Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study
et al., Nutrients, doi:10.3390/nu13010219
, Jan 2021
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 91 hospitalized patients, 36 treated with high-dose cholecalciferol, showing lower combined death/ICU admission with treatment.
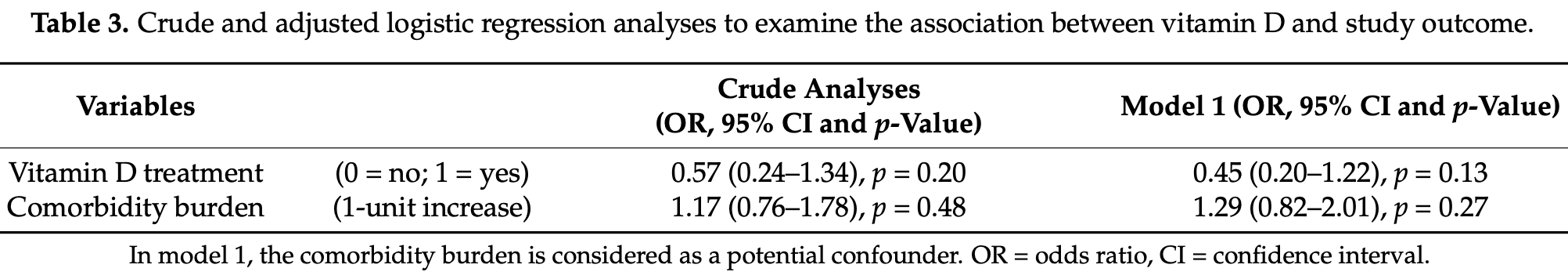
Authors also analyze the relationship with comorbidity burden, finding that the positive effect of high-dose cholecalciferol on the combined endpoint was significantly amplified with increasing comorbidity burden.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 45% [34‑54%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 17th of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
|
risk of death/ICU, 36.6% lower, RR 0.63, p = 0.13, treatment 14 of 36 (38.9%), control 29 of 55 (52.7%), NNT 7.2, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Giannini et al., 14 Jan 2021, retrospective, Italy, peer-reviewed, 21 authors, dosage 200,000IU days 1-2.
Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study
Nutrients, doi:10.3390/nu13010219
Little information is available on the beneficial effects of cholecalciferol treatment in comorbid patients hospitalized for COVID-19. The aim of this study was to retrospectively examine the clinical outcome of patients receiving in-hospital high-dose bolus cholecalciferol. Patients with a positive diagnosis of SARS-CoV-2 and overt COVID-19, hospitalized from 15 March to 20 April 2020, were considered. Based on clinical characteristics, they were supplemented (or not) with 400,000 IU bolus oral cholecalciferol (200,000 IU administered in two consecutive days) and the composite outcome (transfer to intensive care unit; ICU and/or death) was recorded. Ninety-one patients (aged 74 ± 13 years) with COVID-19 were included in this retrospective study. Fifty (54.9%) patients presented with two or more comorbid diseases. Based on the decision of the referring physician, 36 (39.6%) patients were treated with vitamin D. Receiver operating characteristic curve analysis revealed a significant predictive power of the four variables: (a) low (<50 nmol/L) 25(OH) vitamin D levels, (b) current cigarette smoking, (c) elevated D-dimer levels (d) and the presence of comorbid diseases, to explain the decision to administer vitamin D (area under the curve = 0.77, 95% CI: 0.67-0.87, p < 0.0001). Over the follow-up period (14 ± 10 days), 27 (29.7%) patients were transferred to the ICU and 22 (24.2%) died (16 prior to ICU and six in ICU). Overall, 43 (47.3%) patients experienced the combined endpoint of transfer to ICU and/or death. Logistic regression analyses revealed that the comorbidity burden significantly modified the effect of vitamin D treatment on the study outcome, both in crude (p = 0.033) and propensity score-adjusted analyses (p = 0.039), so the positive effect of high-dose cholecalciferol on the combined endpoint was significantly amplified with increasing comorbidity burden. This hypothesis-generating study warrants the formal evaluation (i.e., clinical trial) of the potential benefit that cholecalciferol can offer in these comorbid COVID-19 patients.
