Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study
et al., Nutrients, doi:10.3390/nu12113377, Nov 2020
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective study finding that regular bolus vitamin D supplementation was associated with less severe COVID-19 and better survival in frail elderly.
For those receiving regular supplementation:
Adjusted mortality hazard ratio with supplementation HR 0.07, p = 0.017.
Risk of severe COVID-19 with supplementation OR 0.08, p = 0.033.
Risk of severe COVID-19 with supplementation OR 0.08, p = 0.033.
For supplementation started after COVID-19 diagnosis:
Adjusted mortality hazard ratio HR 0.37, p = 0.28.
Risk of severe COVID-19 with supplementation OR 0.46, p = 0.4.
Risk of severe COVID-19 with supplementation OR 0.46, p = 0.4.
Bolus treatment is less effective.
Pharmacokinetics and the potential side effects of high bolus doses suggest
that ongoing treatment spread over time is more appropriate.
Research has confirmed that lower dose regular treatment with vitamin D is more
effective than intermittent high-dose bolus treatment for various conditions,
including rickets and acute respiratory infections1,2. The biological mechanisms supporting these
findings involve the induction of enzymes such as 24-hydroxylase and
fibroblast growth factor 23 (FGF23) by high-dose bolus treatments. These
enzymes play roles in inactivating vitamin D, which can paradoxically reduce
levels of activated vitamin D and suppress its activation for extended periods
post-dosage. Evidence indicates that 24-hydroxylase activity may remain
elevated for several weeks following a bolus dose, leading to reduced levels
of the activated form of vitamin D. Additionally, FGF23 levels can increase
for at least three months after a large bolus dose, which also contributes to
the suppression of vitamin D activation1.
This is the 8th of 136 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
|
risk of death, 93.0% lower, RR 0.07, p = 0.02, treatment 2 of 29 (6.9%), control 10 of 32 (31.2%), NNT 4.1, adjusted per study, regular bolus supplementation.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Annweiler et al., 2 Nov 2020, retrospective, France, peer-reviewed, mean age 88.0, 7 authors, dosage 50,000IU monthly, dose varies - 50,000 IU/month, or 80,000IU/100,000IU every 2-3 months.
Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study
Nutrients, doi:10.3390/nu12113377
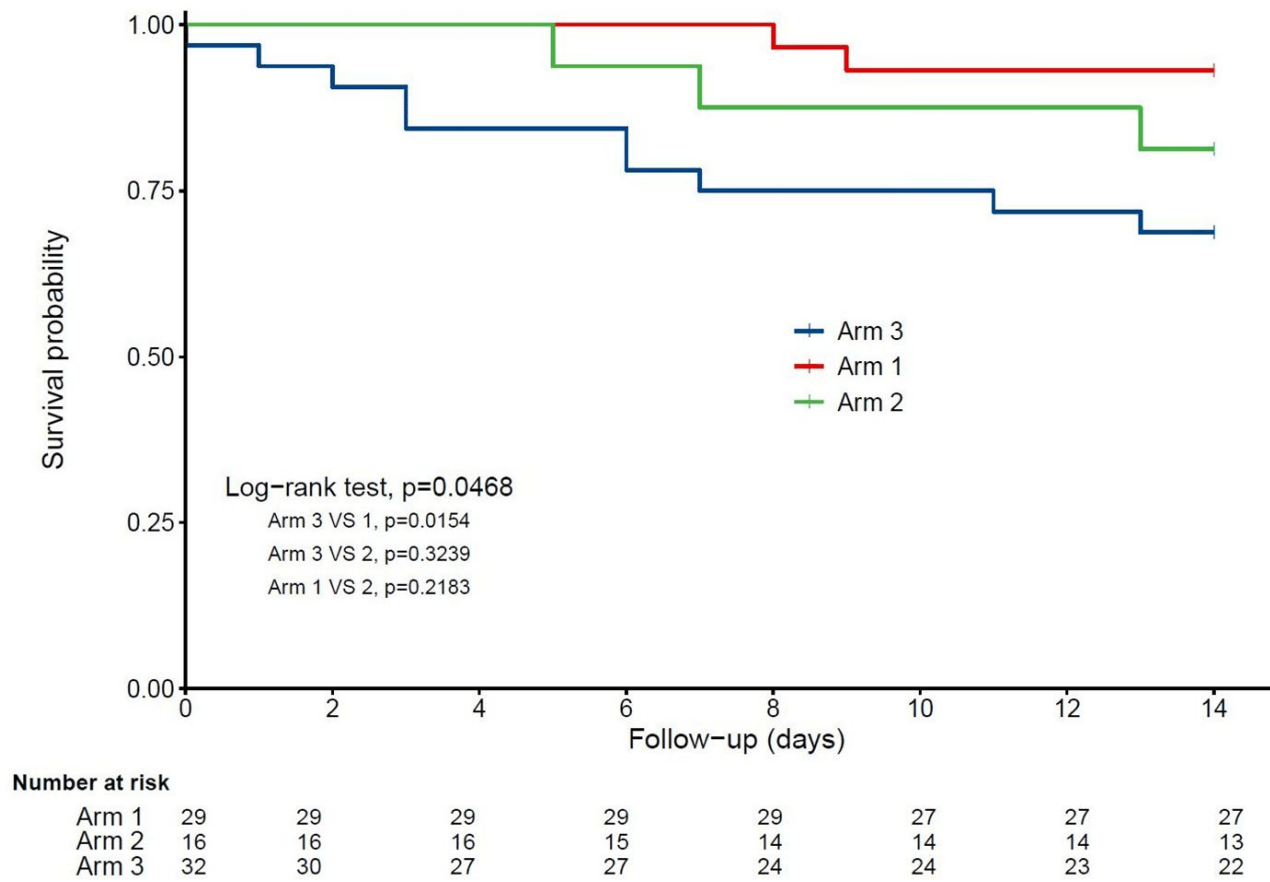
Background. The objective of this quasi-experimental study was to determine whether bolus vitamin D supplementation taken either regularly over the preceding year or after the diagnosis of COVID-19 was effective in improving survival among hospitalized frail elderly COVID-19 patients. Methods. Seventy-seven patients consecutively hospitalized for COVID-19 in a geriatric unit were included. Intervention groups were participants regularly supplemented with vitamin D over the preceding year (Group 1), and those supplemented with vitamin D after COVID-19 diagnosis (Group 2). The comparator group involved participants having received no vitamin D supplements (Group 3). Outcomes were 14-day mortality and highest (worst) score on the ordinal scale for clinical improvement (OSCI) measured during COVID-19 acute phase. Potential confounders were age, gender, functional abilities, undernutrition, cancer, hypertension, cardiomyopathy, glycated hemoglobin, number of acute health issues at admission, hospital use of antibiotics, corticosteroids, and pharmacological treatments of respiratory disorders. Results. The three groups (n = 77; mean ± SD, 88 ± 5 years; 49% women) were similar at baseline (except for woman proportion, p = 0.02), as were the treatments used for COVID-19. In Group 1 (n = 29), 93.1% of COVID-19 participants survived at day 14, compared to 81.2% survivors in Group 2 (n = 16) (p = 0.33) and 68.7% survivors in Group 3 (n = 32) (p = 0.02). While considering Group 3 as reference (hazard ratio (HR) = 1), the fully-adjusted HR for 14-day mortality was HR = 0.07 (p = 0.017) for Group 1 and HR = 0.37 (p = 0.28) for Group 2. Group 1 had longer survival time than Group 3 (log-rank p = 0.015), although there was no difference between Groups 2 and 3 (log-rank p = 0.32). Group 1, but not Group 2 (p = 0.40), was associated with lower risk of OSCI score ≥5 compared to Group 3 (odds ratio = 0.08, p = 0.03). Conclusions. Regular bolus vitamin D supplementation was associated with less severe COVID-19 and better survival in frail elderly.
