Impact of Vitamin D Therapy on the Progress COVID-19: Six Weeks Follow-Up Study of Vitamin D Deficient Elderly Diabetes Patients
et al., Proceedings of Singapore Healthcare, doi:10.1177/20101058211041405, Sep 2021
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small RCT with 56 eldery diabetes patients hospitalized in Egypt, 40 treated with cholecalciferol, not showing significant differences.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 44% [33‑53%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
Bolus treatment is less effective.
Pharmacokinetics and the potential side effects of high bolus doses suggest
that ongoing treatment spread over time is more appropriate.
Research has confirmed that lower dose regular treatment with vitamin D is more
effective than intermittent high-dose bolus treatment for various conditions,
including rickets and acute respiratory infections1,2. The biological mechanisms supporting these
findings involve the induction of enzymes such as 24-hydroxylase and
fibroblast growth factor 23 (FGF23) by high-dose bolus treatments. These
enzymes play roles in inactivating vitamin D, which can paradoxically reduce
levels of activated vitamin D and suppress its activation for extended periods
post-dosage. Evidence indicates that 24-hydroxylase activity may remain
elevated for several weeks following a bolus dose, leading to reduced levels
of the activated form of vitamin D. Additionally, FGF23 levels can increase
for at least three months after a large bolus dose, which also contributes to
the suppression of vitamin D activation1.
This is the 6th of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 51st of 136 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
|
risk of death, 63.4% lower, RR 0.37, p = 0.21, treatment 7 of 40 (17.5%), control 3 of 16 (18.8%), adjusted per study, odds ratio converted to relative risk, logistic regression.
|
|
risk of mechanical ventilation, 20.0% lower, RR 0.80, p = 0.56, treatment 14 of 40 (35.0%), control 7 of 16 (43.8%), NNT 11, unadjusted.
|
|
risk of no recovery, 20.0% lower, RR 0.80, p = 0.56, treatment 14 of 40 (35.0%), control 7 of 16 (43.8%), NNT 11, unadjusted.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Soliman et al., 1 Sep 2021, Randomized Controlled Trial, placebo-controlled, Egypt, peer-reviewed, 3 authors, dosage 200,000IU single dose.
Impact of Vitamin D Therapy on the Progress COVID-19: Six Weeks Follow-Up Study of Vitamin D Deficient Elderly Diabetes Patients
Proceedings of Singapore Healthcare, doi:10.1177/20101058211041405
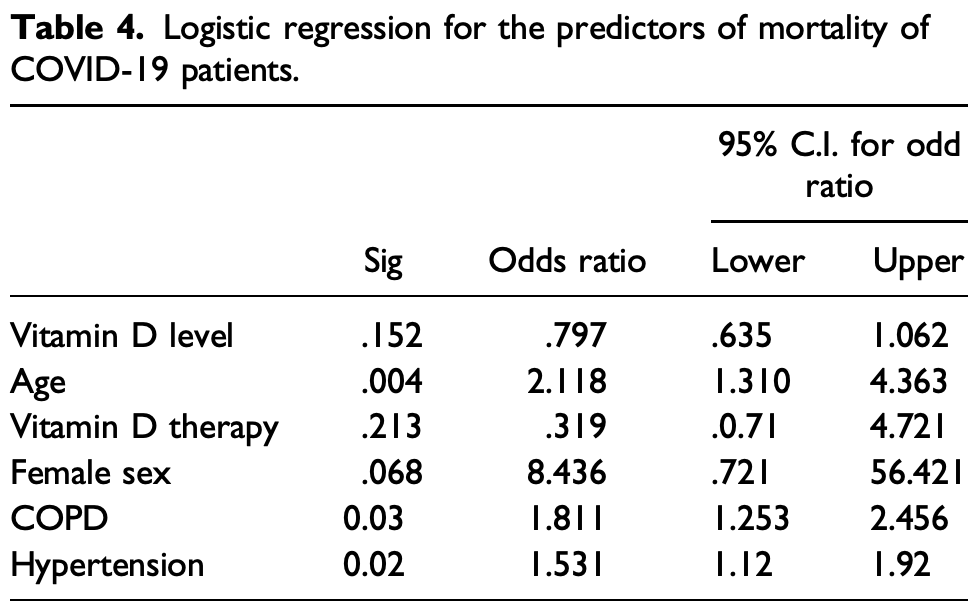
Background: Coronavirus disease-19 (COVID-19) is an ongoing pandemic causing considerable fatalities worldwide. Vitamin D modulates the immune response through effects on various cells, such as: macrophages, B and T lymphocytes, neutrophils, and dendritic cells. Aim: To explore whether supplementation of vitamin D, in the form of a single intramuscular cholecalciferol injection, to patients with diabetes, COVID-19, and low vitamin D levels could improve the prognosis of those patients. Methods: This was a placebo-controlled randomized prospective study. The study has two arms as follows: the intervention arm (40 vitamin D deficient diabetes elderly patients that acquired SARS-CoV-2), compared to the control arm (16 elderly diabetes patients, with deficient vitamin D with SARS-CoV-2). Patients in the intervention arm were given vitamin D as a single intramuscular injection (200,000 IU); patients in the control arm were given placebo. The primary outcome was mortality within 6 weeks of the diagnosis of COVID-19. Clinical, laboratory, treatment, and outcome data were recorded after 6 weeks of follow-up. Results: No significant difference in 6 weeks mortality was observed between patients who received vitamin D and patients who received placebo (17.5% vs 18.8%, p = 0.838). Age, presence of hypertension, and chronic obstructive pulmonary disease were independent predictors of mortality at 6 weeks. Conclusion: Vitamin D supplementation did not reduce the severity or mortality of COVID-19 at 6 weeks. Further large scale studies are required to explore the effect of vitamin D therapy on survival in patients with diabetes mellitus who acquire COVID-19.