Supplementary Materials: The following are available online at https://www.mdpi.com/2072-6 643/13/1/219/s1, Figure S1 : Kaplan-Meier survival curve of in-hospital mortality in vitamin Dtreated and untreated patients, Figure S2 : Kaplan-Meier survival curves of the effect modification by comorbidity burden on the effectiveness of vitamin D treatment for the combined endpoint (death/ICU transfer). Author Contributions: All authors contributed to data analysis, drafting and revising the article. All authors have read and agreed to the published version of the manuscript.
References
Aibana, Huang, Aboud, Arnedo-Pena, Becerra et al., Vitamin D Status and Risk of Incident Tuberculosis Disease: A Nested Case-Control Study, Systematic Review, and Individual-Participant Data Meta-Analysis, PLoS Med, doi:10.1371/journal.pmed.1002907
Al-Badr, Martin, Vitamin, Disease, None, Clin. J. Am. Soc. Nephrol, doi:10.2215/CJN.01150308
Amrein, Schnedl, Holl, Riedl, Christopher et al., Effect of High-Dose Vitamin D3 on Hospital Length of Stay in Critically Ill Patients with Vitamin D Deficiency: The VITdAL-ICU Randomized Clinical Trial, JAMA, doi:10.1001/jama.2014.13204
Annweiler, Corvaisier, Gautier, Dubée, Legrand et al., Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study, Nutrients, doi:10.3390/nu12113377
Autier, Boniol, Pizot, Mullie, Vitamin D Status and Ill Health: A Systematic Review, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(13)70165-7
Bansal, Singh, Jain, Aggarwal, Gupta et al., The Association of D-Dimers with Mortality, Intensive Care Unit Admission or Acute Respiratory Distress Syndrome in Patients Hospitalized with Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis, Heart Lung
Bettica, Bevilacqua, Vago, Norbiato, High Prevalence of Hypovitaminosis D among Free-Living Postmenopausal Women Referred to an Osteoporosis Outpatient Clinic in Northern Italy for Initial Screening, Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found, doi:10.1007/s001980050141
Biesalski, Vitamin D Deficiency and Co-Morbidities in COVID-19 Patients-A Fatal Relationship?, NFS J
Bowles, Mcdonald, Barrón, Kennedy, O'connor et al., Surviving COVID-19 After Hospital Discharge: Symptom, Functional, and Adverse Outcomes of Home Health Recipients, Ann. Intern. Med, doi:10.7326/M20-5206
Castillo, Entrenas Costa, Vaquero Barrios, Alcalá Díaz, López Miranda et al., Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105751
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study, Lancet Lond. Engl, doi:10.1016/S0140-6736(20)30211-7
D'avolio, Avataneo, Manca, Cusato, De Nicolò et al., 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2, Nutrients, doi:10.3390/nu12051359
Daneshkhah, Agrawal, Eshein, Subramanian, Roy et al., Evidence for Possible Association of Vitamin D Status with Cytokine Storm and Unregulated Inflammation in COVID-19 Patients, Aging Clin. Exp. Res, doi:10.1007/s40520-020-01677-y
Demelo-Rodríguez, Cervilla-Muñoz, Ordieres-Ortega, Parra-Virto, Toledano-Macías et al., Incidence of Asymptomatic Deep Vein Thrombosis in Patients with COVID-19 Pneumonia and Elevated D-Dimer Levels, Thromb. Res, doi:10.1016/j.thromres.2020.05.018
Fan, Brodie, Slutsky, Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment, JAMA, doi:10.1001/jama.2017.21907
Grant, Lahore, Mcdonnell, Baggerly, French et al., Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths, Nutrients, doi:10.3390/nu12040988
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy, JAMA, doi:10.1001/jama.2020.5394
Hastie, Mackay, Ho, Celis-Morales, Katikireddi et al., Vitamin D Concentrations and COVID-19 Infection in UK Biobank, Diabetes Metab. Syndr
Holick, Binkley, Bischoff-Ferrari, Gordon, Hanley et al., Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline, J. Clin. Endocrinol. Metab, doi:10.1210/jc.2011-0385
Huang, Wang, Li, Ren, Zhao et al., Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China, Lancet Lond. Engl, doi:10.1016/S0140-6736(20)30183-5
Ilie, Stefanescu, Smith, The Role of Vitamin D in the Prevention of Coronavirus Disease 2019 Infection and Mortality, Aging Clin. Exp. Res, doi:10.1007/s40520-020-01570-8
