.3390/ijerph19010447
This data-based cohort consisted of 26,508 (7%) United States veterans out of the 399,290 who tested positive for SARS-CoV-2 from 1 March to 10 September 2020. We aimed to assess the interaction of post-index vitamin D (Vit D) and corticosteroid (CRT) use on 30-day mortality among hospitalized and non-hospitalized patients with coronavirus disease 2019 (COVID-19). Combination Vit D and CRT drug use was assessed according to four multinomial pairs (−|+, −|−, +|+, +|−). Respective categorical effects were computed on a log-binomial scale as adjusted relative risk (aRR). Approximately 6% of veterans who tested positive for SARS-CoV-2 died within 30 days of their index date. Among hospitalized patients, a significantly decreased aRR was observed for the use of Vit D in the absence of CRTs relative to patients who received CRTs but not Vit D (aRR = 0.30; multiplicity corrected, p = 0.0004). Among patients receiving systemically administered CRTs (e.g., dexamethasone), the use of Vit D was associated with fewer deaths in hospitalized patients (aRR = 0.51) compared with non-hospitalized patients (aRR = 2.5) (P-for-Interaction = 0.0071). Evaluating the effect of modification of these compounds in the context of hospitalization may aid in the management of COVID-19 and provide a better understanding of the pathophysiological mechanisms underlying this and future infectious disease outbreaks.
Author Contributions: Conceptualization, J.T.E., A.S. and E.J.A.; data curation, T.S.R., A.D.T. and J.T.E.; formal analysis, J.T.E., T.S.R. and A.D.T.; funding acquisition, A.S.; methodology, J.T.E.; project administration, A.S., A.M.P. and J.U.; supervision, A.S. and J.T.E.; validation, A.D.T.; visualization,
Conflicts of Interest: The authors declare no conflict of interest.
References
Aboumrad, Shiner, Riblet, Huizenga, Neupane et al., Trends in COVID-19 cases and clinical management in Veterans Health Administration medical facilities: A national cohort study, PLoS ONE, doi:10.1371/journal.pone.0246217
Abrishami, Dalili, Mohammadi Torbati, Asgari, Arab-Ahmadi et al., Possible association of vitamin D status with lung involvement and outcome in patients with COVID-19: A retrospective study, Eur. J. Nutr, doi:10.1007/s00394-020-02411-0
Agrawal, Evaluation of Corticosteroids-Vitamin D Combination in Asthma
Al-Anouti, Mousa, Karras, Grant, Alhalwachi et al., Associations between Genetic Variants in the Vitamin D Metabolism Pathway and Severity of COVID-19 among UAE Residents, Nutrients, doi:10.3390/nu13113680
Alcala-Diaz, Limia-Perez, Gomez-Huelgas, Martin-Escalante, Cortes-Rodriguez et al., Calcifediol Treatment and Hospital Mortality Due to COVID-19: A Cohort Study, Nutrients, doi:10.3390/nu13061760
Alguwaihes, Sabico, Hasanato, Al-Sofiani, Megdad et al., Severe vitamin D deficiency is not related to SARS-CoV-2 infection but may increase mortality risk in hospitalized adults: A retrospective case-control study in an Arab Gulf country, Aging Clin. Exp. Res
Amrein, Papinutti, Mathew, Vila, Parekh, Vitamin D and critical illness: What endocrinology can learn from intensive care and vice versa, Endocr. Connect, doi:10.1530/EC-18-0184
Annweiler, Corvaisier, Gautier, Dubée, Legrand et al., Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study, Nutrients, doi:10.3390/nu12113377
Annweiler, Hanotte, Grandin De L'eprevier, Sabatier, Lafaie et al., Vitamin D and survival in COVID-19 patients: A quasi-experimental study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105771
Arthur, Vitamine, Covid, -19, Bull. Acad. Natl. Med
Arvinte, Singh, Marik, Serum Levels of Vitamin C and Vitamin D in a Cohort of Critically Ill COVID-19 Patients of a North American Community Hospital Intensive Care Unit in May 2020: A Pilot Study, Med. Drug Discov
Baktash, Hosack, Patel, Shah, Kandiah et al., Vitamin D status and outcomes for hospitalised older patients with COVID-19, Postgrad. Med. J, doi:10.1136/postgradmedj-2020-138712
Barassi, Pezzilli, Mondoni, Rinaldo, Davì et al., Vitamin D in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with non-invasive ventilation support, Panminerva Med
Basheer, Saad, Shlezinger, Assy, Convalescent Plasma Reduces Mortality and Decreases Hospitalization Stay in Patients with Moderate COVID-19 Pneumonia, Metabolites, doi:10.3390/metabo11110761
Bassatne, Basbous, Chakhtoura, El Zein, Rahme et al., The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis, Metabolism, doi:10.1016/j.metabol.2021.154753
Benjamini, Yekutieli, False Discovery Rate-Adjusted Multiple Confidence Intervals for Selected Parameters, J. Am. Stat. Assoc, doi:10.1198/016214504000001907
Benskin, The influence of vitamin D on COVID-19 outcomes
Bianconi, Mannarino, Figorilli, Cosentini, Batori et al., Prevalence of vitamin D deficiency and its prognostic impact on patients hospitalized with COVID-19, Nutrition, doi:10.1016/j.nut.2021.111408
Bilezikian, Bikle, Hewison, Lazaretti-Castro, Formenti et al., Mechanisma in endocrinology: Vitamin D and COVID-19, Eur. J. Endocrinol
Bland, Introduction to Medical Statistics
Boaz, Vitamin D and COVID-19: Partial Evidence, BCHD, doi:10.31989/bchd.v4i3.796
Borsche, Glauner, Von Mendel, COVID-19 mortality risk correlates inversely with vitamin D3 status, and a mortality rate close to zero could theoretically be achieved at 50 ng/ml 25(OH)D3: Results of a systematic review and meta-analysis, medRxiv, doi:10.3390/nu13103596
Brenner, Vitamin D Supplementation to Prevent COVID-19 Infections and Deaths-Accumulating Evidence from Epidemiological and Intervention Studies Calls for Immediate Action, Nutrients, doi:10.3390/nu13020411
