Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study)
et al., Postgraduate Medical Journal, doi:10.1136/postgradmedj-2020-139065, SHADE, NCT04459247, Nov 2020
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
53% reduction in PCR+ with high-dose cholecalciferol supplementation. RCT with 16 treatment patients and 24 control patients. 25(OH)D levels at day 14 were 52 ng/ml vs. 15 ng/ml in the intervention and control group.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 44% [33‑53%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 2nd of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 11th of 136 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
|
risk of no viral clearance, 52.6% lower, RR 0.47, p = 0.02, treatment 6 of 16 (37.5%), control 19 of 24 (79.2%), NNT 2.4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Rastogi et al., 12 Nov 2020, Randomized Controlled Trial, India, peer-reviewed, 8 authors, dosage 60,000IU days 1-7, trial NCT04459247 (history) (SHADE).
Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study)
Postgraduate Medical Journal, doi:10.1136/postgradmedj-2020-139065
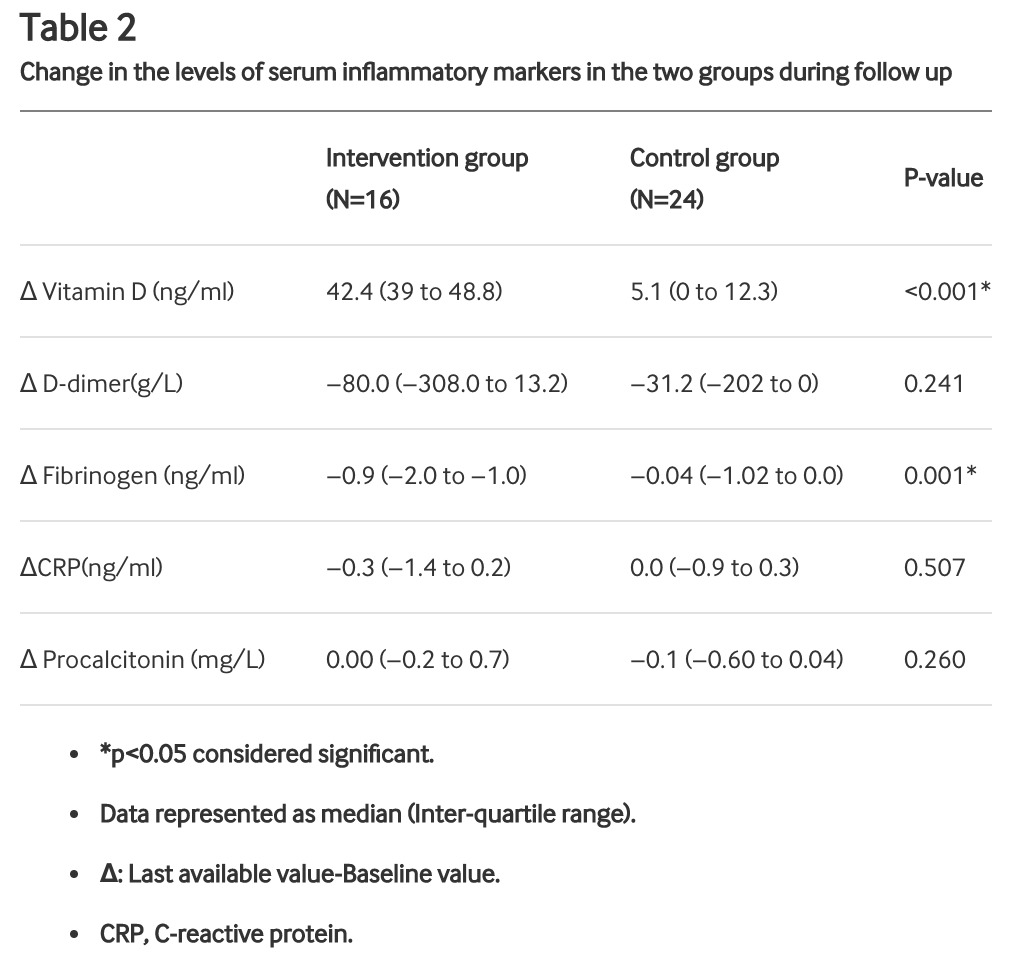
Background Vitamin D has an immunomodulatory role but the effect of therapeutic vitamin D supplementation in SARS-CoV-2 infection is not known. Aim Effect of high dose, oral cholecalciferol supplementation on SARS-CoV-2 viral clearance. Design Randomised, placebo-controlled. Participants Asymptomatic or mildly symptomatic SARS-CoV-2 RNA positive vitamin D deficient (25(OH) D<20 ng/ml) individuals. Intervention Participants were randomised to receive daily 60 000 IU of cholecalciferol (oral nano-liquid droplets) for 7 days with therapeutic target 25(OH) D>50 ng/ml (intervention group) or placebo (control group). Patients requiring invasive ventilation or with significant comorbidities were excluded. 25(OH)D levels were assessed at day 7, and cholecalciferol supplementation was continued for those with 25(OH)D <50 ng/ml in the intervention arm. SARS-CoV-2 RNA and inflammatory markers fibrinogen, D-dimer, procalcitonin and (CRP), ferritin were measured periodically. Outcome measure Proportion of patients with SARS-CoV-2 RNA negative before day-21 and change in inflammatory markers. Results Forty SARS-CoV-2 RNA positive individuals were randomised to intervention (n=16) or control (n=24) group. Baseline serum 25(OH)D was 8.6 (7.1 to 13.1) and 9.54 (8.1 to 12.5) ng/ml (p=0.730), in the intervention and control group, respectively. 10 out of 16 patients could achieve 25(OH)D>50 ng/ml by day-7 and another two by day-14 [day-14 25(OH)D levels 51.7 (48.9 to 59.5) ng/ml and 15.2 (12.7 to 19.5) ng/ml (p<0.001) in intervention and control group, respectively]. 10 (62.5%) participants in the intervention group and 5 (20.8%) participants in the control arm (p<0.018) became SARS-CoV-2 RNA negative. Fibrinogen levels significantly decreased with cholecalciferol supplementation (intergroup difference 0.70 ng/ml; P=0.007) unlike other inflammatory biomarkers. Conclusion Greater proportion of vitamin D-deficient individuals with SARS-CoV-2 infection turned SARS-CoV-2 RNA negative with a significant decrease in fibrinogen on high-dose cholecalciferol supplementation. Trial register number NCT04459247.
Competing interests None declared. Patient consent for publication Not required. Provenance and peer review Not commissioned; externally peer reviewed. Data availability statement Data are available upon reasonable request. Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peerreviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/ or omissions arising from translation and adaptation or otherwise. This article is made freely available for use in accordance with BMJ's website terms and conditions for the duration of the COVID-19 pandemic or until otherwise determined by BMJ. You may use, download and print the article for any lawful, noncommercial purpose (including text and data mining) provided that all copyright notices and trade marks are retained. What is already known on the subject ► Vitamin-D has immunomodulatory effect and may reduce susceptibility and severity of viral infections but its role in SARS-CoV-2 infection is not known.