Isaia, Giorgino, Rini, Bevilacqua, Maugeri et al., Prevalence of Hypovitaminosis D in Elderly Women in Italy: Clinical Consequences and Risk Factors, Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found, doi:10.1007/s00198-003-1390-7
Jain, Chaurasia, Sengar, Singh, Mahor et al., Analysis of Vitamin D Level among Asymptomatic and Critically Ill COVID-19 Patients and Its Correlation with Inflammatory Markers, Sci. Rep, doi:10.1038/s41598-020-77093-z
Kassi, Stavropoulos, Kokkoris, Galanos, Moutsatsou et al., Smoking Is a Significant Determinant of Low Serum Vitamin D in Young and Middle-Aged Healthy Males, Horm. Athens Greece, doi:10.14310/horm.2002.1521
Kearns, Binongo, Watson, Alvarez, Lodin et al., The Effect of a Single, Large Bolus of Vitamin D in Healthy Adults over the Winter and Following Year: A Randomized, Double-Blind, Placebo-Controlled Trial, Eur. J. Clin. Nutr, doi:10.1038/ejcn.2014.209
Kyriakaki, Fragkoulis, The Vitamin D Paradox: High Prevalence of Deficiency in Sunny Athens (Greece), Ann. Res. Hosp, doi:10.21037/arh.2019.06.02
Laird, Rhodes, Kenny, Vitamin, and Inflammation: Potential Implications for Severity of Covid-19, Ir. Med. J
Léonard-Lorant, Delabranche, Séverac, Helms, Pauzet et al., Acute Pulmonary Embolism in Patients with COVID-19 at CT Angiography and Relationship to d-Dimer Levels, Radiology, doi:10.1148/radiol.2020201561
Maghbooli, Sahraian, Ebrahimi, Pazoki, Kafan et al., Vitamin D Sufficiency, a Serum 25-Hydroxyvitamin D at Least 30 Ng/ML Reduced Risk for Adverse Clinical Outcomes in Patients with COVID-19 Infection, PLoS ONE, doi:10.1371/journal.pone.0239799
Mahase, Covid-19: Vaccine Candidate May Be More than 90% Effective, Interim Results Indicate, BMJ, doi:10.1136/bmj.m4347
Malaguarnera, Vitamin D3 as Potential Treatment Adjuncts for COVID-19, Nutrients, doi:10.3390/nu12113512
Martineau, Forouhi, Vitamin D for COVID-19: A Case to Answer?, Lancet Diabetes Endocrinol
Martineau, Jolliffe, Hooper, Greenberg, Aloia et al., Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data, BMJ, doi:10.1136/bmj.i6583
Meltzer, Best, Zhang, Vokes, Arora et al., Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2020.19722
Mitchell, Vitamin-D and COVID-19: Do Deficient Risk a Poorer Outcome?, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(20)30183-2
Mithal, Wahl, Bonjour, Burckhardt, Dawson-Hughes et al., Global Vitamin D Status and Determinants of Hypovitaminosis D, Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found, doi:10.1007/s00198-009-0954-6
Murai, Effect of Vitamin D3 Supplementation vs. Placebo on Hospital Length of Stay in Patients with Severe COVID-19: A Multicenter, Double-Blind, Randomized Controlled Trial|MedRxiv, doi:10.1101/2020.11.16.20232397v1
Negrea, Active Vitamin D in Chronic Kidney Disease: Getting Right Back Where We Started From?, Kidney Dis
Pereira, Dantas Damascena, Galvão Azevedo, De Almeida Oliveira, Da Mota Santana et al., Deficiency Aggravates COVID-19: Systematic Review and Meta-Analysis, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2020.1841090
Polverino, Cigarette Smoking and COVID-19: A Complex Interaction, Am. J. Respir. Crit. Care Med
Quesada-Gomez, Entrenas-Castillo, Bouillon, Vitamin, Receptor Stimulation to Reduce Acute Respiratory Distress Syndrome (ARDS) in Patients with Coronavirus SARS-CoV-2 Infections, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105719
Ranieri, Rubenfeld, Thompson, Ferguson, Caldwell et al., Acute Respiratory Distress Syndrome: The Berlin Definition, JAMA, doi:10.1001/jama.2012.5669
Rastogi, Bhansali, Khare, Suri, Yaddanapudi et al., Short Term, High-Dose Vitamin D Supplementation for COVID-19 Disease: A Randomised, Placebo-Controlled, Study (SHADE Study), Postgrad. Med. J, doi:10.1136/postgradmedj-2020-139065
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Roffman, Buchanan, Allison, Charlson, Comorbidities Index, J. Physiother, doi:10.1016/j.jphys.2016.05.008
Rossato, Russo, Mazzocut, Vincenzo, Fioretto et al., Current Smoking Is Not Associated with COVID-19, Eur. Respir. J, doi:10.1183/13993003.01290-2020
Sabetta, Depetrillo, Cipriani, Smardin, Burns et al., Serum 25-Hydroxyvitamin d and the Incidence of Acute Viral Respiratory Tract Infections in Healthy Adults, PLoS ONE, doi:10.1371/journal.pone.0011088
