Calcifediol Treatment and Hospital Mortality Due to COVID-19: A Cohort Study
et al., Nutrients, doi:10.3390/nu13061760, May 2021
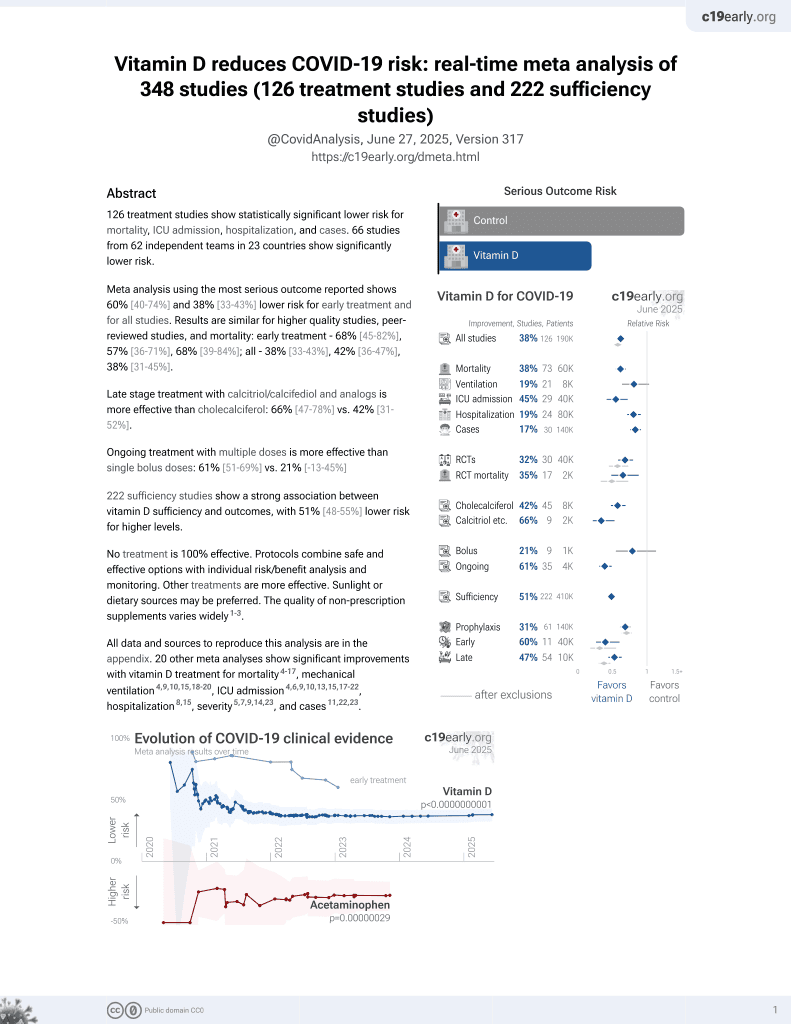
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
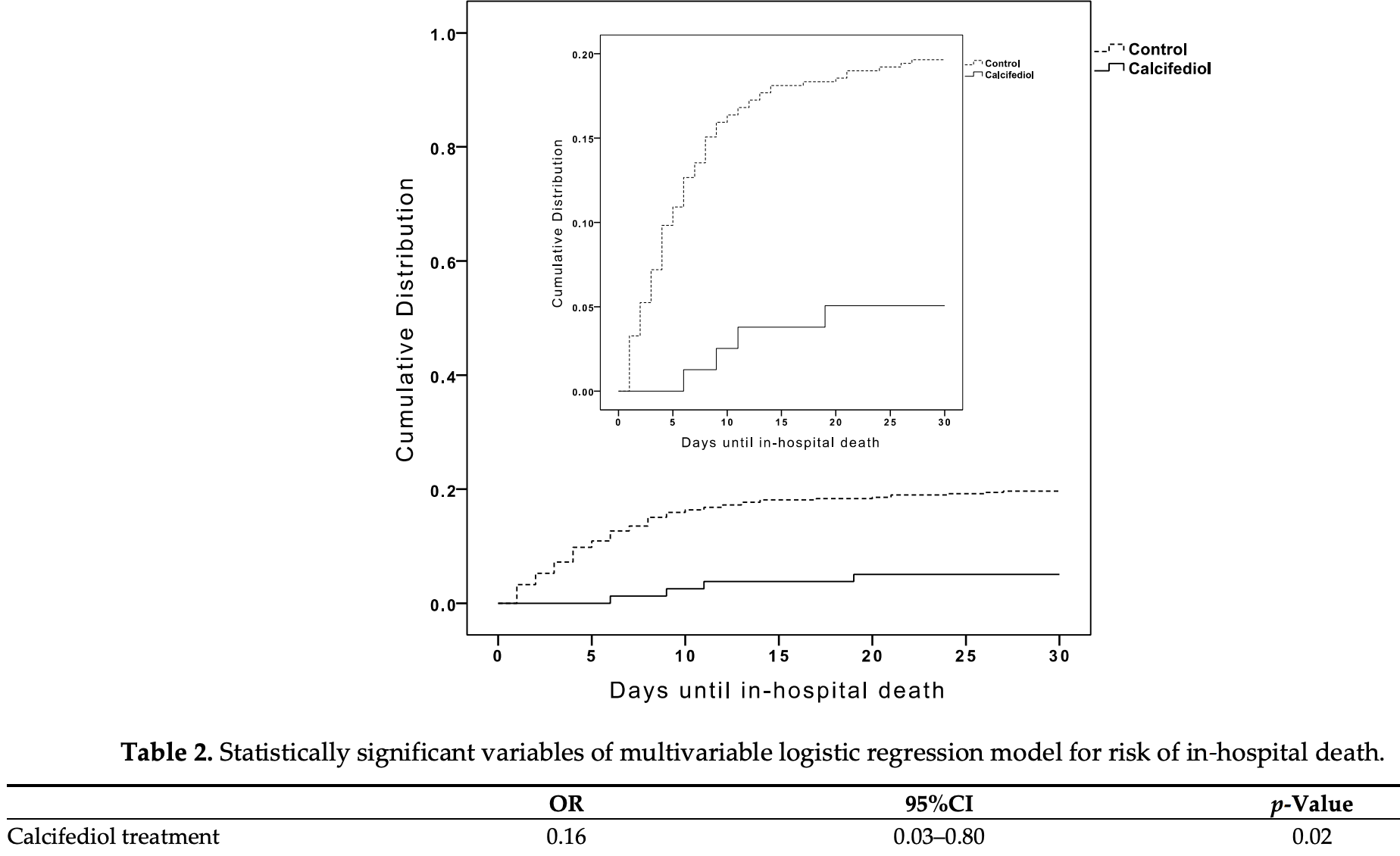
Retrospective 537 patients in Spain, 79 treated with calcifediol, showing significantly lower mortality with treatment. The treated group had a higher risk of comorbidity, whereas the control group had lower O2 saturation, higher CURB-65, and higher ARDS (severity measures were included in the multivariate analysis).
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 44% [33‑53%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 36th of 136 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
|
risk of death, 80.8% lower, RR 0.19, p = 0.04, treatment 4 of 79 (5.1%), control 90 of 458 (19.7%), NNT 6.9, adjusted per study, odds ratio converted to relative risk, day 30, multivariate logistic regression.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Alcala-Diaz et al., 21 May 2021, retrospective, Spain, peer-reviewed, 17 authors, dosage calcifediol 0.5mg day 1, 0.27mg day 3, 0.27mg day 7, 0.27mg day 14, 0.27mg day 21, 0.27mg day 28.
