High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study
et al., Nutrients, doi:10.3390/nu12123799, Dec 2020
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
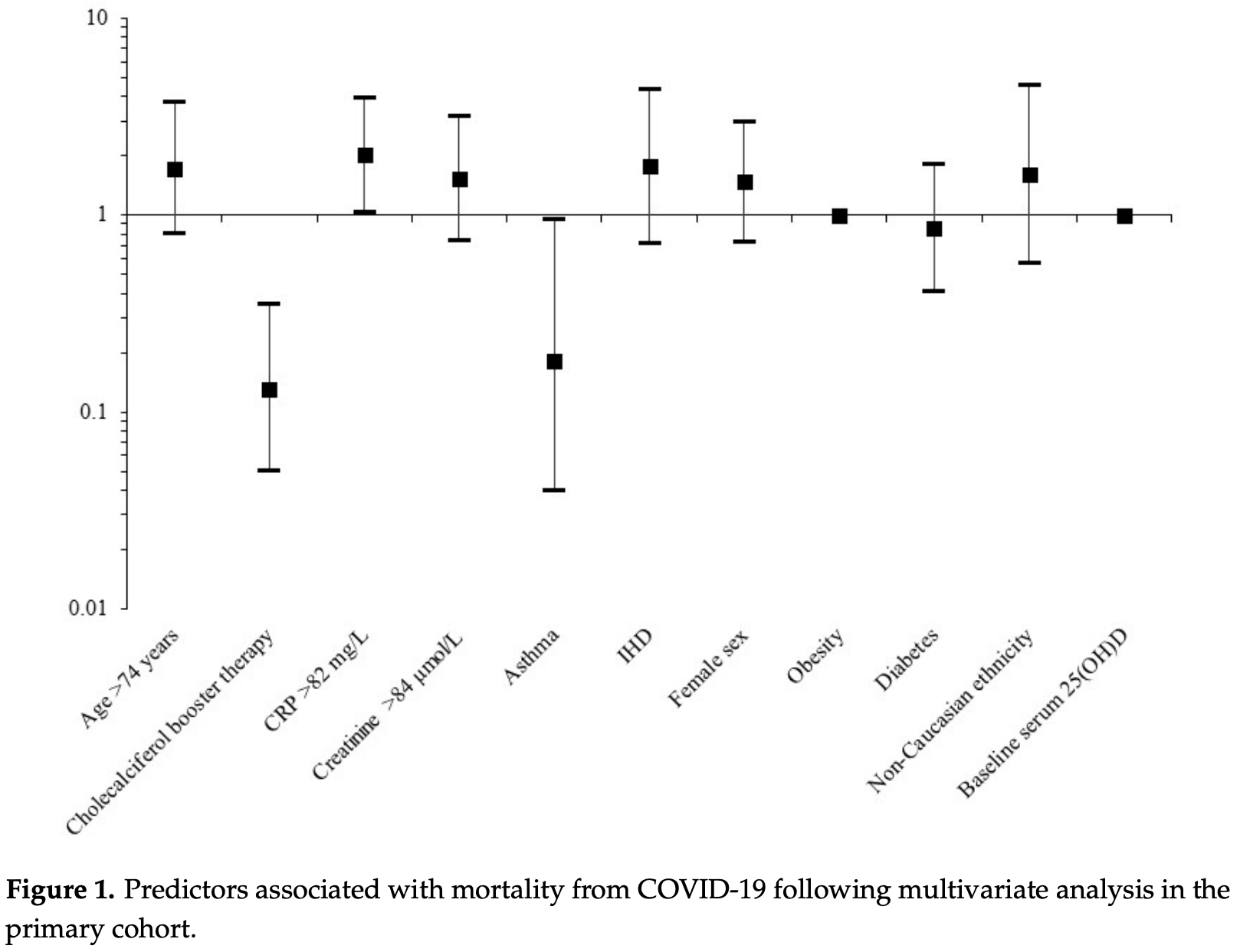
80% lower mortality with cholecalciferol booster therapy. Retrospective 986 hospitalized patients in the UK finding that cholecalciferol booster therapy, regardless of baseline serum levels, was associated with a reduced risk of mortality in acute COVID-19 inpatients.
Primary cohort of 444 patients, adjusted mortality odds ratio aOR 0.13, p < 0.001.
Validation cohort of 541 patients, adjusted mortality odds ratio aOR 0.38, p = 0.018.
Validation cohort of 541 patients, adjusted mortality odds ratio aOR 0.38, p = 0.018.