Sassi, Tamone, D'amelio, Vitamin, Nutrient, Hormone, and Immunomodulator, Nutrients, doi:10.3390/nu10111656
Scragg, Limitations of Vitamin D Supplementation Trials: Why Observational Studies Will Continue to Help Determine the Role of Vitamin D in Health, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2017.06.006
Shaffer, Drugs Being Tested to Treat COVID-19 and How They Would Work, Nat. Med, doi:10.1038/d41591-020-00019-9
Siddiqui, Manansala, Abdulrahman, Nasrallah, Smatti et al., Immune Modulatory Effects of Vitamin D on Viral Infections, Nutrients, doi:10.3390/nu12092879
Simonnet, Chetboun, Poissy, Raverdy, Noulette et al., High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation, Obesity, doi:10.1002/oby.22831
Van Der Wielen, Löwik, Van Den Berg, De Groot, Haller et al., Serum Vitamin D Concentrations among Elderly People in Europe, Lancet Lond. Engl, doi:10.1016/S0140-6736(95)91266-5
Vandenbroucke, Von Elm, Altman, Gøtzsche, Mulrow et al., STROBE initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration, Ann. Intern. Med, doi:10.7326/0003-4819-147-8-200710160-00010-w1
Vena, Giacobbe, Di Biagio, Mikulska, Taramasso et al., Clinical Characteristics, Management and in-Hospital Mortality of Patients with Coronavirus Disease 2019 in Genoa, Italy, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2020.07.049
Vitamina, Analisi Dell'effetto Della Nota 96 nel Primo Trimestre di Applicazione
Von Elm, Altman, Egger, Pocock, Gøtzsche et al., The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies, Lancet Lond. Engl, doi:10.1016/S0140-6736(07)61602-X
Who, WHO Coronavirus Disease (COVID-19) Dashboard
Who, WHO Director-General's Opening Remarks at the Media Briefing on COVID-19-11
Wu, Zhao, Yu, Chen, Wang et al., A New Coronavirus Associated with Human Respiratory Disease in China, Nature, doi:10.1038/s41586-020-2008-3
Zhang, Yan, Fan, Liu, Liu et al., -Dimer Levels on Admission to Predict in-Hospital Mortality in Patients with Covid-19, J. Thromb. Haemost. JTH, doi:10.1111/jth.14859
DOI record:
{
"DOI": "10.3390/nu13010219",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu13010219",
"abstract": "<jats:p>Little information is available on the beneficial effects of cholecalciferol treatment in comorbid patients hospitalized for COVID-19. The aim of this study was to retrospectively examine the clinical outcome of patients receiving in-hospital high-dose bolus cholecalciferol. Patients with a positive diagnosis of SARS-CoV-2 and overt COVID-19, hospitalized from 15 March to 20 April 2020, were considered. Based on clinical characteristics, they were supplemented (or not) with 400,000 IU bolus oral cholecalciferol (200,000 IU administered in two consecutive days) and the composite outcome (transfer to intensive care unit; ICU and/or death) was recorded. Ninety-one patients (aged 74 ± 13 years) with COVID-19 were included in this retrospective study. Fifty (54.9%) patients presented with two or more comorbid diseases. Based on the decision of the referring physician, 36 (39.6%) patients were treated with vitamin D. Receiver operating characteristic curve analysis revealed a significant predictive power of the four variables: (a) low (<50 nmol/L) 25(OH) vitamin D levels, (b) current cigarette smoking, (c) elevated D-dimer levels (d) and the presence of comorbid diseases, to explain the decision to administer vitamin D (area under the curve = 0.77, 95% CI: 0.67–0.87, p < 0.0001). Over the follow-up period (14 ± 10 days), 27 (29.7%) patients were transferred to the ICU and 22 (24.2%) died (16 prior to ICU and six in ICU). Overall, 43 (47.3%) patients experienced the combined endpoint of transfer to ICU and/or death. Logistic regression analyses revealed that the comorbidity burden significantly modified the effect of vitamin D treatment on the study outcome, both in crude (p = 0.033) and propensity score-adjusted analyses (p = 0.039), so the positive effect of high-dose cholecalciferol on the combined endpoint was significantly amplified with increasing comorbidity burden. This hypothesis-generating study warrants the formal evaluation (i.e., clinical trial) of the potential benefit that cholecalciferol can offer in these comorbid COVID-19 patients.</jats:p>",