Calcifediol Treatment and Hospital Mortality Due to COVID-19: A Cohort Study
Nutrients, doi:10.3390/nu13061760
Context. Calcifediol has been proposed as a potential treatment for COVID-19 patients. Objective: To compare the administration or not of oral calcifediol on mortality risk of patients hospitalized because of COVID-19. Design: Retrospective, multicenter, open, non-randomized cohort study. Settings: Hospitalized care. Patients: Patients with laboratory-confirmed COVID-19 between 5 February and 5 May 2020 in five hospitals in the South of Spain. Intervention: Patients received calcifediol (25-hydroxyvitamin D 3 ) treatment (0.266 mg/capsule, 2 capsules on entry and then one capsule on day 3, 7, 14, 21, and 28) or not. Main Outcome Measure: In-hospital mortality during the first 30 days after admission. Results: A total of 537 patients were hospitalized with COVID-19 (317 males (59%), median age, 70 years), and 79 (14.7%) received calcifediol treatment. Overall, in-hospital mortality during the first 30 days was 17.5%. The OR of death for patients receiving calcifediol (mortality rate of 5%) was 0.22 (95% CI, 0.08 to 0.61) compared to patients not receiving such treatment (mortality rate of 20%; p < 0.01). Patients who received calcifediol after admission were more likely than those not receiving treatment to have comorbidity and a lower rate of CURB-65 score for pneumonia severity ≥ 3 (one point for each of confusion, urea > 7 mmol/L, respiratory rate ≥ 30/min, systolic blood pressure < 90 mm Hg or diastolic blood pressure ≤ 60 mm Hg, and age Nutrients 2021, 13, 1760. https://doi.org/10.3390/nu13061760 https://www.mdpi.com/journal/nutrients ≥ 65 years), acute respiratory distress syndrome (moderate or severe), c-reactive protein, chronic kidney disease, and blood urea nitrogen. In a multivariable logistic regression model, adjusting for confounders, there were significant differences in mortality for patients receiving calcifediol compared with patients not receiving it (OR = 0.16 (95% CI 0.03 to 0.80). Conclusion: Among patients hospitalized with COVID-19, treatment with calcifediol, compared with those not receiving calcifediol, was significantly associated with lower in-hospital mortality during the first 30 days. The observational design and sample size may limit the interpretation of these findings.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest: JFAD received lecture fees from Bayer, Grunenthal Pharma, Esteve, Ferrer, and Boehringer Ingelheim outside the submitted work. LLP received lecture fees from Gebro Pharma S.A., Boehringer Ingelheim, Pfizer, Mylan, Almirall, SANOFI, and ESTEVE outside the submitted work. IJ, RGH, MDME, BCR, JLZG, MEC, AIPC, MDLC, JGA, ALRM, MDSAL, and LMPB have nothing to declare. RB received lecture fees from Abiogen, Faes Farma, Fresenius, and Proctor and Gamble outside the submitted work. JMQG received lecture fees from FAES Farma (Spain) and Amgen related to vitamin D-these activities in no way influenced the writing of the present manuscript. JLM received lecture fees from AMGEN, SANOFI, FERRER, Laboratorios Dr. Esteve, and Boehringer Ingelheim-Lilly outside the submitted work. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
Amrein, Sourij, Wagner, Holl, Pieber et al., Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: A randomized, double-blind, placebo-controlled pilot study, Crit. Care, doi:10.1186/cc10120
Andrukhov, Andrukhova, Hulan, Tang, Bantleon et al., Both 25-hydroxyvitamin-D3 and 1,25-dihydroxyvitamin-D3 reduces inflammatory response in human periodontal ligament cells, PLoS ONE, doi:10.1371/journal.pone.0090301
Annweiler, Corvaisier, Gautier, Dubee, Legrand et al., Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study, Nutrients, doi:10.3390/nu12113377
Annweiler, Hanotte, Grandin De L'eprevier, Sabatier, Lafaie et al., Vitamin D and survival in COVID-19 patients: A quasi-experimental study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105771
Annweiler, Mercat, Souberbielle, Learning from previous methodological pitfalls to propose well-designed trials on vitamin D in COVID-19, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2021.105901
Arunachalam, Wimmers, Mok, Perera, Scott et al., Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans, Science, doi:10.1126/science.abc6261
Barker, May, Doty, Lappe, Knowlton et al., Vitamin D supplementation protects against reductions in plasma 25-hydroxyvitamin D induced by open-heart surgery: Assess-d trial, Physiol. Rep, doi:10.14814/phy2.14747
