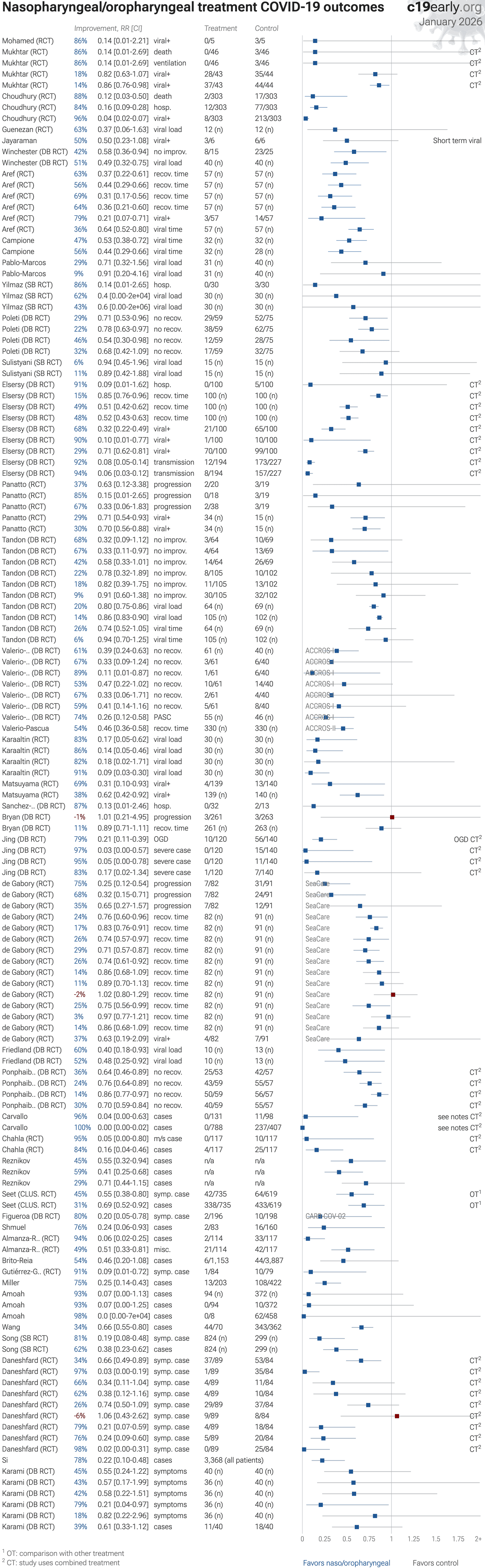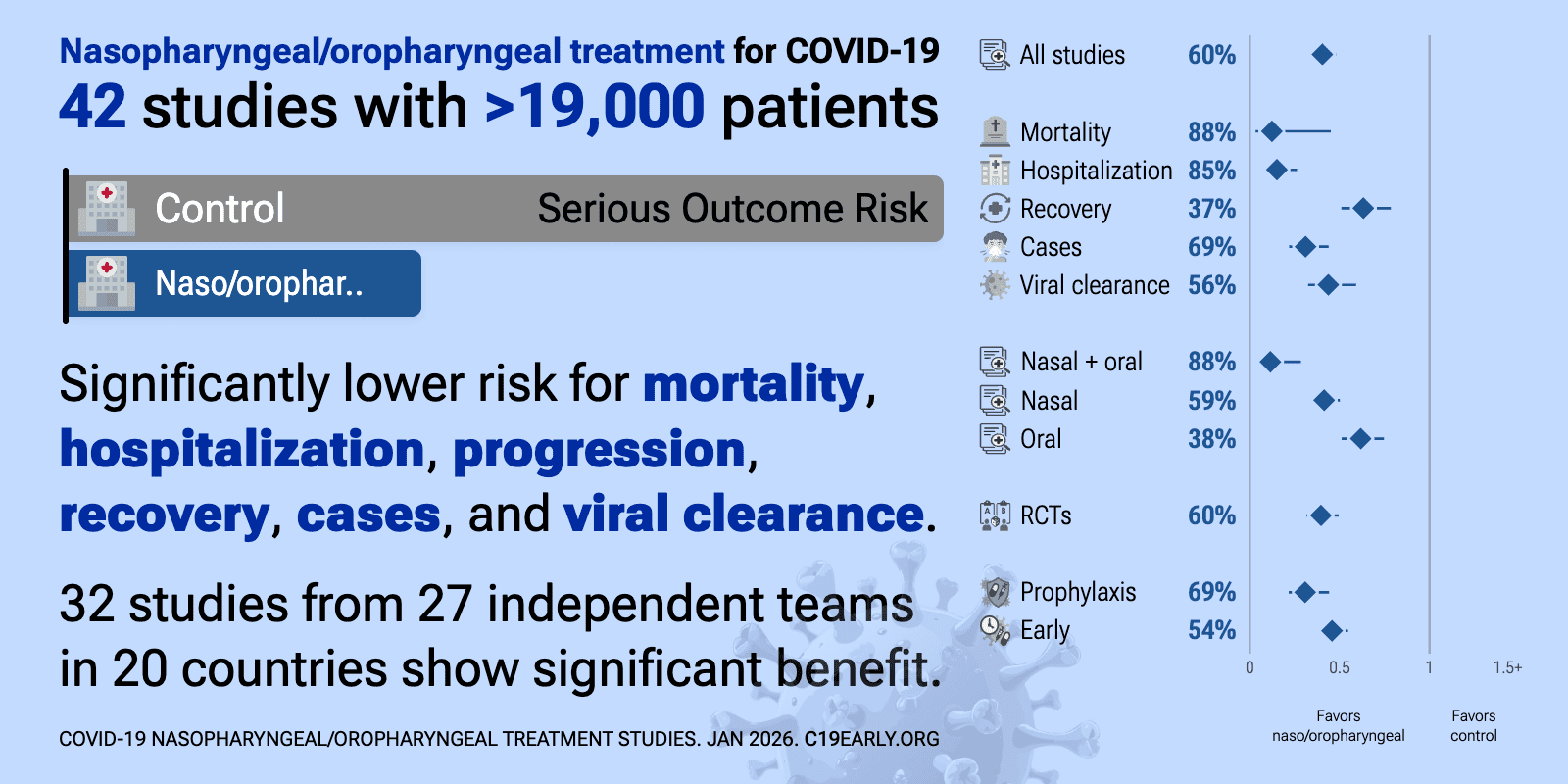
This analysis covers prophylaxis and early treatment with nasal/oral sprays and rinses, covering multiple different treatments. The efficacy of individual treatments varies. For specific treatments, late treatment studies, and alternative administration methods see the individual analyses.
