Efficacy of a nasal spray containing Iota-Carrageenan in the prophylaxis of COVID-19 in hospital personnel dedicated to patients care with COVID-19 disease A pragmatic multicenter, randomized, double-blind, placebo-controlled trial (CARR-COV-02)
et al., International Journal of General Medicine, doi:10.2147/IJGM.S328486, CARR-COV-02, NCT04521322, Apr 2021 (preprint)
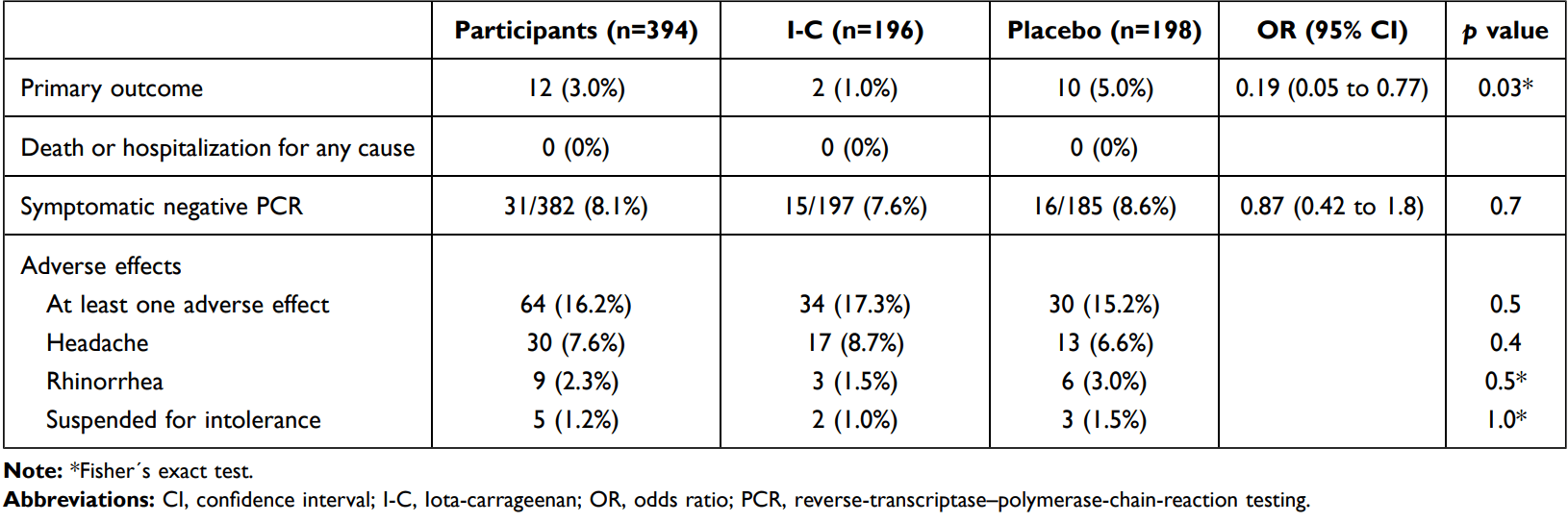
Prophylaxis RCT with 394 healthcare workers, 196 treated with iota-carrageenan, showing significantly lower symptomatic cases with treatment. There were no deaths or hospitalizations. There was a significant number of PCR- symptomatic cases (7.6% treatment and 8.6% control). The two treatment cases occurred shortly after randomization - infection may have occurred before the start of treatment.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
|
risk of symptomatic case, 80.2% lower, RR 0.20, p = 0.03, treatment 2 of 196 (1.0%), control 10 of 198 (5.1%), NNT 25, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Figueroa et al., 15 Apr 2021, Double Blind Randomized Controlled Trial, Argentina, peer-reviewed, 18 authors, study period 24 July, 2020 - 20 December, 2020, trial NCT04521322 (history) (CARR-COV-02).
Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease
International Journal of General Medicine, doi:10.2147/ijgm.s328486
Background: Iota-Carrageenan (I-C) is a sulfate polysaccharide synthesized by red algae, with demonstrated antiviral activity and clinical efficacy as nasal spray in the treatment of common cold. In vitro, I-C inhibits SARS-CoV-2 infection in cell culture. Research Question: Can a nasal spray with Iota-Carrageenan be useful in the prophylaxis of COVID-19 in health care workers managing patients with COVID-19 disease? Study Design and Methods: This is a pilot pragmatic multicenter, randomized, doubleblind, placebo-controlled study assessing the use of a nasal spray containing I-C in the prophylaxis of COVID-19 in hospital personnel dedicated to care of COVID-19 patients. Clinically healthy physicians, nurses, kinesiologists and other health care providers managing patients hospitalized for COVID-19 were assigned in a 1:1 ratio to receive four daily doses of I-C spray or placebo for 21 days. The primary end point was clinical COVID-19, as confirmed by reverse transcriptase polymerase chain reaction testing, over a period of 21 days. The trial is registered at ClinicalTrials.gov (NCT04521322). Results: A total of 394 individuals were randomly assigned to receive I-C or placebo. Both treatment groups had similar baseline characteristics. The incidence of COVID-19 differs significantly between subjects receiving the nasal spray with I-C (2 of 196 [1.0%]) and those receiving placebo (10 of 198 [5.0%]). Relative risk reduction: 79.8% (95% CI 5.3 to 95.4; p=0.03). Absolute risk reduction: 4% (95% CI 0.6 to 7.4). Interpretation: In this pilot study a nasal spray with I-C showed significant efficacy in preventing COVID-19 in health care workers managing patients with COVID-19 disease. Clinical Trials Registration: NCT04521322.
Funding The study did not receive any support for hospitals, staff or patients involved. Publication and administrative costs were supported by: Programa de articulación y fortalecimiento federal de las capacidades en ciencia y tecnología COVID-
International Journal of General Medicine
Dovepress
References
Ahmadi, Moghadamtousi, Abubakar, Zandi, Antiviral potential of algae polysaccharides isolated from marine sources: a review, Biomed Res Int, doi:10.1155/2015/825203
Baden, Sahly, Essink, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine, N Engl J Med, doi:10.1056/NEJMoa2035389
Bansal, Colleen, Taylor, Iota-carrageenan and xylitol inhibit SARS-CoV-2 in cell culture, BioRxiv, doi:10.1101/2020.08.19.225854
Barnabas, Brown, Bershteyn, Hydroxychloroquine as postexposure prophylaxis to prevent severe acute respiratory syndrome coronavirus 2 infection: a randomized trial, Ann Intern Med, doi:10.7326/M20-6519
Boulware, Pullen, Bangdiwala, A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19, N Engl J Med, doi:10.1056/NEJMoa2016638
Buck, Thompson, Roberts, Müller, Lowy et al., Carrageenan is a potent inhibitor of papillomavirus infection, PLoS Pathog, doi:10.1371/journal.ppat.0020069
Eccles, Meier, Jawad, Weinmüllner, Grassauer et al., Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold, Respir Res, doi:10.1186/1465-9921-11-108
Eccles, Winther, Johnston, Robinson, Trampisch et al., Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial, Respir Res, doi:10.1186/s12931-015-0281-8
Figueroa, TCPDF
Graf, Bernkop-Schnürch, Egyed, Koller, Prieschl-Grassauer et al., Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis, Int J Gen Med, doi:10.2147/IJGM.S167123
Grassauer, Weinmuellner, Meier, Interference in dengue virus adsorption and uncoating by carrageenans, Virology, doi:10.1016/j.virol.2007.01.043
Grassauer, Weinmuellner, Meier, Pretsch, Prieschl-Grassauer et al., Iota-Carrageenan is a potent inhibitor of rhinovirus infection, Virol J, doi:10.1186/1743-422X-5-107
Griffin, Brennan-Rieder, Ngo, The importance of understanding the stages of COVID-19 in treatment and trials, AIDS Rev, doi:10.24875/AIDSRev.200001261