Barnaby, Becker, Chelico, Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of Covid-19-Final Report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Bilezikian, Bikle, Hewison, Lazaretti-Castro, Formenti et al., MECHANISMS IN ENDOCRINOLOGY: Vitamin D and COVID-19, Eur. J. Endocrinol
Bouillon, Bikle, Vitamin D Metabolism Revised: Fall of Dogmas, J. Bone Miner. Res, doi:10.1002/jbmr.3884
Bouillon, Marcocci, Carmeliet, Bikle, White et al., Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions, Endocr. Rev, doi:10.1210/er.2018-00126
Cangiano, Fatti, Danesi, Gazzano, Croci et al., Mortality in an Italian nursing home during COVID-19 pandemic: Correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests, Aging, doi:10.18632/aging.202307
Castillo, Entrenas Costa, Vaquero Barrios, Alcala Diaz, Lopez Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105751
Chen, Nirula, Heller, Gottlieb, Boscia et al., SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2029849
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study, Lancet
D'avolio, Avataneo, Manca, Cusato, De Nicolo et al., 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2, Nutrients, doi:10.3390/nu12051359
Dancer, Parekh, Lax, D'souza, Zheng et al., Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS), Thorax, doi:10.1136/thoraxjnl-2014-206680
Fan, Brodie, Slutsky, Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment, JAMA, doi:10.1001/jama.2017.21907
Force, Ranieri, Rubenfeld, Thompson, Ferguson et al., Acute respiratory distress syndrome: The Berlin Definition, JAMA, doi:10.1001/jama.2012.5669
Giustina, Bouillon, Binkley, Sempos, Adler et al., Controversies in Vitamin D: A Statement from the Third International Conference, JBMR Plus
Grant, Boucher, Bhattoa, Lahore, Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2017.08.009
Grant, Lahore, Mcdonnell, Baggerly, French et al., Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths, Nutrients, doi:10.3390/nu12040988
Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in Hospitalized Patients with Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2021436
Hansdottir, Monick, Hinde, Lovan, Look et al., Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense, J. Immunol, doi:10.4049/jimmunol.181.10.7090
Heaney, Guidelines for optimizing design and analysis of clinical studies of nutrient effects, Nutr. Rev
Hernandez, Nan, Fernandez-Ayala, Garcia-Unzueta, Hernandez-Hernandez et al., Vitamin D Status in Hospitalized Patients with SARS-CoV-2
Hill, The Environment and Disease: Association or Causation?, Proc. R. Soc. Med, doi:10.1177/003591576505800503
Ilie, Stefanescu, Smith, The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality, Aging Clin. Exp. Res, doi:10.1007/s40520-020-01570-8
Jetter, Egli, Dawson-Hughes, Staehelin, Stoecklin et al., Pharmacokinetics of oral vitamin D(3) and calcifediol, Bone, doi:10.1016/j.bone.2013.10.014
Jolliffe, Stefanidis, Wang, Kermani, Dimitrov et al., Vitamin D Metabolism Is Dysregulated in Asthma and Chronic Obstructive Pulmonary Disease, Am. J. Respir. Crit. Care Med, doi:10.1164/rccm.201909-1867OC
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2031994
Kaufman, Niles, Kroll, Bi, Holick, SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels, PLoS ONE, doi:10.1371/journal.pone.0239252
Kong, Zhu, Shi, Liu, Chen et al., VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system, Mol. Endocrinol, doi:10.1210/me.2013-1146
Laird, Rhodes, Kenny, Vitamin, and Inflammation: Potential Implications for Severity of Covid-19, Ir. Med. J
Lamontagne, Agoritsas, Macdonald, Leo, Diaz et al., A living WHO guideline on drugs for covid-19, BMJ, doi:10.1136/bmj.m3379
Lim, Van Der Eerden, Laing, Boersma, Karalus et al., Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study, Thorax, doi:10.1136/thorax.58.5.377
Ling, Broad, Murphy, Pappachan, Pardesi-Newton et al., High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study, Nutrients, doi:10.3390/nu12123799
Maghbooli, Sahraian, Ebrahimi, Pazoki, Kafan et al., Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection, PLoS ONE, doi:10.1371/journal.pone.0239799
Martineau, Forouhi, Vitamin D for COVID-19: A case to answer?, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(20)30268-0
Mata-Granados, Luque De Castro, Quesada Gomez, Inappropriate serum levels of retinol, alpha-tocopherol, 25 hydroxyvitamin D3 and 24,25 dihydroxyvitamin D3 levels in healthy Spanish adults: Simultaneous assessment by HPLC, Clin. Biochem, doi:10.1016/j.clinbiochem.2008.02.003