Breslow, Day, The Analysis of Case-control Studies, Statistical Methods in Cancer Research
Burt, Billington, Rose, Raymond, Hanley et al., Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2019.11889
Burton, Kehrli, Jr, Kapil, Horst, Regulation of L-selectin and CD18 on bovine neutrophils by glucocorticoids: Effects of cortisol and dexamethasone, J. Leukoc. Biol, doi:10.1002/jlb.57.2.317
Butler-Laporte, Nakanishi, Mooser, Morrison, Abdullah et al., Vitamin D and COVID-19 susceptibility and severity in the COVID-19 Host Genetics Initiative: A Mendelian randomization study, PLoS Med, doi:10.1371/journal.pmed.1003605
Campi, Gennari, Merlotti, Mingiano, Frosali et al., Vitamin D and COVID-19 severity and related mortality: A prospective study in Italy, BMC Infect. Dis
Cangiano, Fatti, Danesi, Gazzano, Croci et al., Mortality in an Italian nursing home during COVID-19 pandemic: Correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests, Aging, doi:10.18632/aging.202307
Carpagnano, Di Lecce, Quaranta, Zito, Buonamico et al., Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19, J. Endocrinol. Investig, doi:10.1007/s40618-020-01370-x
Castillo, Entrenas Costa, Vaquero Barrios, Alcalá Díaz, López Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105751
Cereda, Bogliolo, Klersy, Lobascio, Masi et al., Vitamin D 25OH deficiency in COVID-19 patients admitted to a tertiary referral hospital, Clin. Nutr
Cereda, Bogliolo, Lobascio, Barichella, Zecchinelli et al., Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy, Nutrition, doi:10.1016/j.nut.2020.111055
Chapman, Peterson, Turano, Box, Wallace et al., A Natural Language Processing System for National COVID-19 Surveillance in the US Department of Veterans Affairs
Chinapaka, Baba, Kandakatla, Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease, Sci. Rep
Cohn, Cirillo, Murphy, Krigbaum, Wallace, SARS-CoV-2 vaccine protection and deaths among US veterans during, Science, doi:10.1126/science.abm0620
Craft, Travassos, Foppiano Palacios, Openshaw, Inadequate Minority Representation within SARS-CoV-2 Vaccine Trials, Am. J. Trop. Med. Hyg, doi:10.4269/ajtmh.20-1294
Cronstein, Kimmel, Levin, Martiniuk, Weissmann, A mechanism for the antiinflammatory effects of corticosteroids: The glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelialleukocyte adhesion molecule 1 and intercellular adhesion molecule 1, Proc. Natl. Acad. Sci, doi:10.1073/pnas.89.21.9991
Da Rocha, Atallah, Aldrighi, Pires, Dos Santos Puga et al., Insufficient evidence for vitamin D use in COVID-19: A rapid systematic review, Int. J. Clin. Pract, doi:10.1111/ijcp.14649
Davidson, Walker, Truby, Do glucocorticosteroids alter vitamin D status? A systematic review with meta-analyses of observational studies, J. Clin. Endocrinol. Metab, doi:10.1210/jc.2011-2757
Dayal, Gupta, Surulinathi, Nanda, COVID-19 and Vitamin D Deficiency: A Scientometric Assessment of Global Publications during 2020-21, J. Young Pharm, doi:10.5530/jyp.2021.13s.77
De Smet, De Smet, Herroelen, Gryspeerdt, Martens, Serum 25(OH)D Level on Hospital Admission Associated with COVID-19 Stage and Mortality, Am. J. Clin. Pathol, doi:10.1093/ajcp/aqaa252
Devoto, Arcurio, Fetta, Ley, Rodney et al., Inflammation Relates to Chronic Behavioral and Neurological Symptoms in Military Personnel with Traumatic Brain Injuries, Cell Transplant, doi:10.1177/0963689717714098
Dhawan, Christakos, Novel regulation of 25-hydroxyvitamin D3 24-hydroxylase (24(OH)ase) transcription by glucocorticoids:Ccooperative effects of the glucocorticoid receptor, C/EBP beta, and the Vitamin D receptor in 24(OH)ase transcription, J. Cell. Biochem, doi:10.1002/jcb.22645
Dramé, Cofais, Hentzien, Proye, Coulibaly et al., Relation between Vitamin D and COVID-19 in Aged People: A Systematic Review, Nutrients
Duvall, Scehnet, VA Informatics and Computing Infrastructure: Introduction to the VA COVID-19 Shared Data Resource and Its Use for Research NW Washington DC 204202020
Efird, Goldilocks Rounding: Achieving Balance between Accuracy and Parsimony in the Reporting of Relative Effect Estimates, Cancer Inform, doi:10.1177/1176935120985132
Escobar, Adams, Liu, Soltesz, Chen et al., Racial Disparities in COVID-19 Testing and Outcomes: Retrospective Cohort Study in an Integrated Health System, Ann. Intern. Med, doi:10.7326/M20-6979
Faul, Kerley, Love, O'neill, Cody et al., Vitamin D Deficiency and ARDS after SARS-CoV-2 Infection, Ir. Med. J
Ferreira, Kleijwegt, Waelkens, Lage, Nikolic et al., Differential protein pathways in 1,25-dihydroxyvitamin d(3) and dexamethasone modulated tolerogenic human dendritic cells, J. Proteome Res, doi:10.1021/pr200724e
Filep, Delalandre, Payette, Földes-Filep, Glucocorticoid receptor regulates expression of L-selectin and CD11/CD18 on human neutrophils, Circulation, doi:10.1161/01.CIR.96.1.295
Fogleman, Janney, Cialdella-Kam, Flint, Vitamin D Deficiency in the Military: It's Time to Act!, Mil. Med, doi:10.1093/milmed/usab402
Garg, Patel, Pham, Whitaker, O'halloran et al., Trends Among U.S. Adults Hospitalized With COVID-19, March to, Ann. Intern. Med, doi:10.7326/M21-1991
Geara, Castellanos, Bassil, Schuller-Levis, Park et al., Effects of parathyroid hormone on immune function, Clin. Dev. Immunol, doi:10.1155/2010/418695
Giannini, Passeri, Tripepi, Sella, Fusaro et al., Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study, Nutrients, doi:10.3390/nu13010219
Gibson-Moore, Vitamin, What's new a year on from the COVID-19 outbreak?, Nutr. Bull, doi:10.1111/nbu.12499