This is the 14th of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 79.8% lower, RR 0.20, p < 0.001, treatment 73, control 253, odds ratio converted to relative risk, primary cohort.
|
|
risk of death, 55.5% lower, RR 0.44, p = 0.02, treatment 80, control 443, odds ratio converted to relative risk, validation cohort.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ling et al., 11 Dec 2020, retrospective, United Kingdom, peer-reviewed, 7 authors, dosage 40,000IU weekly, regimen varied with 77% receiving a total of 40,000IU/week.
VITAMIN D TREATMENT IS ASSOCIATED WITH REDUCED RISK OF MORTALITY IN PATIENTS WITH COVID-19: A CROSS-SECTIONAL MULTI-CENTRE OBSERVATIONAL STUDY
Background: The 2019 novel coronavirus disease (Covid-19) worldwide pandemic has posed the most substantial and severe public health issue for several generations, and therapeutic options for it have not yet been optimised. Vitamin D has been proposed in the pharmacological management of Covid-19 by various sources. This study aimed to determine whether Covid-19 disease outcomes were affected by vitamin D status, and to elucidate any predictors of Covid-19 outcomes. Methods: Patients hospitalised with Covid-19 were opportunistically recruited from three different UK hospitals and their data were collected. Logistic regression was used to determine any relationships between vitamin D status and various predictors, including mortality and ventilation, and to determine any relationships between mortality, ventilation, and various predictors. Findings: Vitamin D status was not associated with any outcomes of Covid-19 investigated, following adjustment for age and sex. However, treatment with vitamin D was significantly associated with a reduced risk of death, following adjustment for age and sex (OR adj 0•48, 95% CI 0•32 -0•70, p = 1•79x10 -4 ). This relationship remained significant when also adjusted for baseline vitamin D levels (OR adj 0•47, 95% CI 0•33 -0•70, p = 1•27x10 -4 ). Interpretation: Treatment with vitamin D, regardless of baseline serum vitamin D levels, appears to be associated with a reduced risk of mortality in acute in-patients admitted with Covid-19. Further work on large population studies needs to be carried out to determine adequate serum levels of vitamin D, as well as multi-dose clinical trials of vitamin D treatment to assess maximum efficacy.
CONFLICT OF INTERESTS The authors have no conflicts of interest.
References
Caccialanza, Laviano, Lobascio, Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol, Nutrition
Calder, Carr, Gombart, Eggersdorfer, Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections, Nutrients
Carter, Baranauskas, Fly, Considerations for Obesity, Vitamin D, and Physical Activity Amid the COVID-19 Pandemic, Obesity (Silver Spring)
Gmmmg, Treatment of Vitamin D Deficiency and Insufficiency in Adults
Grant, Lahore, Mcdonnell, Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths, Nutrients
Hajhashemy, Shahdadian, Ziaei, Saneei, Serum vitamin D levels in relation to abdominal obesity: A systematic review and dose-response meta-analysis of epidemiologic studies, Obes Rev
Hastie, Mackay, Ho, Vitamin D concentrations and COVID-19 infection in UK Biobank, Diabetes Metab Syndr
Iaccarino, Grassi, Borghi, Age and Multimorbidity Predict Death Among COVID-19 Patients: Results of the SARS-RAS Study of the Italian Society of Hypertension, Hypertension
Ilie, Stefanescu, Smith, The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality, Aging Clin Exp Res
Jakovac, COVID-19 and vitamin D-Is there a link and an opportunity for intervention?, Am J Physiol Endocrinol Metab
Lovinsky-Desir, Deshpande, De, TABLE 1. SUMMARY STATISTICS OF THE STUDY POPULATION. Number of participants with available data Age (years), J Allergy Clin Immunol
Meltzer, Best, Zhang, Vokes, Arora et al., Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results, JAMA Netw Open
Merzon, Tworowski, Gorohovski, Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study, FEBS J
Munshi, Hussein, Toraih, Vitamin D insufficiency as a potential culprit in critical COVID-19 patients, J Med Virol
Ons, Deaths involving COVID-19
Panagiotou, Tee, Ihsan, Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity, Clin Endocrinol
Pastor-Barriuso, Perez-Gomez, Hernan, SARS-CoV-2 infection fatality risk in a nationwide seroepidemiological study
Rhodes, Subramanian, Laird, Kenny, Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity, Aliment Pharmacol Ther
Sacn, Vitamin, Health, None
Silberstein, Vitamin, A simpler alternative to tocilizumab for trial in COVID-19?, Med Hypotheses
Stein, Shane, Vitamin D in organ transplantation, Osteoporos Int
Teymoori-Rad, Shokri, Salimi, Marashi, The interplay between vitamin D and viral infections, Rev Med Virol
Vimaleswaran, Berry, Lu, Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts, PLoS Med
Vitamin, Level, None, median
Who, International Guidelines for Certification and Classification (Coding) of Covid-19 as Cause of Death
DOI record:
{
"DOI": "10.3390/nu12123799",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu12123799",