"alternative-id": [
"nu13010219"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-0796-9749",
"affiliation": [],
"authenticated-orcid": false,
"family": "Giannini",
"given": "Sandro",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-4039-1160",
"affiliation": [],
"authenticated-orcid": false,
"family": "Passeri",
"given": "Giovanni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tripepi",
"given": "Giovanni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sella",
"given": "Stefania",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9478-4851",
"affiliation": [],
"authenticated-orcid": false,
"family": "Fusaro",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arcidiacono",
"given": "Gaetano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torres",
"given": "Marco Onofrio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Michielin",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prandini",
"given": "Tancredi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baffa",
"given": "Valeria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aghi",
"given": "Andrea",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6942-7147",
"affiliation": [],
"authenticated-orcid": false,
"family": "Egan",
"given": "Colin Gerard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brigo",
"given": "Martina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zaninotto",
"given": "Martina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Plebani",
"given": "Mario",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vettor",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fioretto",
"given": "Paola",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9692-2293",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rossini",
"given": "Maurizio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vignali",
"given": "Alessandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fabris",
"given": "Fabrizio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bertoldo",
"given": "Francesco",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
1,
15
]
],
"date-time": "2021-01-15T06:33:29Z",
"timestamp": 1610692409000
},
"deposited": {
"date-parts": [
[
2021,
1,
15
]
],
"date-time": "2021-01-15T06:48:52Z",
"timestamp": 1610693332000
},
"indexed": {
"date-parts": [
[
2024,
2,
21
]
],
"date-time": "2024-02-21T09:45:17Z",
"timestamp": 1708508717182
},
"is-referenced-by-count": 53,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
1,
14
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2021,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
14
]
],
"date-time": "2021-01-14T00:00:00Z",
"timestamp": 1610582400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/13/1/219/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "219",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
1,
14
]
]
},
"published-online": {
"date-parts": [
[
2021,
1,
14
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1038/s41586-020-2008-3",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1001/jama.2020.5394",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"key": "ref4",
"unstructured": "WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1001/jama.2017.21907",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1001/jama.2012.5669",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"key": "ref9"
},
{
"key": "ref10",
"unstructured": "WHO Coronavirus Disease (COVID-19) Dashboardhttps://covid19.who.int/table"
},
{
"DOI": "10.1136/bmj.m4347",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1038/d41591-020-00019-9",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/j.jsbmb.2020.105719",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1007/s40520-020-01677-y",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.3390/nu10111656",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.3390/nu12113512",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/S2213-8587(13)70165-7",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1371/journal.pmed.1002907",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1136/bmj.i6583",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1371/journal.pone.0011088",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1210/jc.2011-0385",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1007/s00198-009-0954-6",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1007/s00198-003-1390-7",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1016/j.nfs.2020.06.001",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1016/S2213-8587(20)30183-2",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1016/S2213-8587(20)30268-0",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"article-title": "Vitamin D and Inflammation: Potential Implications for Severity of Covid-19",