Griffin, Hewison, Hopkin, Kenny, Quinton et al., Perspective: Vitamin D supplementation prevents rickets and acute respiratory infections when given as daily maintenance but not as intermittent bolus: Implications for COVID-19, Clin. Med, doi:10.7861/clinmed.2021-0035
Griffith, Morris, Tudball, Herbert, Mancano et al., Collider bias undermines our understanding of COVID-19 disease risk and severity, Nat. Commun
Gönen, Alaylıo Glu, Durcan, Özdemir, Şahin et al., Rapid and Effective Vitamin D Supplementation May Present Better Clinical Outcomes in COVID-19 (SARS-CoV-2) Patients by Altering Serum INOS1, IL1B, IFNg, Cathelicidin-LL37, and ICAM1, Nutrients, doi:10.3390/nu13114047
Güven, Gültekin, The effect of high-dose parenteral vitamin D(3) on COVID-19-related inhospital mortality in critical COVID-19 patients during intensive care unit admission: An observational cohort study, Eur. J. Clin. Nutr
Hahn, Glucocorticosteroids are potential confounders in studies of vitamin D and asthma, Am. J. Respir. Crit. Care Med, doi:10.1164/ajrccm.185.11.1245
Han, Jones, Tangpricha, Brown, Brown et al., High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial, J. Clin. Transl. Endocrinol, doi:10.1016/j.jcte.2016.04.004
Hariyanto, Intan, Hananto, Harapan, Kurniawan, Vitamin D supplementation and Covid-19 outcomes: A systematic review, meta-analysis and meta-regression, Rev. Med. Virol, doi:10.1002/rmv.2269
Hernández, Nan, Fernandez-Ayala, García-Unzueta, Hernández-Hernández et al., Vitamin D Status in Hospitalized Patients with SARS-CoV-2
Hidalgo, Deeb, Pike, Johnson, Trump, Dexamethasone enhances 1alpha,25-dihydroxyvitamin D3 effects by increasing vitamin D receptor transcription, J. Biol. Chem, doi:10.1074/jbc.M111.244061
Hochberg, A sharper Bonferroni procedure for multiple tests of significance, Biometrika, doi:10.1093/biomet/75.4.800
Hoerster, Lehavot, Simpson, Mcfall, Reiber et al., Health and health behavior differences: U.S. Military, veteran, and civilian men, Am. J. Prev. Med, doi:10.1016/j.amepre.2012.07.029
Holick, Vitamin, Deficiency, None, N. Engl. J. Med, doi:10.1056/NEJMra070553
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in Hospitalized Patients with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2021436
Hsu, Prince, Williams, Allen, Burke et al., Clinical and biomarker modifiers of vitamin D treatment response: The multi-ethnic study of atherosclerosis, Am. J. Clin. Nutr, doi:10.1093/ajcn/nqab390
Hung, Lee, Lynch, Li, Poonnen et al., Chemoradiation treatment patterns among United States Veteran Health Administration patients with unresectable stage III non-small cell lung cancer, BMC Cancer, doi:10.1186/s12885-021-08577-y
Iacobucci, COVID-19: NHS bosses told to assess risk to ethnic minority staff who may be at greater risk, BMJ
Ioannou, O'hare, Berry, Fan, Crothers et al., Trends over time in the risk of adverse outcomes among patients with SARS-CoV-2 infection, Clin. Infect. Dis, doi:10.1093/cid/ciab419
Ismailova, White, Vitamin, infections and immunity, Rev. Endocr. Metab. Disord, doi:10.1007/s11154-021-09679-5
Jain, Chaurasia, Sengar, Singh, Mahor et al., Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers, Sci. Rep, doi:10.1038/s41598-020-77093-z
Jevalikar, Mithal, Singh, Sharma, Farooqui et al., Lack of association of baseline 25-hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID-19, Sci. Rep, doi:10.1038/s41598-021-85809-y
Jungreis, Kellis, Mathematical analysis of Córdoba calcifediol trial suggests strong role for Vitamin D in reducing ICU admissions of hospitalized COVID-19 patients, medRxiv, doi:10.1101/2020.11.08.20222638
Karonova, Andreeva, Vashukova, Serum 25 (OH) D level in COVID-19 patients, J. Infectol
Kazemi, Mohammadi, Aghababaee, Golzarand, Clark et al., Association of Vitamin D Status with SARS-CoV-2 Infection or COVID-19 Severity: A Systematic Review and Meta-analysis, Adv. Nutr, doi:10.1093/advances/nmab012
Kerget, Kerget, Kızıltunç, Koçak, Araz et al., Evaluation of the relationship of serum vitamin D levels in COVID-19 patients with clinical course and prognosis, Tuberk. Torak, doi:10.5578/tt.70027
Ketha, Thacher, Oberhelman, Fischer, Singh et al., Comparison of the effect of daily versus bolus dose maternal vitamin D(3) supplementation on the 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) ratio, Bone, doi:10.1016/j.bone.2018.02.024
Kishore, Grimes, Anderson, Bharti, Joseph et al., Recommendations for High Vitamin D Supplementation to help Fight the COVID-19 Pandemic and its Inclusion in National COVID-19 Management Guidelines, Epidem. Int
Klinger, Alexiewicz, Linker-Israeli, Pitts, Gaciong et al., Effect of parathyroid hormone on human T cell activation, Kidney Int, doi:10.1038/ki.1990.147
Kojima, Tamai, Masaki, Gatchell, Epure et al., Prevalence of vitamin D deficiency and association with functional status in newly admitted male veteran nursing home residents, J. Am. Geriatr. Soc, doi:10.1111/jgs.12495
Korf, Decallonne, Mathieu, Vitamin D for infections, Curr. Opin. Endocrinol. Diabetes Obes, doi:10.1097/MED.0000000000000108
Kurahashi, Matsunuma, Kawane, Abe, Horiuchi, Dexamethasone enhances vitamin D-24-hydroxylase expression in osteoblastic (UMR-106) and renal (LLC-PK1) cells treated with 1alpha,25-dihydroxyvitamin D3, Endocrine
Lakkireddy, Gadiga, Malathi, Karra, Raju et al., None
Leaf, Ginde, Vitamin D3 to Treat COVID-19: Different Disease, Same Answer, JAMA, doi:10.1001/jama.2020.26850
Lewin, Ladefoged, Brandi, Olgaard, Parathyroid hormone dependent T cell proliferation in uremic rats, Kidney Int, doi:10.1038/ki.1993.255