Sep 19 2025 |
et al., MDPI AG, doi:10.20944/preprints202509.1594.v1 | Efficacy of Nasal Spray, Mouth Spray, and Mouthwash Containing Limonene, Cetylpyridinium Chloride, and Monolaurin in COVID-19 Management: A Double-Blind, Randomized, Placebo-Controlled Trial |
| 36% improved recovery (p=0.006). RCT 120 low-risk COVID-19 patients showing improved recovery with nasal and oral formulations containing cetylpyridinium chloride, D-limonene, and monolaurin (the nasal formulation contained D-limonene and cetylpyridinium chloride, while .. | ||
Nov 26 2024 |
et al., BMC Infectious Diseases, doi:10.1186/s12879-024-10211-8 | Mitigating the risks of post-acute sequelae of SARS-CoV-2 infection (PASC) with intranasal chlorpheniramine: perspectives from the ACCROS studies |
| 90% lower long COVID (p=0.001). Prospective study of 259 COVID-19 outpatients from the ACROSS-I and ACROSS-III RCTs showing significantly lower long COVID with intranasal chlorpheniramine (iCPM) compared to placebo. 72% of placebo patients experienced at least one PASC .. | ||
Mar 30 2024 |
et al., The Laryngoscope, doi:10.1002/lary.31430 | Phase II Trial of the Impact 0.5% Povidone‐Iodine Nasal Spray (Nasodine®) on Shedding of SARS‐CoV‐2 |
| 60% improved viral clearance (p=0.03) and 6% improved recovery. RCT 23 early COVID-19 outpatients showing significantly improved reduction in viral load and significantly faster viral clearance with povidone-iodine nasal spray compared to placebo. The study was underpowered due to low recruitment, enr.. | ||
Feb 20 2024 |
et al., European Archives of Oto-Rhino-Laryngology, doi:10.1007/s00405-024-08518-y | Seawater nasal wash to reduce symptom duration and viral load in COVID-19 and upper respiratory tract infections: a randomized controlled multicenter trial |
| 75% lower progression (p<0.0001), 24% faster recovery (p=0.02), and 37% improved viral clearance (p=0.54). RCT 355 adults with COVID-19 or other upper respiratory tract infections (URTIs). For COVID-19 patients there was lower progression and faster symptom resolution with alkaline seawater nasal wash (pH ~8) 4 times daily for 21 days. There w.. | ||
Jan 9 2024 |
et al., Iranian Journal of Nursing and Midwifery Research, doi:10.4103/ijnmr.ijnmr_38_23 | A Comparison of the Effects of Chlorhexidine and Sodium Bicarbonate Mouthwashes on COVID-19–Related Symptoms |
| 61% lower progression (p=0.04) and 57% fewer cases (p=0.03). RCT 116 healthcare workers comparing 0.2% chlorhexidine mouthwash (n=36), 7.5% sodium bicarbonate mouthwash (n=40), and placebo (n=40) twice daily for 2 weeks, with symptoms followed for 4 weeks. There were lower symtoms and cases in both.. | ||
Dec 31 2023 |
et al., China CDC Weekly, doi:10.46234/ccdcw2023.040 | Safety and Effectiveness of SA58 Nasal Spray Against COVID-19 Infection in Medical Personnel: An Open-Label, Blank-Controlled Study — Hohhot City, Inner Mongolia Autonomous Region, China, 2022 |
| 78% fewer cases (p=0.0001). Prospective study of 3,368 medical personnel in China showing significantly lower COVID-19 cases with SA58 nasal spray use. | ||
Dec 18 2023 |
et al., PeerJ, doi:10.7717/peerj.15080 | Reduction of SARS-CoV-2 viral load in saliva after rinsing with mouthwashes containing cetylpyridinium chloride: a randomized clinical study |
| RCT 95 hospitalized COVID-19 patients showing reduced salivary SARS-CoV-2 viral load after rinsing with cetylpyridinium chloride (CPC) or CPC plus zinc mouthwashes. CPC plus zinc mouthwash reduced viral load by 6.3-fold at 5 minutes, 3.6-.. | ||
Nov 21 2023 |
et al., QJM: An International Journal of Medicine, doi:10.1093/qjmed/hcad262 | Effective early strategy to prevent olfactory and gustatory dysfunction in COVID-19: a randomized controlled trial |
| 71% lower progression (p<0.0001) and 97% lower severe cases (p<0.0001). RCT 379 mild COVID-19 cases showing significantly lower prevalence and severity of olfactory and gustatory dysfunction with budesonide nasal spray, chlorhexidine mouthwash, and saline nasal irrigation. The control group received no interv.. | ||
Aug 31 2023 |
et al., In Vivo, doi:10.21873/invivo.13313 | A Prospective Study of AFree Oral Spray as an Adjuvant Therapy for Mild and Moderate COVID-19 in Community Health Stations: Clinical Progression and Viral Clearance Outcomes |
| RCT 1,252 mild/moderate COVID-19 patients Vietnam, showing faster recovery and faster viral clearance with an oral spray containing zinc, propolis, xylitol, ginger, and DMSO. 5-10 times per day. | ||
Aug 4 2023 |
et al., Scientific Reports, doi:10.1038/s41598-023-39308-x | Efficacy of three antimicrobial mouthwashes in reducing SARS-CoV-2 viral load in the saliva of hospitalized patients: a randomized controlled pilot study |
| RCT 40 late stage (mean 8 days from onset) patients, showing no significant difference in short-term viral load measured by PCR with cetylpyridinium chloride mouthwash. | ||
Jul 16 2023 |
et al., Phytotherapy Research, doi:10.1002/ptr.7915 | Effect of Sinamaz nasal drop on asymptomatic family members of COVID 19 patients: An open-label randomized controlled trial |
| 34% fewer symptomatic cases (p=0.006). RCT 173 family members of COVID-19 patients, showing lower incidence of COVID-19 symptoms with nasal drops containing nigella sativa oil and olea europaea oil. One drop in each nostril twice daily for 7 days. | ||
Jul 3 2023 |