Meltzer, Best, Zhang, Vokes, Arora et al., Association of Vitamin D Levels, Race/Ethnicity, and Clinical Characteristics With COVID-19 Test Results, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.4117
Meltzer, Best, Zhang, Vokes, Arora et al., Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2020.19722
Merzon, Tworowski, Gorohovski, Vinker, Golan Cohen et al., Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study, FEBS J, doi:10.1111/febs.15495
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.26848
Navarro-Valverde, Sosa-Henriquez, Alhambra-Exposito, Quesada-Gomez, Vitamin D3 and calcidiol are not equipotent, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2016.01.014
Park, Lee, Hong, Lim, Koh et al., Effect of vitamin D deficiency in Korean patients with acute respiratory distress syndrome, Korean J. Intern Med, doi:10.3904/kjim.2017.380
Pereira, Dantas Damascena, Galvao Azevedo, De Almeida Oliveira, Da Mota Santana, Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2020.1841090
Quesada-Gomez, Bouillon, Is calcifediol better than cholecalciferol for vitamin D supplementation?, Osteoporos. Int, doi:10.1007/s00198-018-4520-y
Quesada-Gomez, Diaz-Curiel, Sosa-Henriquez, Malouf-Sierra, Nogues-Solan et al., Low calcium intake and inadequate vitamin D status in postmenopausal osteoporotic women, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2012.10.013
Quesada-Gomez, Entrenas-Castillo, Bouillon, Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_166, J. Steroid. Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105719
Radujkovic, Hippchen, Tiwari-Heckler, Dreher, Boxberger et al., Vitamin D Deficiency and Outcome of COVID-19 Patients, Nutrients, doi:10.3390/nu12092757
Rafique, Rejnmark, Heickendorff, Moller, OH)D3 and 1.25(OH)2D3 inhibits TNF-alpha expression in human monocyte derived macrophages, PLoS ONE, doi:10.1371/journal.pone.0215383
Rastogi, Bhansali, Khare, Suri, Yaddanapudi et al., Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study), Postgrad. Med. J, doi:10.1136/postgradmedj-2020-139065
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., the Northwell COVID-19 Research Consortium
Shah Alam, Czajkowsky, Aminul Islam, Ataur Rahman, The role of vitamin D in reducing SARS-CoV-2 infection: An update, Int. Immunopharmacol, doi:10.1016/j.intimp.2021.107686
Shi, Liu, Fu, Xu, Wu et al., Vitamin D/VDR signaling attenuates lipopolysaccharideinduced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier, Mol. Med. Rep, doi:10.3892/mmr.2015.4685
Smolders, Van Den Ouweland, Geven, Pickkers, Kox, Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence, Metabolism Clin. Exp, doi:10.1016/j.metabol.2020.154434
Thickett, Moromizato, Litonjua, Amrein, Quraishi et al., Association between prehospital vitamin D status and incident acute respiratory failure in critically ill patients: A retrospective cohort study, BMJ Open Respir. Res, doi:10.1136/bmjresp-2014-000074
Waldron, Ashby, Cornes, Bechervaise, Razavi et al., Vitamin D: A negative acute phase reactant, J. Clin. Pathol, doi:10.1136/jclinpath-2012-201301
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review, JAMA, doi:10.1001/jama.2020.12839
Xiao, Li, Su, Mu, Qu, Could SARS-CoV-2-induced lung injury be attenuated by vitamin D?, Int. J. Infect Dis, doi:10.1016/j.ijid.2020.10.059
Xu, Yang, Chen, Luo, Zhang et al., Vitamin D alleviates lipopolysaccharideinduced acute lung injury via regulation of the reninangiotensin system, Mol. Med. Rep, doi:10.3892/mmr.2017.7546
Zheng, Yang, Hu, Li, Wang et al., Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-beta induced epithelial to mesenchymal transition, Biochem. Pharmacol, doi:10.1016/j.bcp.2020.113955
Zhu, Zhang, Wang, Li, Yang et al., A Novel Coronavirus from Patients with Pneumonia in China, N. Engl. J. Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.3390/nu13061760",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu13061760",