Hemilä, Chalker, Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: re-analysis of randomized trial data, Pharmacol Res Perspect, doi:10.1002/prp2.810
Klimyte, Smith, Oreste, Lembo, Dutch, Inhibition of human metapneumovirus binding to heparan sulfate blocks infection in human lung cells and airway tissues, J Virol, doi:10.1128/JVI.01362-16
Lauer, Grantz, Bi, The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application, Ann Intern Med, doi:10.7326/M20-0504
Leibbrandt, Meier, König-Schuster, Iota-carrageenan is a potent inhibitor of influenza A virus infection, PLoS One, doi:10.1371/journal.pone.0014320
Logunov, Dolzhikova, Shcheblyakov, -Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled Phase 3 trial in Russia, Lancet, doi:10.1016/S0140-6736(21)00234-8
Ludwig, Enzenhofer, Schneider, Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial, Respir Res, doi:10.1186/1465-9921-14-124
Mitjà, Corbacho-Monné, Ubals, BCN-PEP-CoV2 research group. A cluster-randomized trial of hydroxychloroquine for prevention of covid-19, N Engl J Med, doi:10.1056/NEJMoa2021801
Morokutti-Kurz, Graf, Grassauer, Prieschl-Grassauer, SARS-CoV-2 in-vitro neutralization assay reveals inhibition of virus entry by iota-carrageenan, bioRxiv, doi:10.1101/2020.07.28.224733
Niriella, Ediriweera, Silva, Hydroxychloroquine for post-exposure prophylaxis of COVID-19 among naval personnel in Sri Lanka: study protocol for a randomized, controlled trial, Trials, doi:10.1186/s13063-020-04659-7
Polack, Thomas, Kitchin, C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577
Schütz, Conzelmann, Fois, Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00552.2020
Song, Peng, Wang, Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food Funct, doi:10.1039/d0fo02017f
Varese, Ceballos, Palacios, Figueroa, Dugour, Iotacarrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model, bioRxiv, doi:10.1101/2021.04.27.441512
Voysey, Clemens, Madhi, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet
Wang, Zhang, Hao, Zhang, Cui et al., In vitro inhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus, Antiviral Res, doi:10.1016/j.antiviral.2011.08.010
Weiner, Food additive carrageenan: part II: a critical review of carrageenan in vivo safety studies, International Journal of General Medicine, doi:10.3109/10408444.2013.861798
Xin, Wong, Murphy, the incubation period distribution of coronavirus disease 2019: a systematic review and meta-analysis, Clin Infect Dis, doi:10.1093/cid/ciab501
Zhang, Zhou, Adenoviral vector-based strategies against infectious disease and cancer, Hum Vaccin Immunother, doi:10.1080/21645515.2016.1165908
Zou, Ruan, Huang, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med, doi:10.1056/NEJMc2001737
DOI record:
{
"DOI": "10.2147/ijgm.s328486",
"ISSN": [
"1178-7074"
],
"URL": "http://dx.doi.org/10.2147/IJGM.S328486",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5680-1486",
"affiliation": [],
"authenticated-orcid": true,
"family": "Figueroa",
"given": "Juan Manuel",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lombardo",
"given": "Mónica Edith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dogliotti",
"given": "Ariel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flynn",
"given": "Luis Pedro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giugliano",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simonelli",
"given": "Guido",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valentini",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramos",
"given": "Agñel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romano",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marcote",
"given": "Marcelo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Michelini",
"given": "Alicia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salvado",
"given": "Alejandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sykora",
"given": "Emilio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kniz",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kobelinsky",
"given": "Marcelo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9861-4267",
"affiliation": [],
"authenticated-orcid": true,