ORCID iD..
References
Amrein, Sourij, Wagner, Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study, Crit Care, doi:10.1186/cc10120
Arboleda, Urcuqui-Inchima, Vitamin D supplementation: a potential approach for COVID-19 therapeutics?, Front Immunol, doi:10.3389/fimmu.2020.01523
Bai, Yao, Wei, Presumed asymptomatic carrier transmission of COVID-19, JAMA, doi:10.1001/jama.2020.2565
Camargo, Martineau, Vitamin D to prevent COVID-19: recommendations for the design of clinical trials, Febs J, doi:10.1111/febs.15534
Castillo, Costa, Barrios, Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2020.105751
Dixon, Barker, Mckinnon, Positive correlation between circulating cathelicidin antimicrobial peptide (hCAP18/LL-37) and 25-hydroxyvitamin D levels in healthy adults, BMC Res Notes, doi:10.1186/1756-0500-5-575
He, Lau, Wu, Temporal dynamics in viral shedding and transmissibility of COVID-19, Nat Med, doi:10.1038/s41591-020-0869-5
Illie, Stefanescu, Smith, The role of vitamin D in the prevention of coronavirus disease infection and mortality, Aging Clin Exp Res, doi:10.1007/s40520-020-01570-8
Jakovac, COVID-19 and vitamin D: is there a link and an opportunity for intervention?, Am J Physiol Endocrinol Metab, doi:10.1152/ajpendo.00138.2020
Jing, Liu, Zhang, Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study [published online ahead of print, 2020 Jun 17, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30471-0
Kamboj, Dwivedi, Toteja, Prevalence of hypovitaminosis D in India & way forward, Indian J Med Res, doi:10.4103/ijmr.IJMR_1807_18
Kearns, Alvarez, Tangpricha, Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review, Endocr Pract, doi:10.4158/EP13265.RA
Kempker, Martin, Vitamin D and sepsis: from associations to causal connections, Inflamm Allergy Drug Targets, doi:10.2174/18715281113129990048
Maghbooli, Sahraian, Ebrahimi, Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection, PLoS One, doi:10.1371/journal.pone.0239799
Martineau, Jolliffe, Hooper, Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ, doi:10.1136/bmj.i6583
Meltzer, Best, Zhang, Association of vitamin D status and other clinical characteristics with COVID-19 test results, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.19722
Merzon, Tworowski, Gorohovski, Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study, Febs J, doi:10.1111/febs.15495
Sanders, Monogue, Jodlowski, Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review, JAMA, doi:10.1001/jama.2020.6019
Tellioglu, Basaran, Guzel, Efficacy and safety of high dose intramuscular or oral cholecalciferol in vitamin D deficient/insufficient elderly, Maturitas, doi:10.1016/j.maturitas.2012.04.011
Velavan, Meyer, Mild versus severe COVID-19: laboratory markers, Int J Infect Dis, doi:10.1016/j.ijid.2020.04.061
Zdrenghea, Makrinioti, Bagacean, Vitamin D modulation of innate immune responses to respiratory viral infections, Rev Med Virol, doi:10.2174/18715281113129990046
Zeng, Huang, Guo, Association of inflammatory markers with the severity of COVID-19: a meta-analysis
Zeng, Yu, Chen, Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China, Crit Care, doi:10.1186/s13054-020-03255-0
DOI record:
{
"DOI": "10.1136/postgradmedj-2020-139065",
"ISSN": [
"0032-5473",
"1469-0756"
],