et al., Journal of Personalized Medicine, doi:10.3390/jpm13071093 | A Hypertonic Seawater Nasal Irrigation Solution Containing Algal and Herbal Natural Ingredients Reduces Viral Load and SARS-CoV-2 Detection Time in the Nasal Cavity |
| 42% improved viral clearance (p=0.06). RCT 56 severe COVID-19 patients, showing significantly decreased viral load with Sinomarin Plus Algae nasal irrigation. Sinomarin Plus Algae is a hypertonic seawater solution with algal and herbal natural ingredients with a pH of 7.5-8.. | ||
Jun 27 2023 |
et al., In Vivo, doi:10.21873/invivo.13262 | Therapeutic Efficacy of AFree Oral Spray on the Symptoms and Course of Moderate and Severe COVID-19 in the Field Hospital |
| 84% improved recovery (p<0.0001) and 78% improved viral clearance (p=0.06). RCT 200 hospitalized patients in Vietnam, showing faster recovery with an oral spray containing zinc, propolis, xylitol, ginger, and DMSO. | ||
Jun 24 2023 |
et al., The American Journal of Medicine, doi:10.1016/j.amjmed.2023.05.021 | The Efficacy of Nitric Oxide Generating Lozenges on Outcome in Newly Diagnosed COVID-19 Patients of African American and Hispanic Origin |
| 1% higher progression (p=1) and 11% faster recovery (p=0.3). RCT 524 outpatients in the USA for a nitric oxide generating lozenge, showing no significant difference in combined hospitalization, ICU admission, intubation, dialysis, and death. There were only 3 events in each arm, all occuring in 202.. | ||
May 25 2023 |
et al., Emerging Microbes & Infections, doi:10.1080/22221751.2023.2212806 | Post-exposure prophylaxis with SA58 (anti-SARS-COV-2 monoclonal antibody) nasal spray for the prevention of symptomatic COVID-19 in healthy adult workers: a randomized, single-blind, placebo-controlled clinical study |
| 81% fewer symptomatic cases (p=0.0004) and 62% fewer cases (p=0.0001). RCT 1,222 healthy adult workers in China showing SA58 (anti-SARS-CoV-2 monoclonal antibody) nasal spray reduced symptomatic COVID-19 by 81% and SARS-COV-2 infection by 62% compared to placebo when used as post-exposure prophylaxis within .. | ||
May 22 2023 |
et al., Revista Estomatologia, doi:10.25100/re.v31i1.12669 | A Mouthwash with Cetylpyridinium Chloride is reducing salivary SARS-CoV-2 viral load in +COVID-19 |
| 83% improved viral clearance (p=0.06). RCT 23 patients in Colombia, showing improved viral clearance with cetylpyridinium chloride plus chlorhexidine mouthwash. | ||
Mar 20 2023 |
et al., medRxiv, doi:10.1101/2023.03.19.23287462 | Safety and Effectiveness of SA58 Nasal Spray against SARS-CoV-2 family transmission: an exploratory single-arm trial |
| 34% fewer cases (p<0.0001). Exploratory single-arm trial of 70 family contacts showing a protective effect of SA58 nasal spray against household SARS-CoV-2 transmission. The incidence of infection was 62.9% in the experimental group versus 94.8% in a contemporaneous.. | ||
Mar 15 2023 |
et al., Frontiers in Public Health, doi:10.3389/fpubh.2023.1145669 | Efficacy of nasal irrigation and oral rinse with sodium bicarbonate solution on virus clearance for COVID-19 patients |
| 39% shorter hospitalization (p=0.0009). RCT 55 mild/moderate patients in China, showing shorter hospitalization with sodium bicarbonate nasal irrigation and oral rinsing. Oral rinse with 5% sodium bicarbonate solution three times daily. Nasal irrigation two times with the solut.. | ||
Dec 31 2022 |
et al., Medical Research Archives, doi:10.18103/mra.v10i3.2752 | Intranasal Chlorpheniramine Maleate for the treatment of COVID-19: Translational and Clinical Evidence |
| 87% lower hospitalization (p=0.08). Small RCT showing significantly improved recovery with intranasal chlorpheniramine maleate. Authors also perform an in vitro study showing efficacy with a highly differentiated three-dimensional model of normal, human-derived tracheal/bro.. | ||
Nov 28 2022 |
et al., Scientific Reports, doi:10.1038/s41598-022-24683-8 | A prospective, randomized, open-label trial of early versus late povidone-iodine gargling in patients with COVID-19 |
| 69% improved viral clearance (p=0.03). RCT 430 COVID+ patients in Japan, showing significantly lower viral infectivity from culture, and significantly faster PCR viral clearance with PVP-I. For days 2-4 the study compares treatment with PVP-I vs. water (on day 5 both groups re.. | ||
Nov 18 2022 |
et al., Pharmaceutics, doi:10.3390/pharmaceutics14112502 | Early Negativization of SARS-CoV-2 Infection by Nasal Spray of Seawater plus Additives: The RENAISSANCE Open-Label Controlled Clinical Trial |
| 32% improved viral clearance (p=0.05). 108 patient prospective study showing improved viral clearance with Panthexyl nasal spray (a sterile hypertonic solution containing seawater, xylitol, panthenol and lactic acid). | ||
Nov 18 2022 |
et al., Current Therapeutic Research, doi:10.1016/j.curtheres.2025.100801 (date from preprint) | Efficacy and safety of nebulized Sodium Bicarbonate in adults with COVID-19 (SODIC): a randomized, single center, double-blinded, controlled trial |
| 23% lower mortality (p=0.26) and 28% faster recovery (p<0.0001). RCT 546 patients showing significantly faster recovery and lower mortality with sodium bicarbonate (inhaled and nasal drops). The reduction in mortality is only statistically significant when excluding baseline critical cases. Authors hyp.. | ||
Oct 31 2022 |