Conflicts of Interest: C.A. serves as an editor for Nutrients. All authors declare that they do not have any other financial and personal conflicts of interest with this manuscript.
References
Ahn, Shin, Kim, Lee, Kim et al., Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease
Annweiler, Cao, Sabatier, Point of view: Should COVID-19 patients be supplemented with vitamin D?, Maturitas, doi:10.1016/j.maturitas.2020.06.003
Annweiler, Cao, Wu, Faucon, Mouhat et al., Counter-regulatory 'Renin-Angiotensin' System-based Candidate Drugs to Treat COVID-19 Diseases in SARS-CoV-2-infected patients, Infect. Disord. Drug Targets, doi:10.2174/1871526520666200518073329
Benhamou, Souberbielle, Cortret, Fardellone, Gauvain et al., Vitamin D in adults: GRIO guidelines, La Presse Médicale
Bonanad, García-Blas, Tarazona-Santabalbina, Sanchis, Bertomeu-González et al., The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects, J. Am. Med. Dir. Assoc, doi:10.1016/j.jamda.2020.05.045
Castillo, Costa, Barrios, Díaz, Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105751
Chan, Arai, Park, Gunaratne, Setiati et al., Prevention of COVID-19 in Older Adults: A Brief Guidance from the International Association for Gerontology and Geriatrics (IAGG) Asia/Oceania Region, J. Nutr. Heal. Aging, doi:10.1007/s12603-020-1359-7
Chhetri, International Association for Gerontology and Geriatrics-Asia/Oceania Region
D'avolio, Avataneo, Manca, Cusato, De Nicolò et al., 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2, Nutrients, doi:10.3390/nu12051359
Dancer, Parekh, Lax, D'souza, Zheng et al., Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS), Thorax, doi:10.1136/thoraxjnl-2014-206680
Del Galy, Bertrand, Bigot, Abraham, Thomlinson et al., Vitamin D Insufficiency and Acute Care in Geriatric Inpatients, J. Am. Geriatr. Soc, doi:10.1111/j.1532-5415.2009.02408.x
Dijkman, Jebbink, Deijs, Milewska, Pyrc et al., Replication-Dependent Downregulation of Cellular Angiotensin-Converting Enzyme 2 Protein Expression by Human Coronavirus NL63, J. Gen. Virol, doi:10.1099/vir.0.043919-0
Fabbri, Infante, Ricordi, Editorial-Vitamin D status: A key modulator of innate immunity and natural defense from acute viral respiratory infections, Eur. Rev. Med. Pharmacol. Sci
Glinsky, Tripartite Combination of Candidate Pandemic Mitigation Agents: Vitamin D, Quercetin, and Estradiol Manifest Properties of Medicinal Agents for Targeted Mitigation of the COVID-19 Pandemic Defined by Genomics-Guided Tracing of SARS-CoV-2 Targets in Human Cells, Biomed, doi:10.3390/biomedicines8050129
Grant, Lahore, Mcdonnell, Baggerly, French et al., Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths, Nutrienrs, doi:10.3390/nu12040988
Hastie, Mackay, Ho, Celis-Morales, Katikireddi et al., Vitamin D concentrations and COVID-19 infection in UK Biobank, Diabetes Metab. Syndr. Clin. Res. Rev, doi:10.1016/j.dsx.2020.04.050
Heaney, Guidelines for optimizing design and analysis of clinical studies of nutrient effects, Nutr. Rev, doi:10.1111/nure.12090
Hewitt, Carter, Vilches-Moraga, Quinn, Braude et al., The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study, Lancet Public Health, doi:10.1016/S2468-2667(20)30146-8
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Ilie, Stefanescu, Smith, The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality, Aging Clin. Exp. Res, doi:10.1007/s40520-020-01570-8
Ji, Zhang, Zhai, Zhang, Zhang et al., an Automated Topic-Wise Inference Method Based on Massive Literature, Suggests a Possible Mechanism via ACE2 for the Pathological Changes in the Human Host after Coronavirus Infection, bioRxiv, doi:10.1101/2020.02.27.967588