Ling, Broad, Murphy, Pappachan, Pardesi-Newton et al., High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study, Nutrients, doi:10.3390/nu12123799
Lohia, Nguyen, Patel, Kapur, Exploring the link between vitamin D and clinical outcomes in COVID-19, Am. J. Physiol. Metab, doi:10.1152/ajpendo.00517.2020
Loucera, Peña-Chilet, Esteban-Medina, Muñoyerro-Muñiz, Villegas et al., Real world evidence of calcifediol or vitamin D prescription and mortality rate of COVID-19 in a retrospective cohort of hospitalized Andalusian patients, Sci. Rep, doi:10.1038/s41598-021-02701-5
Luo, Liao, Shen, Li, Cheng, Vitamin D Deficiency Is Associated with COVID-19 Incidence and Disease Severity in Chinese People, J. Nutr, doi:10.1093/jn/nxaa332
Macaya, Espejo Paeres, Valls, Fernández-Ortiz, González Del Castillo et al., Interaction between age and vitamin D deficiency in severe COVID-19 infection, Nutr. Hosp
Maghbooli, Sahraian, Jamalimoghadamsiahkali, Asadi, Zarei et al., Treatment with 25-Hydroxyvitamin D(3) (Calcifediol) Is Associated with a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients with COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial, Endocr. Pract, doi:10.1016/j.eprac.2021.09.016
Maloney, Goolkasian, Vitamin, States Observed in U.S. Marines and Navy Sailors with Early Multi-Symptom Illness, Biomolecules, doi:10.3390/biom10071032
Mandal, Wenban, Heer, Baktash, Missouris, Does Vitamin D have a role to play in COVID-19 in the dexamethasone era?, Diabetes Metab. Syndr. Clin. Res. Rev, doi:10.1016/j.dsx.2021.102237
Mardani, Alamdary, Mousavi Nasab, Gholami, Ahmadi et al., Association of vitamin D with the modulation of the disease severity in COVID-19, Virus Res, doi:10.1016/j.virusres.2020.198148
Mariani, Giménez, Bergam, Tajer, Antonietti et al., Association Between Vitamin D Deficiency and COVID-19 Incidence, Complications, and Mortality in 46 Countries: An Ecological Study, Health Secur, doi:10.1089/hs.2020.0137
Martineau, Jolliffe, Greenberg, Aloia, Bergman et al., Vitamin D supplementation to prevent acute respiratory infections: Individual participant data meta-analysis, Health Technol. Assess, doi:10.3310/hta23020
Mazess, Bischoff-Ferrari, Dawson-Hughes, Vitamin, Bolus Is Bogus-A Narrative Review, JBMR Plus, doi:10.1002/jbm4.10567
Mehta, Agrawal, Appanna, Chaudagar, Vitamin D improves corticosteroid efficacy and attenuates its side-effects in an animal model of asthma, Can. J. Physiol. Pharmacol, doi:10.1139/cjpp-2014-0323
Mendy, Apewokin, Wells, Morrow, Factors Associated with Hospitalization and Disease Severity in a Racially and Ethnically Diverse Population of COVID-19 Patients, medRxiv, doi:10.1101/2020.06.25.20137323
Munshi, Hussein, Toraih, Elshazli, Jardak et al., Vitamin D insufficiency as a potential culprit in critical COVID-19 patients, J. Med. Virol, doi:10.1002/jmv.26360
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.26848
Ohaegbulam, Swalih, Patel, Smith, Perrin, Vitamin D Supplementation in COVID-19 Patients: A Clinical Case Series, Am. J. Ther, doi:10.1097/MJT.0000000000001222
Pal, Banerjee, Bhadada, Shetty, Singh et al., Vitamin D supplementation and clinical outcomes in COVID-19: A systematic review and meta-analysis, J. Endocrinol. Investig, doi:10.1007/s40618-021-01614-4
Panagiotou, Tee, Ihsan, Athar, Marchitelli et al., Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity, Clin. Endocrinol, doi:10.1111/cen.14276
Pearce, Rhodes, Stocking, Pembrey, Van Veldhoven et al., Occupational differences in COVID-19 incidence, severity, and mortality in the United Kingdom: Available data and framework for analyses, Wellcome Open Res, doi:10.12688/wellcomeopenres.16729.1
Pecina, Merry, Park, Thacher, Vitamin D Status and Severe COVID-19 Disease Outcomes in Hospitalized Patients, J. Prim. Care Community Health, doi:10.1177/21501327211041206
Penna, Amuchastegui, Giarratana, Daniel, Vulcano et al., 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells, J. Immunol
Pizzini, Aichner, Sahanic, Böhm, Egger et al., Impact of Vitamin D Deficiency on COVID-19-A Prospective Analysis from the CovILD Registry, Nutrients
Pouramini, Kafi, Hassanzadeh, Vitamin D and COVID-19 infection; recent findings, J. Ren. Endocrinol
Radujkovic, Hippchen, Tiwari-Heckler, Dreher, Boxberger et al., Vitamin D Deficiency and Outcome of COVID-19 Patients, Nutrients, doi:10.3390/nu12092757
Raisi-Estabragh, Martineau, Curtis, Moon, Darling et al., Vitamin D and coronavirus disease 2019 (COVID-19): Rapid evidence review, Aging Clin. Exp. Res, doi:10.1007/s40520-021-01894-z
Rastogi, Bhansali, Khare, Suri, Yaddanapudi et al., Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study), Postgrad. Med. J, doi:10.1136/postgradmedj-2020-139065
Razjouyan, Helmer, Lynch, Hanania, Klotman et al., Smoking Status and Factors associated with COVID-19 In-hospital Mortality among U.S. Veterans, Nicotine Tob. Res, doi:10.1093/ntr/ntab223
Reijven, Soeters, Vitamin D: A magic bullet or a myth?, Clin. Nutr, doi:10.1016/j.clnu.2019.12.028
Reis, Fernandes, Sales, Santos, Dos Santos et al., Influence of vitamin D status on hospital length of stay and prognosis in hospitalized patients with moderate to severe COVID-19: A multicenter prospective cohort study, Am. J. Clin. Nutr, doi:10.1093/ajcn/nqab151
Santaolalla, Beckmann, Kibaru, Josephs, Van Hemelrijck et al., Association Between Vitamin D and Novel SARS-CoV-2 Respiratory Dysfunction-A Scoping Review of Current Evidence and Its Implication for COVID-19 Pandemic, Front. Physiol, doi:10.3389/fphys.2020.564387
Seal, Bertenthal, Carey, Impact of Low Vitamin D Levels on COVID19-Related Hospitalization and Mortality: Results From a National Cohort of Veterans Affairs Patients, Glob. Adv. Health Med, doi:10.1177/21649561211003689