"URL": "http://dx.doi.org/10.1136/postgradmedj-2020-139065",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Vitamin D has an immunomodulatory role but the effect of therapeutic vitamin D supplementation in SARS-CoV-2 infection is not known.</jats:p></jats:sec><jats:sec><jats:title>Aim</jats:title><jats:p>Effect of high dose, oral cholecalciferol supplementation on SARS-CoV-2 viral clearance.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>Randomised, placebo-controlled.</jats:p></jats:sec><jats:sec><jats:title>Participants</jats:title><jats:p>Asymptomatic or mildly symptomatic SARS-CoV-2 RNA positive vitamin D deficient (25(OH)D&lt;20 ng/ml) individuals.</jats:p></jats:sec><jats:sec><jats:title>Intervention</jats:title><jats:p>Participants were randomised to receive daily 60 000 IU of cholecalciferol (oral nano-liquid droplets) for 7 days with therapeutic target 25(OH)D&gt;50 ng/ml (intervention group) or placebo (control group). Patients requiring invasive ventilation or with significant comorbidities were excluded. 25(OH)D levels were assessed at day 7, and cholecalciferol supplementation was continued for those with 25(OH)D &lt;50 ng/ml in the intervention arm. SARS-CoV-2 RNA and inflammatory markers fibrinogen, D-dimer, procalcitonin and (CRP), ferritin were measured periodically.</jats:p></jats:sec><jats:sec><jats:title>Outcome measure</jats:title><jats:p>Proportion of patients with SARS-CoV-2 RNA negative before day-21 and change in inflammatory markers.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Forty SARS-CoV-2 RNA positive individuals were randomised to intervention (n=16) or control (n=24) group. Baseline serum 25(OH)D was 8.6 (7.1 to 13.1) and 9.54 (8.1 to 12.5) ng/ml (p=0.730), in the intervention and control group, respectively. 10 out of 16 patients could achieve 25(OH)D&gt;50 ng/ml by day-7 and another two by day-14 [day-14 25(OH)D levels 51.7 (48.9 to 59.5) ng/ml and 15.2 (12.7 to 19.5) ng/ml (p&lt;0.001) in intervention and control group, respectively]. 10 (62.5%) participants in the intervention group and 5 (20.8%) participants in the control arm (p&lt;0.018) became SARS-CoV-2 RNA negative. Fibrinogen levels significantly decreased with cholecalciferol supplementation (intergroup difference 0.70 ng/ml; P=0.007) unlike other inflammatory biomarkers.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Greater proportion of vitamin D-deficient individuals with SARS-CoV-2 infection turned SARS-CoV-2 RNA negative with a significant decrease in fibrinogen on high-dose cholecalciferol supplementation.</jats:p></jats:sec><jats:sec><jats:title>Trial register number</jats:title><jats:p>NCT04459247.</jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Endocrinology, Post Graduate Institute of Medical Education and Research , Chandigarh, India"
}
],
"family": "Rastogi",
"given": "Ashu",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Endocrinology, Post Graduate Institute of Medical Education and Research , Chandigarh, India"
}
],
"family": "Bhansali",
"given": "Anil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine, Post Graduate Institute of Medical Education and Research , Chandigarh, India"
}
],
"family": "Khare",
"given": "Niranjan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine, Post Graduate Institute of Medical Education and Research , Chandigarh, India"
}
],
"family": "Suri",
"given": "Vikas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Anaesthesia, Post Graduate Institute of Medical Education and Research , Chandigarh, India"
}
],
"family": "Yaddanapudi",
"given": "Narayana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Endocrinology, Post Graduate Institute of Medical Education and Research , Chandigarh, India"
}
],
"family": "Sachdeva",
"given": "Naresh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Anaesthesia, Post Graduate Institute of Medical Education and Research , Chandigarh, India"
}
],
"family": "Puri",
"given": "G D",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9375-3102",
"affiliation": [
{
"name": "Internal Medicine, Post Graduate Institute of Medical Education and Research , Chandigarh, India"
}
],
"authenticated-orcid": false,
"family": "Malhotra",
"given": "Pankaj",
"sequence": "additional"
}
],
"container-title": "Postgraduate Medical Journal",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2020,
11,
12
]
],
"date-time": "2020-11-12T22:20:59Z",
"timestamp": 1605219659000
},
"deposited": {
"date-parts": [
[
2023,
10,
12
]
],
"date-time": "2023-10-12T06:43:43Z",
"timestamp": 1697093023000