"abstract": "<jats:p>Context. Calcifediol has been proposed as a potential treatment for COVID-19 patients. Objective: To compare the administration or not of oral calcifediol on mortality risk of patients hospitalized because of COVID-19. Design: Retrospective, multicenter, open, non-randomized cohort study. Settings: Hospitalized care. Patients: Patients with laboratory-confirmed COVID-19 between 5 February and 5 May 2020 in five hospitals in the South of Spain. Intervention: Patients received calcifediol (25-hydroxyvitamin D3) treatment (0.266 mg/capsule, 2 capsules on entry and then one capsule on day 3, 7, 14, 21, and 28) or not. Main Outcome Measure: In-hospital mortality during the first 30 days after admission. Results: A total of 537 patients were hospitalized with COVID-19 (317 males (59%), median age, 70 years), and 79 (14.7%) received calcifediol treatment. Overall, in-hospital mortality during the first 30 days was 17.5%. The OR of death for patients receiving calcifediol (mortality rate of 5%) was 0.22 (95% CI, 0.08 to 0.61) compared to patients not receiving such treatment (mortality rate of 20%; p < 0.01). Patients who received calcifediol after admission were more likely than those not receiving treatment to have comorbidity and a lower rate of CURB-65 score for pneumonia severity ≥ 3 (one point for each of confusion, urea > 7 mmol/L, respiratory rate ≥ 30/min, systolic blood pressure < 90 mm Hg or diastolic blood pressure ≤ 60 mm Hg, and age ≥ 65 years), acute respiratory distress syndrome (moderate or severe), c-reactive protein, chronic kidney disease, and blood urea nitrogen. In a multivariable logistic regression model, adjusting for confounders, there were significant differences in mortality for patients receiving calcifediol compared with patients not receiving it (OR = 0.16 (95% CI 0.03 to 0.80). Conclusion: Among patients hospitalized with COVID-19, treatment with calcifediol, compared with those not receiving calcifediol, was significantly associated with lower in-hospital mortality during the first 30 days. The observational design and sample size may limit the interpretation of these findings.</jats:p>",
"alternative-id": [
"nu13061760"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4572-3611",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alcala-Diaz",
"given": "Juan F.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Limia-Perez",
"given": "Laura",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9909-3555",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gomez-Huelgas",
"given": "Ricardo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1932-8682",
"affiliation": [],
"authenticated-orcid": false,
"family": "Martin-Escalante",
"given": "Maria D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cortes-Rodriguez",
"given": "Begoña",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zambrana-Garcia",
"given": "Jose L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Entrenas-Castillo",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perez-Caballero",
"given": "Ana I.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9333-076X",
"affiliation": [],
"authenticated-orcid": false,
"family": "López-Carmona",
"given": "Maria D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia-Alegria",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lozano Rodríguez-Mancheño",
"given": "Aquiles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arenas-de Larriva",
"given": "Maria del Sol",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9512-8274",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pérez-Belmonte",
"given": "Luis M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3197-5367",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jungreis",
"given": "Irwin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouillon",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Quesada-Gomez",
"given": "Jose Manual",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopez-Miranda",
"given": "Jose",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
5,
24
]
],
"date-time": "2021-05-24T04:01:20Z",
"timestamp": 1621828880000
},
"deposited": {
"date-parts": [
[
2021,
5,
24
]
],
"date-time": "2021-05-24T04:20:14Z",
"timestamp": 1621830014000
},
"indexed": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T15:10:11Z",
"timestamp": 1712070611912
},
"is-referenced-by-count": 66,
"issue": "6",
"issued": {
"date-parts": [
[
2021,
5,
21
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2021,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
21
]
],
"date-time": "2021-05-21T00:00:00Z",
"timestamp": 1621555200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/13/6/1760/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1760",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
5,
21
]
]
},
"published-online": {
"date-parts": [
[
2021,
5,
21
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1001/jama.2020.12839",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"key": "ref3",
"unstructured": "COVID-19 Coronavirus Pandemichttps://www.worldometers.info/coronavirus/?"