Searing, Zhang, Murphy, Hauk, Goleva et al., Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use, J. Allergy Clin. Immunol, doi:10.1016/j.jaci.2010.03.008
Shurtz-Swirski, Shkolnik, Shasha, Parathyroid hormone and the cellular immune system, Nephron, doi:10.1159/000188538
Skversky, Kumar, Abramowitz, Kaskel, Melamed, Association of glucocorticoid use and low 25-hydroxyvitamin D levels: Results from the National Health and Nutrition Examination Survey (NHANES): 2001-2006, J. Clin. Endocrinol. Metab, doi:10.1210/jc.2011-1600
Smith, Endothelial adhesion molecules and their role in inflammation, Can. J. Physiol. Pharmacol, doi:10.1139/y93-012
Soliman, Abdelaziz, Fathy, Impact of Vitamin D Therapy on the Progress COVID-19: Six Weeks Follow-Up Study of Vitamin D Deficient Elderly Diabetes Patients, Proc. Singap. Health, doi:10.1177/20101058211041405
Song, Ho, Schubert, Park, Posner et al., Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program, PLoS ONE, doi:10.1371/journal.pone.0251651
Speeckaert, Speeckaert, Delanghe, Vitamin D Sufficiency and COVID-19: Is Vitamin D Binding Protein (and Its Polymorphism) the Missing Link?, Endocr. Pract, doi:10.1016/j.eprac.2021.03.011
Sterne, Murthy, Diaz, Slutsky, Villar et al., Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-analysis, JAMA, doi:10.1001/jama.2020.17023
Sundberg, An iterative method for solution of the likelihood equations for incomplete data from exponential families, Commun. Stat.-Simul. Comput, doi:10.1080/03610917608812007
Suzuki, Efird, Redding, Thompson, Jr et al., COVID-19-Associated Mortality in US Veterans with and without SARS-CoV-2 Infection, Int. J. Environ. Res. Public Health
Szeto, Zucker, Lasota, Rubin, Walker et al., Vitamin D Status and COVID-19 Clinical Outcomes in Hospitalized Patients, Endocr. Res, doi:10.1080/07435800.2020.1867162
Sánchez-Zuno, González-Estevez, Matuz-Flores, Macedo-Ojeda, Hernández-Bello et al., Vitamin D Levels in COVID-19 Outpatients from Western Mexico: Clinical Correlation and Effect of Its Supplementation, J. Clin. Med, doi:10.3390/jcm10112378
Tahir, Zahra, Neutrophilia, StatPearls Publishing Copyright
Tan, Ho, Kalimuddin, Cherng, Teh et al., Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B(12) in combination on progression to severe outcomes in older patients with coronavirus (COVID-19), Nutrition, doi:10.1016/j.nut.2020.111017
Tehrani, Khabiri, Moradi, Mosavat, Khabiri, Evaluation of vitamin D levels in COVID-19 patients referred to Labafinejad hospital in Tehran and its relationship with disease severity and mortality, Clin. Nutr. ESPEN
Tran, Vu, Le, Pham, Phan et al., Understanding health seeking behaviors to inform COVID-19 surveillance and detection in resource-scarce settings, J. Glob. Health, doi:10.7189/jogh.10.0203106
Turrubiates-Hernández, Sánchez-Zuno, González-Estevez, Hernández-Bello, Macedo-Ojeda et al., Potential immunomodulatory effects of vitamin D in the prevention of severe coronavirus disease 2019: An ally for Latin America (Review), Int. J. Mol. Med, doi:10.3892/ijmm.2021.4865
Török, Lundahl, Hed, Lagercrantz, Diversity in regulation of adhesion molecules (Mac-1 and L-selectin) in monocytes and neutrophils from neonates and adults, Arch. Dis. Child, doi:10.1136/adc.68.5_Spec_No.561
Van Eeden, Miyagashima, Haley, Hogg, L-selectin expression increases on peripheral blood polymorphonuclear leukocytes during active marrow release, Am. J. Respir. Crit. Care Med, doi:10.1164/ajrccm.151.2.7531098
Vasheghani, Jannati, Baghaei, Rezaei, Aliyari et al., The relationship between serum 25-hydroxyvitamin D levels and the severity of COVID-19 disease and its mortality, Sci. Rep, doi:10.1038/s41598-021-97017-9
Vaughan, Trott, Sapkota, Premi, Roberts et al., Changes in 25-hydroxyvitamin D levels post-vitamin D supplementation in people of Black and Asian ethnicities and its implications during COVID-19 pandemic: A systematic review, J. Hum. Nutr. Diet, doi:10.1111/jhn.12949
Vitamin, Fact Sheet for Consumers
Weber, Toelboell, Chang, Tirrell, Saama et al., Mechanisms of glucocorticoid-induced down-regulation of neutrophil L-selectin in cattle: Evidence for effects at the gene-expression level and primarily on blood neutrophils, J. Leukoc. Biol, doi:10.1189/jlb.1003505
Wenban, Heer, Baktash, Kandiah, Katsanouli et al., Dexamethasone treatment may mitigate adverse effects of vitamin D deficiency in hospitalized COVID-19 patients, J. Med. Virol, doi:10.1002/jmv.27215
Xystrakis, Kusumakar, Boswell, Peek, Urry et al., Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients, J. Clin. Investig, doi:10.1172/JCI21759
Ye, Tang, Liao, Shaw, Deng et al., Does Serum Vitamin D Level Affect COVID-19 Infection and Its Severity? A Case-Control Study, J. Am. Coll. Nutr, doi:10.1080/07315724.2020.1826005
Yin, Agrawal, Vitamin D and inflammatory diseases, J. Inflamm. Res, doi:10.2147/jir.S63898
Zella, Meyer, Nerenz, Lee, Martowicz et al., Multifunctional enhancers regulate mouse and human vitamin D receptor gene transcription, Mol. Endocrinol, doi:10.1210/me.2009-0140
Zhang, Wu, Sun, Vitamin, Vitamin, Receptor, and Tissue Barriers, Tissue Barriers, doi:10.4161/tisb.23118
Zoorob, Cender, A different look at corticosteroids, Am. Fam. Physician
DOI record:
{
"DOI": "10.3390/ijerph19010447",
"ISSN": [
"1660-4601"
],
"URL": "http://dx.doi.org/10.3390/ijerph19010447",