et al., Journal of Family Medicine and Primary Care, doi:10.4103/jfmpc.jfmpc_446_22 | Effect of 0.5% povidone-iodine on the nasopharyngeal and oropharyngeal viral loads in patients with COVID-19: A double-blind placebo-controlled randomized clinical trial |
| 100% improved viral load (p=0.5). RCT 32 patients in India, showing greater reduction in viral load with PVP-I treatment, without statistical significance. | ||
Oct 26 2022 |
et al., Authorea, doi:10.22541/au.166675335.56566797/v1 | Effect of the povidone iodine, hypertonic alkaline solution and saline nasal lavage on nasopharyngeal viral load in COVID-19 |
| 83% improved viral load (p=0.007). RCT 120 outpatients in Turkey, showing improved reduction in viral load with PVP-I nasal irrigation. PVP-I prepared with hypertonic alkaline solution had better results. [Kreutzberger] show that SARS-CoV-2 requires acidic pH to infect cel.. | ||
Oct 18 2022 |
et al., Research Square, doi:10.21203/rs.3.rs-2167465/v1 | Chlorpheniramine Intranasal Spray to Accelerate COVID-19 Clinical Recovery in an Outpatient Setting: The ACCROS Trials |
| 54% faster recovery (p<0.0001). RCT and retrospective study of chlorpheniramine nasal spray for COVID-19. The retrospective study included 660 outpatients showing fewer days with general COVID-19 symptoms, cough, anosmia, and ageusia compared to standard of care alone. .. | ||
Sep 19 2022 |
et al., Infection and Drug Resistance, doi:10.2147/IDR.S381715 | Possible Role of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Recovery of Post-COVID-19 Anosmia |
| 74% faster recovery (p=0.0005). 96 patient RCT showing faster resolution of post-COVID anosmia with an ivermectin nanosuspension nasal spray. | ||
Aug 31 2022 |
et al., Journal of Hospital Infection, doi:10.1016/j.jhin.2022.05.007 | Further observations on hydrogen peroxide antisepsis and COVID-19 cases among healthcare workers and inpatients |
| 93% fewer cases (p=0.06). Retrospective 458 healthcare workers in Ghana, showing lower COVID-19 cases with hydrogen peroxide prophylaxis (oral and nasal rinse), without statistical significance. | ||
Aug 25 2022 |
et al., Ear, Nose & Throat Journal, doi:10.1177/01455613221123737 | Rapid initiation of nasal saline irrigation to reduce severity in high-risk COVID+ outpatients |
| Small RCT 79 PCR+ patients 55+ comparing pressure-based nasal irrigation with povidone-iodine and sodium bicarbonate, showing significantly lower hospitalization when compared with CDC data. | ||
Aug 22 2022 |
et al., medRxiv, doi:10.1101/2022.08.18.22278340 | A pilot study of 0.4% povidone-iodine nasal spray to eradicate SARS-CoV-2 in the nasopharynx |
| 33% improved viral load (p=0.58). Small single-arm trial testing short-term viral load change after a single administration of three puffs of 0.4% PVP-I, showing lower viral titer at 3 minutes and 4 hours, not reaching statistical significance. Authors note that one reaso.. | ||
Jul 29 2022 |
et al., Medicine, doi:10.1097/MD.0000000000028925 | The short-term effect of different chlorhexidine forms versus povidone iodine mouth rinse in minimizing the oral SARS-CoV-2 viral load: An open label randomized controlled clinical trial study |
| 60 patient RCT comparing chlorhexidine, PVP-I, and saline in Saudi Arabia with a single mouth rinse treatment and PCR testing 5 minutes later, showing statistically significant improvement in Ct value for PVP-I. PVP-I showed greater impro.. | ||
Jul 29 2022 |
et al., Dental and Medical Problems, doi:10.17219/dmp/150831 | Effect of oral antiseptics on the viral load of SARS-CoV-2: A randomized controlled trial |
| RCT with 21 PVP-I, 20 HOCl, and 20 saline patients gargling for 30 seconds and testing PCR Ct after 30 minutes, showing greater improvement with PVP-I and HOCl, without statistical significance. Ct values differ across testing platforms, .. | ||
Jul 28 2022 |
et al., American Journal of Otolaryngology, doi:10.1016/j.amjoto.2022.103549 | Efficacy of antiseptic mouthrinses against SARS-CoV-2: A prospective randomized placebo-controlled pilot study |
| Mouthrinse RCT in Italy comparing short-term viral load after a single 60 second treatment with povidone-iodine, hydrogen peroxide, chlorhexidine, and saline. The greatest efficacy was seen with povidone-iodine, especially for patients wi.. | ||
Jul 27 2022 |
et al., Emerging Microbes & Infections, doi:10.1080/22221751.2022.2098059 | Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: evidence from a randomized double-blind clinical trial |
| 73% improved viral load. RCT hospitalized patients testing viral load shortly after a single mouthwash with PVP-I, hydrogen peroxide, cetylpyridinium chloride, chlorhexidine, and water. For CPC, there was significantly lower culture-based viral load one hour late.. | ||
Jun 29 2022 |
et al., The Lancet Regional Health - Southeast Asia, doi:10.1016/j.lansea.2022.100036 | SARS-CoV-2 accelerated clearance using a novel nitric oxide nasal spray (NONS) treatment: A randomized trial |
| 68% greater improvement (p=0.08) and 20% improved viral clearance (p<0.0001). RCT with 153 patients treated with a nitric oxide nasal spray, and 153 placebo patients, showing faster viral clearance with treatment. NO generated by a nasal spray (NONS) self-administered six times daily as two sprays per nostril (0.45.. | ||
Jun 12 2022 |