},
"indexed": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T15:07:35Z",
"timestamp": 1712070455089
},
"is-referenced-by-count": 182,
"issue": "1156",
"issued": {
"date-parts": [
[
2020,
11,
12
]
]
},
"journal-issue": {
"issue": "1156",
"published-online": {
"date-parts": [
[
2020,
11,
12
]
]
},
"published-print": {
"date-parts": [
[
2022,
2,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 787,
"start": {
"date-parts": [
[
2023,
1,
8
]
],
"date-time": "2023-01-08T00:00:00Z",
"timestamp": 1673136000000
}
},
{
"URL": "https://bmj.com/coronavirus/usage",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
11,
12
]
],
"date-time": "2020-11-12T00:00:00Z",
"timestamp": 1605139200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/pmj/article-pdf/98/1156/87/49919102/postgradmedj-98-87.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/pmj/article-pdf/98/1156/87/49919102/postgradmedj-98-87.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "87-90",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2020,
11,
12
]
]
},
"published-online": {
"date-parts": [
[
2020,
11,
12
]
]
},
"published-other": {
"date-parts": [
[
2022,
2
]
]
},
"published-print": {
"date-parts": [
[
2022,
2,
1
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1038/s41591-020-0869-5",
"article-title": "Temporal dynamics in viral shedding and transmissibility of COVID-19",
"author": "He",
"doi-asserted-by": "crossref",
"first-page": "672",
"journal-title": "Nat Med",
"key": "2023041705590501800_",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2565",
"article-title": "Presumed asymptomatic carrier transmission of COVID-19",
"author": "Bai",
"doi-asserted-by": "crossref",
"first-page": "1406",
"journal-title": "JAMA",
"key": "2023041705590501800_",
"volume": "323",
"year": "2020"
},
{
"article-title": "Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study [published online ahead of print, 2020 Jun 17]",
"author": "Jing",
"first-page": "30471",
"journal-title": "Lancet Infect Dis",
"key": "2023041705590501800_",
"volume": "S1473-3099",
"year": "2020"
},
{
"article-title": "Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review",
"author": "Sanders",
"first-page": "1824",
"journal-title": "JAMA",
"key": "2023041705590501800_",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1007/s40520-020-01570-8",
"article-title": "The role of vitamin D in the prevention of coronavirus disease infection and mortality",
"author": "Illie",
"doi-asserted-by": "crossref",
"first-page": "1195",
"journal-title": "Aging Clin Exp Res",
"key": "2023041705590501800_",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.19722",
"article-title": "Association of vitamin D status and other clinical characteristics with COVID-19 test results",
"author": "Meltzer",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "2023041705590501800_",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1111/febs.15495",
"article-title": "Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study",
"author": "Merzon",
"doi-asserted-by": "crossref",
"first-page": "3693",
"journal-title": "Febs J",
"key": "2023041705590501800_",
"volume": "287",
"year": "2020"
},
{
"article-title": "Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data",
"author": "Martineau",
"first-page": "356:i6583",
"journal-title": "BMJ",
"key": "2023041705590501800_",
"volume": "15",
"year": "2017"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"article-title": "Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study",
"author": "Entrenas Castillo",
"doi-asserted-by": "crossref",
"first-page": "105751",
"journal-title": "J Steroid Biochem Mol Biol",
"key": "2023041705590501800_",
"volume": "203",
"year": "2020"
},
{
"DOI": "10.1111/febs.15534",
"article-title": "Vitamin D to prevent COVID-19: recommendations for the design of clinical trials",
"author": "Camargo",
"doi-asserted-by": "crossref",
"first-page": "3689",
"journal-title": "Febs J",
"key": "2023041705590501800_",
"volume": "287",
"year": "2020"
},
{