"abstract": "<jats:p>This data-based cohort consisted of 26,508 (7%) United States veterans out of the 399,290 who tested positive for SARS-CoV-2 from 1 March to 10 September 2020. We aimed to assess the interaction of post-index vitamin D (Vit D) and corticosteroid (CRT) use on 30-day mortality among hospitalized and non-hospitalized patients with coronavirus disease 2019 (COVID-19). Combination Vit D and CRT drug use was assessed according to four multinomial pairs (−|+, −|−, +|+, +|−). Respective categorical effects were computed on a log-binomial scale as adjusted relative risk (aRR). Approximately 6% of veterans who tested positive for SARS-CoV-2 died within 30 days of their index date. Among hospitalized patients, a significantly decreased aRR was observed for the use of Vit D in the absence of CRTs relative to patients who received CRTs but not Vit D (aRR = 0.30; multiplicity corrected, p = 0.0004). Among patients receiving systemically administered CRTs (e.g., dexamethasone), the use of Vit D was associated with fewer deaths in hospitalized patients (aRR = 0.51) compared with non-hospitalized patients (aRR = 2.5) (P-for-Interaction = 0.0071). Evaluating the effect of modification of these compounds in the context of hospitalization may aid in the management of COVID-19 and provide a better understanding of the pathophysiological mechanisms underlying this and future infectious disease outbreaks.</jats:p>",
"alternative-id": [
"ijerph19010447"
],
"author": [
{
"affiliation": [],
"family": "Efird",
"given": "Jimmy T.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Anderson",
"given": "Ethan J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jindal",
"given": "Charulata",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Redding",
"given": "Thomas S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thompson",
"given": "Andrew D.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7359-0505",
"affiliation": [],
"authenticated-orcid": false,
"family": "Press",
"given": "Ashlyn M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2668-2535",
"affiliation": [],
"authenticated-orcid": false,
"family": "Upchurch",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Williams",
"given": "Christina D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choi",
"given": "Yuk Ming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suzuki",
"given": "Ayako",
"sequence": "additional"
}
],
"container-title": "International Journal of Environmental Research and Public Health",
"container-title-short": "IJERPH",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
4
]
],
"date-time": "2022-01-04T03:51:50Z",
"timestamp": 1641268310000
},
"deposited": {
"date-parts": [
[
2022,
1,
4
]
],
"date-time": "2022-01-04T04:40:26Z",
"timestamp": 1641271226000
},
"indexed": {
"date-parts": [
[
2024,
3,
20
]
],
"date-time": "2024-03-20T11:53:13Z",
"timestamp": 1710935593442
},
"is-referenced-by-count": 8,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
12,
31
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
31
]
],
"date-time": "2021-12-31T00:00:00Z",
"timestamp": 1640908800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1660-4601/19/1/447/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "447",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
12,
31
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
31
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1097/MED.0000000000000108",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.2147/jir.S63898",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1007/s11154-021-09679-5",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1056/NEJMra070553",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1016/j.clnu.2019.12.028",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.3390/nu13020411",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1007/s40520-021-01894-z",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1530/EJE-20-0665",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"article-title": "Vitamine D et COVID-19",
"author": "Arthur",
"first-page": "721",
"journal-title": "Bull. Acad. Natl. Med.",
"key": "ref9",
"volume": "204",
"year": "2020"
},
{
"DOI": "10.1111/jhn.12949",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1136/bmj.m1820",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.3892/ijmm.2021.4865",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"article-title": "Recommendations for High Vitamin D Supplementation to help Fight the COVID-19 Pandemic and its Inclusion in National COVID-19 Management Guidelines",
"author": "Kishore",
"first-page": "6",
"journal-title": "Epidem. Int.",
"key": "ref13",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1002/jmv.26360",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1093/jn/nxaa332",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1016/j.clnesp.2021.01.014",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1093/ajcn/nqab151",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.3390/nu13103596",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"article-title": "The influence of vitamin D on COVID-19 outcomes",
"author": "Benskin",
"key": "ref19",
"series-title": "COVID-19 and Nutraceuticals: A Guidebook",
"year": "2021"
},
{