et al., Indian Journal of Respiratory Care, doi:10.4103/ijrc.ijrc_48_21 | Role of Sodium Bicarbonate as Adjuvant Treatment of Nonsevere Computed Tomography-identified COVID-19 Pneumonia: A Preliminary Report |
| 57% lower mortality (p=0.37), 39% lower progression (p=0.52), and 19% improved recovery (p=0.03). Prospective study of 182 COVID-19 pneumonia patients, 127 treated with sodium bicarbonate inhalation and nasal drops, showing significantly faster recovery and improved CT scores with treatment. Authors note that contacts of index cases a.. | ||
May 19 2022 |
et al., Journal of Cellular and Molecular Medicine, doi:10.1111/jcmm.17337 | Effect of ArtemiC in patients with COVID-19: A Phase II prospective study |
| 77% improved recovery (p=0.04), 92% lower need for oxygen therapy (p=0.01), 13% shorter hospitalization (p=0.92), and 10% improved viral clearance (p=0.77). RCT 50 hospitalized patients in Israel, 33 treated with curcumin, vitamin C, artemisinin, and frankincense oral spray, showing improved recovery with treatment. | ||
May 12 2022 |
et al., Viruses, doi:10.3390/v14051033 | Efficacy of the Sentinox Spray in Reducing Viral Load in Mild COVID-19 and Its Virucidal Activity against Other Respiratory Viruses: Results of a Randomized Controlled Trial and an In Vitro Study |
| 29% improved viral clearance (p=0.01). RCT 57 mild COVID-19 patients showing non-significant viral load reduction with Sentinox (STX), a hypochlorous acid nasal spray. The proportion of COVID negative patients by day 5 was significantly higher in the STX-3 group than controls... | ||
Apr 30 2022 |
et al., Respiratory Therapy, 18:2, 2023 (date from earlier release of results) | Epidemiological Analysis of Nitric Oxide Nasal Spray (VirX™) Use in Students Exposed to COVID-19 Infected Individuals |
| 75% fewer cases (p<0.0001). Retrospective 625 high-risk university students in Thailand showing reduced SARS-CoV-2 infection rates with nitric oxide nasal spray prophylaxis. Among students exposed to infected individuals, those voluntarily using treatment (n=203) ha.. | ||
Apr 19 2022 |
et al., Frontiers in Medicine, doi:10.3389/fmed.2022.863917 | Combined Nasal, Oropharyngeal Povidone Iodine Plus Glycyrrhizic Acid Sprays, Accelerate Clinical and Laboratory Recovery and Reduces Household Transmission of SARS-CoV-2: A Randomized Placebo-Controlled Clinical Trial |
| 91% lower hospitalization (p=0.06), 15% faster recovery (p=0.008), 68% improved viral clearance (p<0.0001), and 92% lower transmission (p<0.0001). RCT with 200 patients and 421 contacts, with 100 patients and their contacts treated with nasal and oropharyngeal sprays containing povidone-iodine and glycyrrhizic acid, showing significantly faster viral clearance and recovery, and sign.. | ||
Mar 15 2022 |
et al., F1000Research, doi:10.12688/f1000research.110843.1 (date from preprint) | The effects of mouth rinsing and gargling with mouthwash containing povidone-iodine and hydrogen peroxide on the cycle threshold value of Severe Acute Respiratory Syndrome Coronavirus 2: A randomized controlled trial of asymptomatic and mildly symptomatic patients |
| 6% improved viral clearance (p=0.74). Small mouth rinsing and gargling RCT with 15 1% PVP-I, 12 0.5% PVP-I, 15 3% hydrogen peroxide, 12 1.5% hydrogen peroxide, and 15 water patients, showing rapid improvement in Ct value in all groups, and no significant differences between g.. | ||
Feb 17 2022 |
et al., Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2022.112729 | Efficacy of a multiple-indication antiviral herbal drug (Saliravira®) for COVID-19 outpatients: A pre-clinical and randomized clinical trial study |
| 98% lower hospitalization (p<0.0001), 35% faster recovery (p<0.0001), and 63% improved viral clearance. RCT COVID-19 outpatients showing faster recovery and lower hospitalization with Saliravira, an antiviral drug combining oral tablets, a nasal spray, an oral spray, and inhalation, and containing glycyrrhiza glabra, echinacea purpurea, rhe.. | ||
Feb 9 2022 |
et al., Australian Journal of Otolaryngology, doi:10.21037/ajo-21-40 | In vivo (human) and in vitro inactivation of SARS-CoV-2 with 0.5% povidone-iodine nasal spray |
| Small study of povidone-iodine nasal spray with 14 patients, showing rapid reduction in viral load for the 6 patients that had culturable virus at baseline. All patients remained PCR+ despite no culturable virus detected for 3 of 6 patien.. | ||
Dec 22 2021 |
et al., Scientific Reports, doi:10.1038/s41598-021-03461-y | Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2 |
| 32% improved viral load (p=0.7). Small very late (>50% 7+ days from symptom onset, 9 PVP-I patients) RCT testing mouthwashing with cetylpyridinium chloride, chlorhexidine, povidone-iodine, hydrogen peroxide, and distilled water, showing no significant differences. Over 3.. | ||
Dec 15 2021 |
et al., Biomedical Reports, doi:10.3892/br.2021.1494 | Nasopharyngeal and oropharyngeal rinses with neutral electrolyzed water prevents COVID‑19 in front‑line health professionals: A randomized, open‑label, controlled trial in a general hospital in Mexico City |
| 91% fewer symptomatic cases (p=0.004). RCT 170 front-line healthcare workers in Mexico showing significantly lower COVID-19 cases with neutral electrolyzed water (SES) nasal and oral rinses. Authors hypothesize that SES inactivates viral particles through its oxidizing potenti.. | ||
Dec 13 2021 |