},
{
"DOI": "10.1126/science.abc6261",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1136/bmj.m3379",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"key": "ref8",
"unstructured": "Therapeutics and COVID-19: Living Guidelinehttps://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1056/NEJMoa2031994",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1210/er.2018-00126",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1001/jama.2017.21907",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.3390/nu12040988",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1530/EJE-20-0665",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/j.jsbmb.2020.105719",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1016/j.intimp.2021.107686",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1007/s40520-020-01570-8",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1002/jbm4.10417",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1016/j.bone.2013.10.014",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.14814/phy2.14747",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1001/jama.2012.5669",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1136/thorax.58.5.377",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1016/j.ijid.2020.10.059",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.3892/mmr.2017.7546",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.3892/mmr.2015.4685",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1210/me.2013-1146",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1016/j.bcp.2020.113955",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.4049/jimmunol.181.10.7090",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1371/journal.pone.0215383",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1371/journal.pone.0090301",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1136/thoraxjnl-2014-206680",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1136/bmjresp-2014-000074",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.3904/kjim.2017.380",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"article-title": "Vitamin D and Inflammation: Potential Implications for Severity of Covid-19",
"author": "Laird",
"first-page": "81",
"journal-title": "Ir. Med. J.",
"key": "ref37",
"volume": "113",
"year": "2020"
},
{
"DOI": "10.3390/nu12051359",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1001/jamanetworkopen.2020.19722",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1371/journal.pone.0239252",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1210/clinem/dgaa733",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1371/journal.pone.0239799",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.3390/nu12092757",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1111/febs.15495",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1001/jamanetworkopen.2021.4117",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1016/j.metabol.2020.154434",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1080/10408398.2020.1841090",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1016/S2213-8587(20)30268-0",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1136/jclinpath-2012-201301",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1186/cc10120",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.1136/postgradmedj-2020-139065",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.1016/j.jsbmb.2016.01.014",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1007/s00198-018-4520-y",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.1002/jbmr.3884",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.1164/rccm.201909-1867OC",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.1001/jama.2020.26848",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.3390/nu12113377",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.1016/j.jsbmb.2021.105901",
"doi-asserted-by": "publisher",
"key": "ref58"
},
{
"DOI": "10.3390/nu12123799",
"doi-asserted-by": "publisher",
"key": "ref59"
},
{
"DOI": "10.18632/aging.202307",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.1016/j.jsbmb.2020.105771",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"DOI": "10.1111/nure.12090",
"doi-asserted-by": "publisher",
"key": "ref62"
},
{
"DOI": "10.1016/j.jsbmb.2017.08.009",
"doi-asserted-by": "publisher",
"key": "ref63"
},
{
"DOI": "10.1016/j.clinbiochem.2008.02.003",
"doi-asserted-by": "publisher",
"key": "ref64"
},
{
"DOI": "10.1016/j.jsbmb.2012.10.013",
"doi-asserted-by": "publisher",
"key": "ref65"
},
{
"DOI": "10.1177/003591576505800503",
"doi-asserted-by": "publisher",
"key": "ref66"
}
],
"reference-count": 66,
"references-count": 66,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/13/6/1760"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Food Science",
"Nutrition and Dietetics"
],
"subtitle": [],
"title": "Calcifediol Treatment and Hospital Mortality Due to COVID-19: A Cohort Study",
"type": "journal-article",
"volume": "13"
}