"DOI": "10.3390/nu13041339",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1007/s40618-021-01614-4",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1002/rmv.2269",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1016/j.jcte.2016.04.004",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1111/ijcp.14649",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.31989/bchd.v4i3.796",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1016/j.clnu.2020.10.055",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1016/j.nut.2020.111055",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1186/s12879-021-06281-7",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1089/hs.2020.0137",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1136/postgradmedj-2020-138712",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.3390/nu12092757",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1016/j.nut.2021.111408",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1177/21501327211041206",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1371/journal.pmed.1003605",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1177/20101058211041405",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1001/jama.2020.26848",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1038/s41430-021-00984-5",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1016/j.metabol.2021.154753",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1093/advances/nmab012",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.3389/fphys.2020.564387",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.3390/jcm10112378",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1111/nbu.12499",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.4269/ajtmh.20-1294",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1080/07435800.2020.1867162",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1007/s40618-020-01370-x",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1016/j.medidd.2020.100064",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1007/s00394-020-02411-0",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.3390/nu12092775",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.5578/tt.70027",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.22625/2072-6732-2020-12-3-21-27",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"article-title": "Vitamin D Deficiency and ARDS after SARS-CoV-2 Infection",
"author": "Faul",
"first-page": "84",
"journal-title": "Ir. Med. J.",
"key": "ref51",
"volume": "113",
"year": "2020"
},
{
"DOI": "10.1093/ajcp/aqaa252",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1080/07315724.2020.1826005",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.1016/j.virusres.2020.198148",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.1016/j.dsx.2021.102237",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.1002/jmv.27215",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.1164/ajrccm.185.11.1245",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.1139/cjpp-2014-0323",
"doi-asserted-by": "publisher",
"key": "ref58"
},
{
"key": "ref59"
},
{
"DOI": "10.1002/jcb.22645",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.1210/jc.2011-1600",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"DOI": "10.1210/jc.2011-2757",
"doi-asserted-by": "publisher",
"key": "ref62"
},
{
"article-title": "A different look at corticosteroids",
"author": "Zoorob",
"first-page": "443",
"journal-title": "Am. Fam. Physician",
"key": "ref63",
"volume": "58",
"year": "1998"
},
{
"DOI": "10.1074/jbc.M111.244061",
"doi-asserted-by": "publisher",
"key": "ref64"
},
{
"DOI": "10.1210/me.2009-0140",
"doi-asserted-by": "publisher",
"key": "ref65"
},
{
"DOI": "10.1385/ENDO:17:2:109",
"doi-asserted-by": "publisher",
"key": "ref66"
},
{
"DOI": "10.1172/JCI21759",
"doi-asserted-by": "publisher",
"key": "ref67"
},
{
"DOI": "10.1016/j.jaci.2010.03.008",
"doi-asserted-by": "publisher",
"key": "ref68"
},
{
"DOI": "10.4161/tisb.23118",
"doi-asserted-by": "publisher",
"key": "ref69"
},
{
"DOI": "10.1021/pr200724e",
"doi-asserted-by": "publisher",
"key": "ref70"
},
{
"DOI": "10.4049/jimmunol.178.1.145",
"doi-asserted-by": "publisher",
"key": "ref71"
},
{
"DOI": "10.7326/M21-1991",
"doi-asserted-by": "publisher",
"key": "ref72"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "ref73"
},
{
"DOI": "10.3390/ijerph18168486",
"doi-asserted-by": "publisher",
"key": "ref74"
},
{
"key": "ref75"
},
{
"key": "ref76",
"unstructured": "A Natural Language Processing System for National COVID-19 Surveillance in the US Department of Veterans Affairshttps://openreview.net/forum?id=ZQ_HvBxcdCv"
},
{
"DOI": "10.3390/metabo11110761",
"doi-asserted-by": "publisher",
"key": "ref77"
},
{
"article-title": "The Analysis of Case-control Studies",
"author": "Breslow",
"key": "ref78",
"series-title": "Statistical Methods in Cancer Research",
"volume": "Volume 1",
"year": "1980"
},
{
"author": "Bland",
"first-page": "464",
"key": "ref79",
"series-title": "Introduction to Medical Statistics",
"year": "2015"
},
{
"DOI": "10.1177/1176935120985132",
"doi-asserted-by": "publisher",
"key": "ref80"
},
{
"DOI": "10.1198/016214504000001907",
"doi-asserted-by": "publisher",
"key": "ref81"
},
{
"DOI": "10.1093/biomet/75.4.800",
"doi-asserted-by": "publisher",
"key": "ref82"
},
{
"DOI": "10.1080/03610917608812007",
"doi-asserted-by": "publisher",
"key": "ref83"
},
{
"DOI": "10.1016/j.eprac.2021.09.016",
"doi-asserted-by": "publisher",
"key": "ref84"
},
{
"DOI": "10.1097/MJT.0000000000001222",
"doi-asserted-by": "publisher",
"key": "ref85"
},
{
"DOI": "10.1038/s41598-021-90189-4",
"doi-asserted-by": "publisher",
"key": "ref86"
},
{
"DOI": "10.1016/j.jsbmb.2020.105771",
"doi-asserted-by": "publisher",
"key": "ref87"
},
{
"DOI": "10.3390/nu12113377",
"doi-asserted-by": "publisher",
"key": "ref88"
},
{
"DOI": "10.3390/nu12123799",
"doi-asserted-by": "publisher",
"key": "ref89"
},
{
"DOI": "10.3390/nu13010219",
"doi-asserted-by": "publisher",
"key": "ref90"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"doi-asserted-by": "publisher",