Kong, Zhu, Shi, Liu, Chen et al., VDR Attenuates Acute Lung Injury by Blocking Ang-2-Tie-2 Pathway and Renin-Angiotensin System, Mol. Endocrinol, doi:10.1210/me.2013-1146
Maghbooli, Sahraian, Ebrahimi, Pazoki, Kafan et al., Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection, PLoS ONE, doi:10.1371/journal.pone.0239799
Martineau, Jolliffe, Hooper, Greenberg, Aloia et al., Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data, BMJ
Meltzer, Best, Zhang, Vokes, Arora et al., Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2020.19722
Rochon, Gondan, Kieser, To test or not to test: Preliminary assessment of normality when comparing two independent samples, BMC Med. Res. Methodol, doi:10.1186/1471-2288-12-81
Vetel, Leroux, Ducoudray, Aggir, Practical use. Geriatric Autonomy Group Resources Needs
Who, Coronavirus Disease (COVID
Yang, Zhang, Xu, Effect of Vitamin D on ACE2 and Vitamin D receptor expression in rats with LPS-induced acute lung injury, Chin. J. Emerg. Med
DOI record:
{
"DOI": "10.3390/nu12113377",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu12113377",
"abstract": "<jats:p>Background. The objective of this quasi-experimental study was to determine whether bolus vitamin D supplementation taken either regularly over the preceding year or after the diagnosis of COVID-19 was effective in improving survival among hospitalized frail elderly COVID-19 patients. Methods. Seventy-seven patients consecutively hospitalized for COVID-19 in a geriatric unit were included. Intervention groups were participants regularly supplemented with vitamin D over the preceding year (Group 1), and those supplemented with vitamin D after COVID-19 diagnosis (Group 2). The comparator group involved participants having received no vitamin D supplements (Group 3). Outcomes were 14-day mortality and highest (worst) score on the ordinal scale for clinical improvement (OSCI) measured during COVID-19 acute phase. Potential confounders were age, gender, functional abilities, undernutrition, cancer, hypertension, cardiomyopathy, glycated hemoglobin, number of acute health issues at admission, hospital use of antibiotics, corticosteroids, and pharmacological treatments of respiratory disorders. Results. The three groups (n = 77; mean ± SD, 88 ± 5 years; 49% women) were similar at baseline (except for woman proportion, p = 0.02), as were the treatments used for COVID-19. In Group 1 (n = 29), 93.1% of COVID-19 participants survived at day 14, compared to 81.2% survivors in Group 2 (n = 16) (p = 0.33) and 68.7% survivors in Group 3 (n = 32) (p = 0.02). While considering Group 3 as reference (hazard ratio (HR) = 1), the fully-adjusted HR for 14-day mortality was HR = 0.07 (p = 0.017) for Group 1 and HR = 0.37 (p = 0.28) for Group 2. Group 1 had longer survival time than Group 3 (log-rank p = 0.015), although there was no difference between Groups 2 and 3 (log-rank p = 0.32). Group 1, but not Group 2 (p = 0.40), was associated with lower risk of OSCI score ≥5 compared to Group 3 (odds ratio = 0.08, p = 0.03). Conclusions. Regular bolus vitamin D supplementation was associated with less severe COVID-19 and better survival in frail elderly.</jats:p>",
"alternative-id": [
"nu12113377"
],
"author": [
{
"affiliation": [],
"family": "Annweiler",
"given": "Gaëlle",
"sequence": "first"
},
{
"affiliation": [],
"family": "Corvaisier",
"given": "Mathieu",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0200-9378",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gautier",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dubée",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Legrand",
"given": "Erick",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6881-1649",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sacco",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Annweiler",
"given": "Cédric",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
11,
3
]
],
"date-time": "2020-11-03T00:51:31Z",
"timestamp": 1604364691000