"DOI": "10.1186/1756-0500-5-575",
"article-title": "Positive correlation between circulating cathelicidin antimicrobial peptide (hCAP18/LL-37) and 25-hydroxyvitamin D levels in healthy adults",
"author": "Dixon",
"doi-asserted-by": "crossref",
"first-page": "575",
"journal-title": "BMC Res Notes",
"key": "2023041705590501800_",
"volume": "5",
"year": "2012"
},
{
"DOI": "10.1371/journal.pone.0239799",
"article-title": "Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection",
"author": "Maghbooli",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "2023041705590501800_",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03255-0",
"article-title": "Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China",
"author": "Zeng",
"doi-asserted-by": "crossref",
"first-page": "525",
"journal-title": "Crit Care",
"key": "2023041705590501800_",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.2174/18715281113129990048",
"article-title": "Vitamin D and sepsis: from associations to causal connections",
"author": "Kempker",
"doi-asserted-by": "crossref",
"first-page": "000",
"journal-title": "Inflamm Allergy Drug Targets",
"key": "2023041705590501800_",
"volume": "12",
"year": "2013"
},
{
"DOI": "10.1002/rmv.1909",
"article-title": "Vitamin D modulation of innate immune responses to respiratory viral infections",
"author": "Zdrenghea",
"doi-asserted-by": "crossref",
"journal-title": "Rev Med Virol",
"key": "2023041705590501800_",
"volume": "27",
"year": "2017"
},
{
"DOI": "10.1152/ajpendo.00138.2020",
"article-title": "COVID-19 and vitamin D: is there a link and an opportunity for intervention?",
"author": "Jakovac",
"doi-asserted-by": "crossref",
"journal-title": "Am J Physiol Endocrinol Metab",
"key": "2023041705590501800_",
"volume": "318",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.01523",
"article-title": "Vitamin D supplementation: a potential approach for COVID-19 therapeutics?",
"author": "Arboleda",
"doi-asserted-by": "crossref",
"journal-title": "Front Immunol",
"key": "2023041705590501800_",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.4103/ijmr.IJMR_1807_18",
"article-title": "Prevalence of hypovitaminosis D in India & way forward",
"author": "Kamboj",
"doi-asserted-by": "crossref",
"first-page": "548",
"journal-title": "Indian J Med Res",
"key": "2023041705590501800_",
"volume": "148",
"year": "2018"
},
{
"DOI": "10.1016/j.maturitas.2012.04.011",
"article-title": "Efficacy and safety of high dose intramuscular or oral cholecalciferol in vitamin D deficient/insufficient elderly",
"author": "Tellioglu",
"doi-asserted-by": "crossref",
"first-page": "332",
"journal-title": "Maturitas",
"key": "2023041705590501800_",
"volume": "72",
"year": "2012"
},
{
"DOI": "10.1186/cc10120",
"article-title": "Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study",
"author": "Amrein",
"doi-asserted-by": "crossref",
"journal-title": "Crit Care",
"key": "2023041705590501800_",
"volume": "15",
"year": "2011"
},
{
"DOI": "10.4158/EP13265.RA",
"article-title": "Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review",
"author": "Kearns",
"doi-asserted-by": "crossref",
"first-page": "341",
"journal-title": "Endocr Pract",
"key": "2023041705590501800_",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1016/j.ijid.2020.04.061",
"article-title": "Mild versus severe COVID-19: laboratory markers",
"author": "Velavan",
"doi-asserted-by": "crossref",
"first-page": "304",
"journal-title": "Int J Infect Dis",
"key": "2023041705590501800_",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.05.055",
"article-title": "Association of inflammatory markers with the severity of COVID-19: a meta-analysis [published online ahead of print, 2020 May 18]",
"author": "Zeng",
"doi-asserted-by": "crossref",
"first-page": "467",
"journal-title": "Int J Infect Dis",
"key": "2023041705590501800_",
"volume": "96",
"year": "2020"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/pmj/article/98/1156/87/6958877"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "98"
}