"key": "ref91"
},
{
"DOI": "10.3390/nu13061760",
"doi-asserted-by": "publisher",
"key": "ref92"
},
{
"DOI": "10.18632/aging.202307",
"doi-asserted-by": "publisher",
"key": "ref93"
},
{
"DOI": "10.1038/s41598-021-97017-9",
"doi-asserted-by": "publisher",
"key": "ref94"
},
{
"DOI": "10.1016/j.nut.2020.111017",
"doi-asserted-by": "publisher",
"key": "ref95"
},
{
"DOI": "10.1136/postgradmedj-2020-139065",
"doi-asserted-by": "publisher",
"key": "ref96"
},
{
"DOI": "10.1101/2020.11.08.20222638",
"doi-asserted-by": "publisher",
"key": "ref97"
},
{
"DOI": "10.1007/s40520-021-01831-0",
"doi-asserted-by": "publisher",
"key": "ref98"
},
{
"DOI": "10.1038/s41598-020-77093-z",
"doi-asserted-by": "publisher",
"key": "ref99"
},
{
"DOI": "10.20960/nh.03193",
"doi-asserted-by": "publisher",
"key": "ref100"
},
{
"DOI": "10.1101/2020.06.25.20137323",
"doi-asserted-by": "publisher",
"key": "ref101"
},
{
"DOI": "10.1111/cen.14276",
"doi-asserted-by": "publisher",
"key": "ref102"
},
{
"DOI": "10.23736/S0031-0808.21.04277-4",
"doi-asserted-by": "publisher",
"key": "ref103"
},
{
"DOI": "10.1177/21649561211003689",
"doi-asserted-by": "publisher",
"key": "ref104"
},
{
"DOI": "10.3390/nu13114047",
"doi-asserted-by": "publisher",
"key": "ref105"
},
{
"DOI": "10.1038/s41598-021-02701-5",
"doi-asserted-by": "publisher",
"key": "ref106"
},
{
"DOI": "10.1038/s41598-021-85809-y",
"doi-asserted-by": "publisher",
"key": "ref107"
},
{
"DOI": "10.1210/clinem/dgaa733",
"doi-asserted-by": "publisher",
"key": "ref108"
},
{
"DOI": "10.1152/ajpendo.00517.2020",
"doi-asserted-by": "publisher",
"key": "ref109"
},
{
"DOI": "10.34172/jre.2021.19",
"doi-asserted-by": "publisher",
"key": "ref110"
},
{
"DOI": "10.5530/jyp.2021.13s.77",
"doi-asserted-by": "publisher",
"key": "ref111"
},
{
"DOI": "10.1161/01.CIR.96.1.295",
"doi-asserted-by": "publisher",
"key": "ref112"
},
{
"DOI": "10.1002/jlb.57.2.317",
"doi-asserted-by": "publisher",
"key": "ref113"
},
{
"DOI": "10.1189/jlb.1003505",
"doi-asserted-by": "publisher",
"key": "ref114"
},
{
"DOI": "10.1139/y93-012",
"doi-asserted-by": "publisher",
"key": "ref115"
},
{
"DOI": "10.1164/ajrccm.151.2.7531098",
"doi-asserted-by": "publisher",
"key": "ref116"
},
{
"DOI": "10.1136/adc.68.5_Spec_No.561",
"doi-asserted-by": "publisher",
"key": "ref117"
},
{
"DOI": "10.1073/pnas.89.21.9991",
"doi-asserted-by": "publisher",
"key": "ref118"
},
{
"article-title": "Neutrophilia. StatPearls Publishing Copyright © 2021",
"author": "Tahir",
"key": "ref119",
"series-title": "StatPearls",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17023",
"doi-asserted-by": "publisher",
"key": "ref120"
},
{
"DOI": "10.1126/science.abm0620",
"doi-asserted-by": "publisher",
"key": "ref121"
},
{
"DOI": "10.1002/jbm4.10567",
"doi-asserted-by": "publisher",
"key": "ref122"
},
{
"DOI": "10.3310/hta23020",
"doi-asserted-by": "publisher",
"key": "ref123"
},
{
"DOI": "10.7861/clinmed.2021-0035",
"doi-asserted-by": "publisher",
"key": "ref124"
},
{
"DOI": "10.1016/j.bone.2018.02.024",
"doi-asserted-by": "publisher",
"key": "ref125"
},
{
"DOI": "10.1001/jama.2019.11889",
"doi-asserted-by": "publisher",
"key": "ref126"
},
{
"DOI": "10.1038/ki.1990.147",
"doi-asserted-by": "publisher",
"key": "ref127"
},
{
"DOI": "10.1159/000188538",
"doi-asserted-by": "publisher",
"key": "ref128"
},
{
"DOI": "10.1038/ki.1993.255",
"doi-asserted-by": "publisher",
"key": "ref129"
},
{
"DOI": "10.1155/2010/418695",
"doi-asserted-by": "publisher",
"key": "ref130"
},
{
"DOI": "10.1093/ajcn/nqab390",
"doi-asserted-by": "publisher",
"key": "ref131"
},
{
"DOI": "10.1001/jama.2020.26850",
"doi-asserted-by": "publisher",
"key": "ref132"
},
{
"DOI": "10.1186/s12885-021-08577-y",
"doi-asserted-by": "publisher",
"key": "ref133"
},
{
"DOI": "10.1093/ntr/ntab223",
"doi-asserted-by": "publisher",
"key": "ref134"
},
{
"DOI": "10.1038/s41467-020-19478-2",
"doi-asserted-by": "publisher",
"key": "ref135"
},
{
"DOI": "10.1371/journal.pone.0251651",
"doi-asserted-by": "publisher",
"key": "ref136"
},
{
"DOI": "10.7326/M20-6979",
"doi-asserted-by": "publisher",
"key": "ref137"
},
{
"DOI": "10.12688/wellcomeopenres.16729.1",
"doi-asserted-by": "publisher",
"key": "ref138"
},
{
"key": "ref139"
},
{
"DOI": "10.1371/journal.pone.0246217",
"doi-asserted-by": "publisher",
"key": "ref140"
},
{
"DOI": "10.1093/cid/ciab419",
"doi-asserted-by": "publisher",
"key": "ref141"
},
{
"DOI": "10.7189/jogh.10.0203106",
"doi-asserted-by": "publisher",
"key": "ref142"
},
{
"DOI": "10.1016/j.amepre.2012.07.029",
"doi-asserted-by": "publisher",
"key": "ref143"
},
{
"DOI": "10.3390/biom10071032",
"doi-asserted-by": "publisher",
"key": "ref144"
},
{
"DOI": "10.1177/0963689717714098",
"doi-asserted-by": "publisher",
"key": "ref145"
},
{
"DOI": "10.1093/milmed/usab402",
"doi-asserted-by": "publisher",
"key": "ref146"
},
{
"DOI": "10.1111/jgs.12495",
"doi-asserted-by": "publisher",
"key": "ref147"
},
{
"DOI": "10.3390/nu13113680",
"doi-asserted-by": "publisher",
"key": "ref148"
},
{
"DOI": "10.1016/j.eprac.2021.03.011",
"doi-asserted-by": "publisher",
"key": "ref149"
},
{
"DOI": "10.1530/EC-18-0184",
"doi-asserted-by": "publisher",
"key": "ref150"
}
],
"reference-count": 150,
"references-count": 150,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1660-4601/19/1/447"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Health, Toxicology and Mutagenesis",
"Public Health, Environmental and Occupational Health"
],
"subtitle": [],
"title": "The Interaction of Vitamin D and Corticosteroids: A Mortality Analysis of 26,508 Veterans Who Tested Positive for SARS-CoV-2",
"type": "journal-article",
"volume": "19"
}