"abstract": "<jats:p>The worldwide pandemic of 2019 novel coronavirus disease (COVID-19) has posed the most substantial and severe public health issue for several generations, and therapeutic options have not yet been optimised. Vitamin D (in its “parent” form, cholecalciferol) has been proposed in the pharmacological management of COVID-19 by various sources. We aimed to determine whether COVID-19 mortality was affected by serum 25-hydroxyvitamin D (25(OH)D) levels, vitamin D status, or cholecalciferol therapy, and to elucidate any other predictors of COVID-19 mortality. Patients hospitalised with COVID-19 were opportunistically recruited from three UK hospitals, and their data were collected retrospectively. Logistic regression was used to determine any relationships between COVID-19 mortality and potential predictors, including 25(OH)D levels and cholecalciferol booster therapy. A total of 986 participants with COVID-19 were studied, of whom 151 (16.0%) received cholecalciferol booster therapy. In the primary cohort of 444 patients, cholecalciferol booster therapy was associated with a reduced risk of COVID-19 mortality, following adjustment for potential confounders (ORadj 0.13, 95% CI 0.05–0.35, p < 0.001). This finding was replicated in a validation cohort of 541 patients (ORadj 0.38, 95% CI 0.17–0.84, p = 0.018). In this observational study, treatment with cholecalciferol booster therapy, regardless of baseline serum 25(OH)D levels, appears to be associated with a reduced risk of mortality in acute in-patients admitted with COVID-19. Further work with large population studies needs to be carried out to determine adequate serum 25(OH)D levels, as well as multi-dose clinical trials of cholecalciferol therapy to assess maximum efficacy.</jats:p>",
"alternative-id": [
"nu12123799"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8148-2344",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ling",
"given": "Stephanie F.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Broad",
"given": "Eleanor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murphy",
"given": "Rebecca",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0886-5255",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pappachan",
"given": "Joseph M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pardesi-Newton",
"given": "Satveer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kong",
"given": "Marie-France",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jude",
"given": "Edward B.",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
12,
14
]
],
"date-time": "2020-12-14T01:56:57Z",
"timestamp": 1607911017000
},
"deposited": {
"date-parts": [
[
2020,
12,
14
]
],
"date-time": "2020-12-14T04:08:59Z",
"timestamp": 1607918939000
},
"indexed": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T15:07:40Z",
"timestamp": 1712070460095
},
"is-referenced-by-count": 111,
"issue": "12",
"issued": {
"date-parts": [
[
2020,
12,
11
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2020,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
11
]
],
"date-time": "2020-12-11T00:00:00Z",
"timestamp": 1607644800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/12/12/3799/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "3799",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2020,
12,
11
]
]
},
"published-online": {
"date-parts": [
[
2020,
12,
11
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1152/ajpendo.00138.2020",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1016/j.nut.2020.110835",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1002/oby.22838",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.3390/nu12113512",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1002/rmv.2032",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.3390/nu12040988",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.3390/nu12041181",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1016/j.mehy.2020.109767",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1111/cen.14276",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1007/s40520-020-01570-8",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1002/jmv.26360",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"key": "ref12",
"unstructured": "Extension of COVID-19 COPI Noticehttps://www.hra.nhs.uk/about-us/news-updates/extension-covid-19-copi-notice/"
},
{
"key": "ref13",
"unstructured": "International Guidelines for Certification and Classification (Coding) of Covid-19 as Cause of Deathhttps://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19-20200420-EN.pdf?ua=1"
},
{
"key": "ref14",
"unstructured": "Treatment of Vitamin D Deficiency and Insufficiency in Adultshttp://gmmmg.nhs.uk/docs/nts/NTS-Recommendation-on-Vitamin-D-deficiency-and-insufficiency-adults.pdf"
},
{
"key": "ref15",
"unstructured": "https://www.randox.com/external-quality-assessment/"
},
{
"key": "ref16",
"unstructured": "http://www.deqas.org/"
},
{
"key": "ref17",
"unstructured": "https://ukneqas.org.uk/"
},
{
"DOI": "10.1016/j.dsx.2020.04.050",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1111/apt.15777",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1111/febs.15495",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1001/jamanetworkopen.2020.19722",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"key": "ref22"
},
{
"DOI": "10.1016/j.jsbmb.2016.12.011",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1371/journal.pone.0071042",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1016/j.jsbmb.2016.06.003",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1136/postgradmedj-2020-139065",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"key": "ref28"
},
{
"DOI": "10.1161/HYPERTENSIONAHA.120.15324",
"doi-asserted-by": "publisher",
"key": "ref29"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/12/12/3799"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Food Science",
"Nutrition and Dietetics"
],
"subtitle": [],
"title": "High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study",
"type": "journal-article",
"volume": "12"
}