"author": "Laird",
"first-page": "81",
"journal-title": "Ir. Med. J.",
"key": "ref27",
"volume": "113",
"year": "2020"
},
{
"DOI": "10.1007/s40520-020-01570-8",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1016/S0140-6736(95)91266-5",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.3390/nu12113377",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"key": "ref31",
"unstructured": "Effect of Vitamin D3 Supplementation vs. Placebo on Hospital Length of Stay in Patients with Severe COVID-19: A Multicenter, Double-Blind, Randomized Controlled Trial|MedRxivhttps://www.medrxiv.org/content/10.1101/2020.11.16.20232397v1"
},
{
"DOI": "10.1136/postgradmedj-2020-139065",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.7326/M20-5206",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1001/jama.2014.13204",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"key": "ref36",
"unstructured": "Clinical Management of COVID-19https://www.who.int/publications-detail-redirect/clinical-management-of-covid-19"
},
{
"DOI": "10.3390/nu12092879",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.3390/nu12040988",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1001/jamanetworkopen.2020.19722",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.3390/nu12051359",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1371/journal.pone.0239799",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1016/j.dsx.2020.04.050",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1038/s41598-020-77093-z",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1080/10408398.2020.1841090",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1111/jth.14859",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1164/rccm.202005-1646LE",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1183/13993003.01290-2020",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.14310/horm.2002.1521",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1016/j.thromres.2020.05.018",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1148/radiol.2020201561",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.1016/j.hrtlng.2020.08.024",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.1159/000495138",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.2215/CJN.01150308",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.1002/oby.22831",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.1007/s001980050141",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.21037/arh.2019.06.02",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"key": "ref57"
},
{
"key": "ref58"
},
{
"key": "ref59",
"unstructured": "Get Vitamin D Supplements If You’re at High Risk from Coronavirus (COVID-19)https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/get-vitamin-d-supplements/"
},
{
"DOI": "10.1016/S0140-6736(07)61602-X",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.7326/0003-4819-147-8-200710160-00010-w1",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"DOI": "10.1016/j.jsbmb.2017.06.006",
"doi-asserted-by": "publisher",
"key": "ref62"
},
{
"DOI": "10.1016/j.cmi.2020.07.049",
"doi-asserted-by": "publisher",
"key": "ref63"
},
{
"DOI": "10.1038/ejcn.2014.209",
"doi-asserted-by": "publisher",
"key": "ref64"
},
{
"DOI": "10.1016/j.jphys.2016.05.008",
"doi-asserted-by": "publisher",
"key": "ref65"
}
],
"reference-count": 65,
"references-count": 65,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/13/1/219"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Food Science",
"Nutrition and Dietetics"
],
"subtitle": [],
"title": "Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study",
"type": "journal-article",
"volume": "13"
}