et al., Cureus, doi:10.7759/cureus.20394 | Determinants of Outcome Among Critically Ill Police Personnel With COVID-19: A Retrospective Observational Study From Andhra Pradesh, India |
| 57% lower mortality (p=0.0004). Retrospective 266 COVID-19 ICU patients in India, showing significantly lower mortality with PVP-I oral gargling and topical nasal use, and non-statistically significant higher mortality with ivermectin and lower mortality with remdesivir.. | ||
Dec 8 2021 |
et al., Journal of Evidence-Based Dental Practice, doi:10.1016/j.jebdp.2022.101777 (date from preprint) | Use of mouthwash and dentifrice containing antimicrobial phthalocyanine derivative for the reduction of clinical symptoms of COVID-19: A randomized triple-blinded clinical trial |
| 29% improved recovery (p=0.02). RCT 500 patients in Brazil, showing improved recovery with a phthalocyanine derivative mouthwash and toothpaste. Toothbrushing for 2 minutes, three times per day, and gargling/rising (5ml) for one minute, three times a day, for 7 days. | ||
Nov 19 2021 |
et al., Laryngoscope Investigative Otolaryngology, doi:10.1002/lio2.686 | Effects of hypertonic alkaline nasal irrigation on COVID‐19 |
| 86% lower hospitalization (p=0.24) and 62% improved viral clearance (p=0.87). RCT 60 outpatients with mild COVID-19 showing improved viral clearance with hypertonic alkaline (pH 9.3) nasal irrigation. All patients received HCQ. The nasal irrigation group had no hospitalizations, while 3 patients in the control grou.. | ||
Nov 15 2021 |
et al., German Medical Science GMS Publishing House, doi:10.3205/dgkh000426 (date from preprint) | Population-based virucidal phthalocyanine gargling/rinsing protocol to reduce the risk of coronavirus disease-2019: a community trial |
| 54% fewer cases (p=0.08). Comparison of two similar communities in Brazil, with one using a phthalocyanine derivative mouthwash, suggesting efficacy of the treatment in lowering COVID-19 cases. There was 54% lower risk of confirmed cases during the intervention in.. | ||
Nov 1 2021 |
et al., Laryngoscope, doi:10.1002/lary.29935 | The Effect of Povidone-Iodine Nasal Spray on COVID-19 Nasopharyngeal Viral Load in Patients: A Randomized Control Trial |
| 27% worse recovery (p=1) and no change in viral clearance (p=1). Very late treatment (7 days from onset) RCT comparing 11 & 13 PVP-I (0.5% and 2%), and 11 saline spray patients in the USA, showing no significant differences. There was no control group (saline is likely not a placebo, showing efficacy i.. | ||
Oct 25 2021 |
et al., Enfermedades Infecciosas y Microbiología Clínica, doi:10.1016/j.eimc.2021.10.005 | Utility of mouth rinses with povidone-iodine and hydrogen peroxide in patients with COVID-19 |
| 12% improved viral clearance (p=0.67). Small prospective study with 31 patients gargling povidone-iodine, 17 hydrogen peroxide, and 40 control patients, showing lower viral load mid-recovery with povidone-iodine, without reaching statistical significance. Oropharyngeal only, a.. | ||
Oct 19 2021 |
et al., International Journal of Environmental Research and Public Health, doi:10.3390/ijerph182010985 | Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence |
| 47% faster viral clearance (p=0.0001). Small prospective study in Italy with 32 lactoferrin patients, 32 SOC, and 28 patients with no treatment, showing significantly faster viral clearance and improved recovery with treatment in unadjusted results. Oral and intranasal lactofe.. | ||
Oct 7 2021 |
et al., Scientific Reports, doi:10.1038/s41598-021-99013-5 | Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: randomised trial |
| 85% lower mortality (p=0.23), 92% lower ICU admission (p=0.02), and 54% lower hospitalization (p=0.03). RCT 41 patients in Brazil, 20 treated with a phthalocyanine derivative mouthwash, showing shorter hosptalization and lower ICU admission with treatment. One minute gargling/rinsing 5 times per day. | ||
Aug 23 2021 |
et al., The Journal of Allergy and Clinical Immunology: In Practice, doi:10.1016/j.jaip.2021.08.007 | Intranasal Corticosteroids Are Associated with Better Outcomes in Coronavirus Disease 2019 |
| 24% lower mortality (p=0.01), 22% lower ICU admission (p=0.003), and 19% lower hospitalization (p<0.0001). Retrospective 72,147 COVID-19+ patients in the USA, showing lower mortality, ICU admission, and hospitalization with intranasal corticosteroid use. | ||
Aug 19 2021 |
et al., PLOS ONE, doi:10.1371/journal.pone.0256401 | Evaluation of silver nanoparticles for the prevention of SARS-CoV-2 infection in health workers: In vitro and in vivo |
| 94% fewer cases (p<0.0001) and 49% improvement (p=0.003). RCT 231 healthcare workers showing significantly lower COVID-19 infection rates with silver nanoparticle (AgNPs) oral and nasal rinses. Authors also report in vitro experiments showing dose-dependent inhibition in cell cultures. | ||
Aug 3 2021 |
et al., Epidemiology and Health, doi:10.4178/epih.e2021051 | Hydrogen peroxide as an auxiliary treatment for COVID-19 in Brazil: a randomized double-blind clinical trial |
| 34% lower ICU admission (p=1), 1% worse recovery (p=0.97), and 31% lower long COVID (p=0.54). RCT very late treatment (>9 days from onset) comparing hydrogen peroxide + mint essence with water + mint essence, showing no significant differences. | ||
Jul 7 2021 |