Author Contributions ARS contributed to study design, data collection, and final revision of the manuscript
Declaration of Conflicting Interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval The study protocol was approved by the Kasr Alainy Research Ethics Committee (REC), number KA (KA-2020/151). The study is registered to clinicaltrials.gov (NCT04733625).
Informed Consent Yes. According to Declaration of Helsink Trial Registration NCT04733625
ORCID iD Tarek Samy Abdelaziz https://orcid.org/0000-0002-1238-1045
References
Bleakley, Licciardi, Binks, Vitamin D modulation of the innate immune response to paediatric respiratory pathogens associated with acute lower respiratory infections, Nutrients
Bombardini, Picano, Angiotensin-converting enzyme 2 as the molecular bridge between epidemiologic and clinical features of COVID-19, Can J Cardiol
Cantorna, Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease, Progress in Biophysics Mol Biol
D'avolio, Avataneo, Manca, 25-hydroxyvitamin d concentrations are lower in patients with positive PCR for SARS-CoV-2, Nutrients
Grant, Lahore, Mcdonnell, Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths, Nutrients
Han, Jones, Tangpricha, High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial, J Clin Translational Endocrinol
Hastie, Mackay, Ho, Vitamin D concentrations and COVID-19 infection in UK Biobank, Diabetes Metab Syndr Clin Res Rev
Jakovac, Covid-19 and vitamin D-is there a link and an opportunity for intervention?, Am J Physiology-Endocrinology Metab
Ketha, Thacher, Oberhelman, Comparison of the effect of daily versus bolus dose maternal vitamin D3 supplementation on the 24, 25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 ratio, Bone
Kralj, Jakovac, Vitamin D and COVID-19 in an immunocompromised patient with multiple comorbidities-a case report, Clin Case Rep
Laird, Rhodes, Kenny, Vitamin D and inflammation: potential implications for severity of COVID-19, Irish Med J
Liu, Stenger, Li, Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response, Science
Martineau, Jolliffe, Hooper, Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ
Mishra, Singh, Singh, Hyperinflammation and Immune Response Generation in COVID-19, Neuroimmunomodulation
Murai, Fernandes, Sales, Effect of a single high dose of Vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19, JAMA
Pham, Rahman, Majidi, Acute respiratory tract infection and 25-hydroxyvitamin D concentration: a systematic review and meta-analysis, Int J Environ Res Public Health
Phokela, Peleg, Moya, Regulation of human pulmonary surfactant protein gene expression by 1α,25-dihydroxyvitamin D3, Am J Physiology-Lung Cell Mol Physiol
DOI record:
{
"DOI": "10.1177/20101058211041405",
"ISSN": [
"2010-1058",
"2059-2329"
],
"URL": "http://dx.doi.org/10.1177/20101058211041405",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p> Coronavirus disease-19 (COVID-19) is an ongoing pandemic causing considerable fatalities worldwide. Vitamin D modulates the immune response through effects on various cells, such as: macrophages, B and T lymphocytes, neutrophils, and dendritic cells. </jats:p></jats:sec><jats:sec><jats:title>Aim</jats:title><jats:p> To explore whether supplementation of vitamin D, in the form of a single intramuscular cholecalciferol injection, to patients with diabetes, COVID-19, and low vitamin D levels could improve the prognosis of those patients. </jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p> This was a placebo-controlled randomized prospective study. The study has two arms as follows: the intervention arm (40 vitamin D deficient diabetes elderly patients that acquired SARS-CoV-2), compared to the control arm (16 elderly diabetes patients, with deficient vitamin D with SARS-CoV-2). Patients in the intervention arm were given vitamin D as a single intramuscular injection (200,000 IU); patients in the control arm were given placebo. The primary outcome was mortality within 6 weeks of the diagnosis of COVID-19. Clinical, laboratory, treatment, and outcome data were recorded after 6 weeks of follow-up. </jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p> No significant difference in 6 weeks mortality was observed between patients who received vitamin D and patients who received placebo (17.5% vs 18.8%, p = 0.838). Age, presence of hypertension, and chronic obstructive pulmonary disease were independent predictors of mortality at 6 weeks. </jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p> Vitamin D supplementation did not reduce the severity or mortality of COVID-19 at 6 weeks. Further large scale studies are required to explore the effect of vitamin D therapy on survival in patients with diabetes mellitus who acquire COVID-19. </jats:p></jats:sec>",