},
"deposited": {
"date-parts": [
[
2020,
11,
10
]
],
"date-time": "2020-11-10T17:03:03Z",
"timestamp": 1605027783000
},
"indexed": {
"date-parts": [
[
2024,
3,
20
]
],
"date-time": "2024-03-20T17:52:01Z",
"timestamp": 1710957121318
},
"is-referenced-by-count": 182,
"issue": "11",
"issued": {
"date-parts": [
[
2020,
11,
2
]
]
},
"journal-issue": {
"issue": "11",
"published-online": {
"date-parts": [
[
2020,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
11,
2
]
],
"date-time": "2020-11-02T00:00:00Z",
"timestamp": 1604275200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/12/11/3377/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "3377",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2020,
11,
2
]
]
},
"published-online": {
"date-parts": [
[
2020,
11,
2
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.4014/jmb.2003.03011",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.3390/biomedicines8050129",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"article-title": "Vitamin D in adults: GRIO guidelines",
"author": "Benhamou",
"first-page": "673",
"journal-title": "La Presse Médicale.",
"key": "ref3",
"volume": "40",
"year": "2011"
},
{
"DOI": "10.3390/nu12040988",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1016/j.maturitas.2020.06.003",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1007/s40520-020-01570-8",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"key": "ref7",
"unstructured": "Coronavirus Disease (COVID-2019) R&D. WHOhttps://www.who.int/teams/blueprint/covid-19"
},
{
"article-title": "[AGGIR. Practical use. Geriatric Autonomy Group Resources Needs]",
"author": "Vetel",
"first-page": "23",
"journal-title": "Soins Gérontologie",
"key": "ref8",
"year": "1998"
},
{
"DOI": "10.1111/j.1532-5415.2009.02408.x",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1186/1471-2288-12-81",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1001/jamanetworkopen.2020.19722",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.3390/nu12051359",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1371/journal.pone.0239799",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1016/j.dsx.2020.04.050",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1136/bmj.i6583",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1007/s12603-020-1359-7",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"key": "ref18",
"unstructured": "Vitamin D and Covid-19—Press Releasehttp://www.academie-medecine.fr/wp-content/uploads/2020/05/20.5.22-Vitamine-D-et-coronavirus-ENG.pdf."
},
{
"DOI": "10.1210/me.2013-1146",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1099/vir.0.043919-0",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.2174/1871526520666200518073329",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1101/2020.02.27.967588",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"article-title": "Effect of Vitamin D on ACE2 and Vitamin D receptor expression in rats with LPS-induced acute lung injury",
"author": "Yang",
"first-page": "1284",
"journal-title": "Chin. J. Emerg. Med.",
"key": "ref24",
"volume": "25",
"year": "2016"
},
{
"article-title": "Editorial—Vitamin D status: A key modulator of innate immunity and natural defense from acute viral respiratory infections",
"author": "Fabbri",
"first-page": "4048",
"journal-title": "Eur. Rev. Med. Pharmacol. Sci.",
"key": "ref25",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1136/thoraxjnl-2014-206680",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1016/j.jamda.2020.05.045",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1016/S2468-2667(20)30146-8",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1111/nure.12090",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "ref30"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/12/11/3377"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Food Science",
"Nutrition and Dietetics"
],
"subtitle": [],
"title": "Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study",
"type": "journal-article",
"volume": "12"
}