, M., Indian Journal of Critical Care Medicine, doi:10.5005/jp-journals-10071-23901 | Gargling with 7.5% Sodium Bicarbonate Solution for SARS-CoV-2 Viremia Clearance: Our Institutional Clinical Experience |
| Report on 10 patients treated with sodium bicarbonate gargling, suggesting no significant improvements. There was no control group and gargling only, without inhalation or nasal spray/rinse. | ||
Jun 15 2021 |
et al., International Journal of Nanomedicine, doi:10.2147/IJN.S313093 | Clinical, Biochemical and Molecular Evaluations of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Reducing Upper Respiratory Symptoms of Mild COVID-19 |
| 63% improved recovery (p=0.0001) and 79% improved viral clearance (p=0.004). RCT 114 patients, 57 treated with ivermectin mucoadhesive nanosuspension intranasal spray, showing faster recovery and viral clearance with treatment. | ||
May 18 2021 |
et al., Indian Journal of Otolaryngology and Head & Neck Surgery, doi:10.1007/s12070-021-02616-7 | Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: an open-label randomized clinical trial |
| 79% improved viral clearance (p=0.02). RCT with 189 patients showing significantly greater viral clearance with a single application of PVP-I. Authors recommend using PVP-I prophylactically in the nasopharynx and oropharynx. NCT04549376 [trialsjournal.biomedcentral.com]. | ||
May 13 2021 |
et al., Journal of Infection, doi:10.1016/j.jinf.2021.05.009 | Clinical efficacy of nitric oxide nasal spray (NONS) for the treatment of mild COVID-19 infection |
| 42% greater improvement (p=0.008) and 51% improved viral clearance (p=0.001). RCT with 40 nitric oxide and 40 placebo patients in the UK, showing faster viral clearance and greater improvement with treatment. | ||
May 1 2021 |
et al., Epidemiology and Health, doi:10.4178/epih.e2021032 | Effectiveness of hydrogen peroxide as auxiliary treatment for hospitalized COVID-19 patients in Brazil: preliminary results of a randomized double-blind clinical trial |
| 50% lower ICU admission (p=1), 6% greater improvement (p=0.91), and 7% higher hospital discharge (p=0.61). RCT very late treatment (>10 days from onset) comparing hydrogen peroxide + mint essence with water + mint essence, showing no significant differences. | ||
Apr 30 2021 |
et al., Journal of Medical Virology, doi:10.1002/jmv.26954 | Use of chlorhexidine to eradicate oropharyngeal SARS‐CoV‐2 in COVID‐19 patients |
| 75% improved viral clearance (p<0.0001). RCT 294 hospitalized patients in the USA, showing faster oropharyngeal viral clearance with chlorhexidine. Results were better with a combination of oropharyngeal rinse and posterior oropharyngeal spray compared with the rinse alone. | ||
Apr 30 2021 |
et al., Expert Review of Anti-infective Therapy, doi:10.1080/14787210.2021.1908127 | Low pH Hypromellose (Taffix) nasal powder spray could reduce SARS-CoV-2 infection rate post mass-gathering event at a highly endemic community: an observational prospective open label user survey |
| 76% fewer cases (p=0.04). Prospective observational study of 243 community members showing significantly lower SARS-CoV-2 infection with Taffix nasal spray during a high-risk mass gathering event. During the 14-day follow-up, 0% of per-protocol Taffix users (0/81).. | ||
Apr 15 2021 |
et al., International Journal of General Medicine, doi:10.2147/IJGM.S328486 (date from preprint) | Efficacy of a nasal spray containing Iota-Carrageenan in the prophylaxis of COVID-19 in hospital personnel dedicated to patients care with COVID-19 disease A pragmatic multicenter, randomized, double-blind, placebo-controlled trial (CARR-COV-02) |
| 80% fewer symptomatic cases (p=0.03). Prophylaxis RCT with 394 healthcare workers, 196 treated with iota-carrageenan, showing significantly lower symptomatic cases with treatment. There were no deaths or hospitalizations. There was a significant number of PCR- symptomatic cas.. | ||
Apr 14 2021 |
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2021.04.035 | Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial |
| 45% fewer symptomatic cases (p=0.002) and 31% fewer cases (p=0.01). Prophylaxis RCT in Singapore with 3,037 low risk patients, showing lower serious cases, lower symptomatic cases, and lower confirmed cases of COVID-19 with all treatments (ivermectin, HCQ, PVP-I, and Zinc + vitamin C) compared to vitamin .. | ||
Mar 17 2021 |
et al., Journal of Evidence Based Dental Practice, doi:10.1016/j.jebdp.2021.101584 (date from preprint) | In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial |
| Small RCT comparing mouthwashing with PVP-I, chlorhexidine, and water, showing significant efficacy for both PVP-I and chlorhexidine, with PVP-I increasing Ct by a mean of 4.45 (p < 0.0001) and chlorhexidine by a mean of 5.69 (p < 0.0001).. | ||
Mar 1 2021 |
et al., medRxiv, doi:10.1101/2021.02.25.21252488 | Povidone iodine, hydrogen peroxide and chlorhexidine mouthwashes reduce SARS-CoV2 burden in whole mouth fluid and respiratory droplets |
| Study of SARS-CoV-2 burden in whole mouth fluid and respiratory droplets with povidone iodine, hydrogen peroxide, and chlorhexidine mouthwashes in 36 hospitalized COVID-19 patients using PCR and rapid antigen testing. There were significa.. | ||
Feb 4 2021 |
et al., JAMA Otolaryngol Head Neck Surg., doi:10.1001/jamaoto.2020.5490 | Povidone Iodine Mouthwash, Gargle, and Nasal Spray to Reduce Nasopharyngeal Viral Load in Patients With COVID-19: A Randomized Clinical Trial |