"alternative-id": [
"10.1177/20101058211041405"
],
"author": [
{
"affiliation": [
{
"name": "Internal Medicine Department, Kasr al Ainy School of Medicine Cairo University, Egypt"
}
],
"family": "Soliman",
"given": "Amin R.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1238-1045",
"affiliation": [
{
"name": "Internal Medicine Department, Kasr al Ainy School of Medicine Cairo University, Egypt"
}
],
"authenticated-orcid": false,
"family": "Abdelaziz",
"given": "Tarek Samy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, Kasr al Ainy School of Medicine Cairo University, Egypt"
}
],
"family": "Fathy",
"given": "Ahmed",
"sequence": "additional"
}
],
"container-title": "Proceedings of Singapore Healthcare",
"container-title-short": "Proceedings of Singapore Healthcare",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2021,
9,
1
]
],
"date-time": "2021-09-01T11:21:23Z",
"timestamp": 1630495283000
},
"deposited": {
"date-parts": [
[
2022,
6,
14
]
],
"date-time": "2022-06-14T17:27:19Z",
"timestamp": 1655227639000
},
"indexed": {
"date-parts": [
[
2024,
2,
12
]
],
"date-time": "2024-02-12T14:44:49Z",
"timestamp": 1707749089061
},
"is-referenced-by-count": 12,
"issued": {
"date-parts": [
[
2021,
9,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
1
]
],
"date-time": "2021-09-01T00:00:00Z",
"timestamp": 1630454400000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/20101058211041405",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/20101058211041405",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/20101058211041405",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "201010582110414",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2021,
9,
1
]
]
},
"published-online": {
"date-parts": [
[
2021,
9,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
6
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1159/000513198",
"doi-asserted-by": "publisher",
"key": "bibr1-20101058211041405"
},
{
"DOI": "10.1016/j.pbiomolbio.2006.02.020",
"doi-asserted-by": "publisher",
"key": "bibr2-20101058211041405"
},
{
"DOI": "10.1152/ajplung.00129.2004",
"doi-asserted-by": "publisher",
"key": "bibr3-20101058211041405"
},
{
"DOI": "10.1016/j.cjca.2020.03.026",
"doi-asserted-by": "publisher",
"key": "bibr4-20101058211041405"
},
{
"DOI": "10.1152/ajpendo.00138.2020",
"doi-asserted-by": "publisher",
"key": "bibr5-20101058211041405"
},
{
"DOI": "10.1002/ccr3.4010",
"doi-asserted-by": "publisher",
"key": "bibr6-20101058211041405"
},
{
"DOI": "10.3390/nu12040988",
"doi-asserted-by": "publisher",
"key": "bibr7-20101058211041405"
},
{
"DOI": "10.1126/science.1123933",
"doi-asserted-by": "publisher",
"key": "bibr8-20101058211041405"
},
{
"DOI": "10.3390/nu13010276",
"doi-asserted-by": "publisher",
"key": "bibr9-20101058211041405"
},
{
"DOI": "10.1016/j.jcte.2016.04.004",
"doi-asserted-by": "publisher",
"key": "bibr10-20101058211041405"
},
{
"DOI": "10.3390/ijerph16173020",
"doi-asserted-by": "publisher",
"key": "bibr11-20101058211041405"
},
{
"DOI": "10.1136/bmj.i6583",
"doi-asserted-by": "publisher",
"key": "bibr12-20101058211041405"
},
{
"DOI": "10.1016/j.bone.2018.02.024",
"doi-asserted-by": "publisher",
"key": "bibr13-20101058211041405"
},
{
"DOI": "10.1016/j.dsx.2020.04.050",
"doi-asserted-by": "publisher",
"key": "bibr14-20101058211041405"
},
{
"author": "Laird E",
"first-page": "81",
"journal-title": "Irish Med J",
"key": "bibr15-20101058211041405",
"volume": "113",
"year": "2020"
},
{
"DOI": "10.3390/nu12051359",
"doi-asserted-by": "publisher",
"key": "bibr16-20101058211041405"
},
{
"DOI": "10.1001/jama.2020.26848",
"doi-asserted-by": "publisher",
"key": "bibr17-20101058211041405"
},
{
"author": "National Institute for Health and Care Excellence",
"key": "bibr18-20101058211041405",
"volume-title": "Evidence reviews for the use of vitamin D supplementation as prevention and treatment of COVID-19",
"year": "2020"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/20101058211041405"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Impact of Vitamin D Therapy on the Progress COVID-19: Six Weeks Follow-Up Study of Vitamin D Deficient Elderly Diabetes Patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "31"
}