| 63% improved viral load (p=0.25). RCT of PCR+ patients with Ct ≤20 with 12 treatment and 12 control patients, concluding that nasopharyngeal decolonization may reduce the carriage of infectious SARS-CoV-2 in adults with mild to moderate COVID-19. All patients but 1 had ne.. | ||
Jan 31 2021 |
et al., Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2020.11.095 | Identification of antiviral antihistamines for COVID-19 repurposing |
| 45% fewer cases (p=0.03). Retrospective 219,000 patients showing lower risk of COVID-19 with antihistamine H1RA use. In vitro study showing these drugs exhibit direct antiviral activity against SARS-CoV-2. Molecular docking suggests hydroxyzine and azelastine may .. | ||
Jan 23 2021 |
et al., Journal of Pharmaceutical Sciences, doi:10.1016/j.xphs.2021.01.017 | Safety and Pharmacokinetic Assessments of a Novel Ivermectin Nasal Spray Formulation in a Pig Model |
| Animal study of a novel spray formulation of ivermectin, showing an advantage of the spray formulation in terms of fast attainment of high and persistent ivermectin concentrations in nasopharyngeal tissue. | ||
Jan 11 2021 |
et al., American Journal of Therapeutics, doi:10.1097/MJT.0000000000001433 | Intensive Treatment With Ivermectin and Iota-Carrageenan as Pre-exposure Prophylaxis for COVID-19 in Health Care Workers From Tucuman, Argentina |
| 95% fewer moderate/severe cases (p=0.002) and 84% fewer cases (p=0.004). Prophylaxis RCT for ivermectin and iota-carrageenan in Argentina, 117 healthcare workers treated with ivermectin and iota-carrageenan, and 117 controls, showing significantly lower cases with treatment. There were no moderate/severe cases.. | ||
Jan 3 2021 |
et al., American Journal of Otolaryngology, doi:10.1016/j.amjoto.2020.102880 | Tolerability and usability of 0.5% PVP-I gargles and nasal drops in 6692 patients: Observational study |
| Study of the use of PVP-I gargles and nasal drops before and after ENT examinations with a total of 6,692 patients, finding high usability and good tolerance for use. 21 patients (0.76%) reported an itching sensation in the nose on the fi.. | ||
Dec 14 2020 |
et al., Infection, doi:10.1007/s15010-020-01563-9 | Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore |
| Small mouthwash RCT with 4 PVP-I patients and 2 water patients concluding that PVP-I may have a sustained effect on reducing the salivary SARS-CoV-2 level in COVID-19 patients. ISRCTN95933274. | ||
Dec 3 2020 |
et al., Bioresearch Communications, doi:10.3329/brc.v7i1.54245 | Effect of 1% Povidone Iodine Mouthwash/Gargle, Nasal and Eye Drop in COVID-19 patient |
| 88% lower mortality (p=0.0006), 84% lower hospitalization (p<0.0001), and 96% improved viral clearance (p<0.0001). RCT 606 patients in Bangladesh for povidone iodine mouthwash/gargle, nasal drops and eye drops showing significantly lower death, hospitalization, and PCR+ at day 7. | ||
Nov 30 2020 |
et al., medRxiv, doi:10.1101/2020.11.27.20234997 | A Randomized trial on the regular use of potent mouthwash in COVID-19 treatment |
| 86% lower mortality (p=0.24), 86% lower ventilation (p=0.24), and 18% improved viral clearance (p=0.16). RCT for mouthwash containing hydrogen peroxide 2% and chlorhexidine gluconate, showing higher discharge, shorter hospital stay, less intubation, and lower mortality with treatment. | ||
Nov 17 2020 |
et al., Journal of Biomedical Research and Clinical Investigation, doi:10.31546/2633-8653.1007 | Study of the Efficacy and Safety of Topical Ivermectin + Iota-Carrageenan in the Prophylaxis against COVID-19 in Health Personnel |
| 100% fewer cases (p<0.0001). Prophylaxis study using ivermectin and iota-carrageenan showing 0 of 788 cases from treated healthcare workers, compared to 237 of 407 control. See [doyourownresearch.substack.com] for discussion of issues with this trial. | ||
Oct 19 2020 |
et al., NCT04425850 | Usefulness of Topical Ivermectin and Carrageenan to Prevent Contagion of Covid 19 (IVERCAR) |
| 96% fewer cases (p<0.0001). Prophylaxis study using ivermectin and carrageenan showing 0 of 131 cases from treated healthcare workers, compared to 11 of 98 control. The effect is likely to be primarily due to ivermectin - the author has later reported that carrageen.. | ||
Sep 9 2020 |
et al., medRxiv, doi:10.1101/2020.09.07.20180448 | Early viral clearance among COVID-19 patients when gargling with povidone-iodine and essential oils: a pilot clinical trial |
| 86% improved viral clearance (p=0.17). Tiny RCT with 5 PVP-I patients, gargling 30 seconds, 3x per day, and 5 control patients (essential oils and tap water were also tested), showing improved viral clearance with PVP-I. | ||
Jul 2 2020 |
et al., Oral Diseases, doi:10.1111/odi.13526 | Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests |
| Small study analyzing the impact of PVP-I mouthwash on the salivary viral load of SARS-CoV-2 in 4 patients with COVID-19. In 2 of the 4 patients (those with a higher initial viral load), PVP-I resulted in a significant drop in viral load,.. | ||
Jun 18 2020 |
et al., Am J Otolaryngol, doi:10.1016/j.amjoto.2020.102618 | Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in Covid 19 pandemic |
| Study of the use of PVP-I gargles and nasal drops before ENT appointments finding good tolerability. | ||