"family": "Salzberg",
"given": "David Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jerusalinsky",
"given": "Diana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Uchitel",
"given": "Osvaldo",
"sequence": "additional"
}
],
"container-title": "International Journal of General Medicine",
"container-title-short": "IJGM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
9,
30
]
],
"date-time": "2021-09-30T13:25:31Z",
"timestamp": 1633008331000
},
"deposited": {
"date-parts": [
[
2021,
9,
30
]
],
"date-time": "2021-09-30T13:25:36Z",
"timestamp": 1633008336000
},
"indexed": {
"date-parts": [
[
2024,
4,
9
]
],
"date-time": "2024-04-09T18:24:49Z",
"timestamp": 1712687089512
},
"is-referenced-by-count": 37,
"issued": {
"date-parts": [
[
2021,
10
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/getfile.php?fileID=74193",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/getfile.php?fileID=74193",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "6277-6286",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2021,
10
]
]
},
"published-online": {
"date-parts": [
[
2021,
10
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1056/NEJMoa2034577",
"author": "Polack",
"doi-asserted-by": "publisher",
"first-page": "2603",
"journal-title": "N Engl J Med",
"key": "ref1",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)00234-8",
"author": "Logunov",
"doi-asserted-by": "publisher",
"first-page": "671",
"journal-title": "Lancet",
"key": "ref2",
"volume": "397",
"year": "2021"
},
{
"key": "ref3",
"unstructured": "World Health Organization Draft landscape of COVID-19 candidate vaccines; Jan 22, 2021. Available from: https://www.who.int/publications/m/item/draft-landscape-of-cOVID-19-candidate-vaccines. Accessed September 16, 2021."
},
{
"DOI": "10.1016/S0140-6736(20)32661-1",
"author": "Voysey",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "Lancet",
"key": "ref4",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035389",
"author": "Baden",
"doi-asserted-by": "publisher",
"first-page": "403",
"journal-title": "N Engl J Med",
"key": "ref5",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1080/21645515.2016.1165908",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "2064",
"journal-title": "Hum Vaccin Immunother",
"key": "ref6",
"volume": "12",
"year": "2016"
},
{
"DOI": "10.1155/2015/825203",
"author": "Ahmadi",
"doi-asserted-by": "publisher",
"first-page": "825203",
"journal-title": "Biomed Res Int",
"key": "ref7",
"volume": "2015",
"year": "2015"
},
{
"DOI": "10.1371/journal.pone.0014320",
"author": "Leibbrandt",
"doi-asserted-by": "publisher",
"first-page": "e14320",
"journal-title": "PLoS One",
"key": "ref8",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.1016/j.antiviral.2011.08.010",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "237",
"journal-title": "Antiviral Res",
"key": "ref9",
"volume": "92",
"year": "2011"
},
{
"DOI": "10.1186/1743-422X-5-107",
"author": "Grassauer",
"doi-asserted-by": "publisher",
"first-page": "107",
"journal-title": "Virol J",
"key": "ref10",
"volume": "5",
"year": "2008"
},
{
"DOI": "10.1016/j.virol.2007.01.043",
"author": "Grassauer",
"doi-asserted-by": "publisher",
"first-page": "473",
"journal-title": "Virology",
"key": "ref11",
"volume": "363",
"year": "2007"
},
{
"DOI": "10.1371/journal.ppat.0020069",
"author": "Buck",
"doi-asserted-by": "publisher",
"first-page": "e69",
"journal-title": "PLoS Pathog",
"key": "ref12",
"volume": "2",
"year": "2006"
},
{
"DOI": "10.1128/JVI.01362-16",
"author": "Klimyte",
"doi-asserted-by": "publisher",
"first-page": "9237",
"journal-title": "J Virol",
"key": "ref13",
"volume": "90",
"year": "2016"
},
{
"DOI": "10.1002/prp2.810",
"author": "Hemilä",
"doi-asserted-by": "publisher",
"first-page": "e00810",
"journal-title": "Pharmacol Res Perspect",
"key": "ref14",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1186/1465-9921-11-108",
"author": "Eccles",
"doi-asserted-by": "publisher",
"first-page": "108",
"journal-title": "Respir Res",
"key": "ref15",
"volume": "11",
"year": "2010"
},
{
"DOI": "10.1186/1465-9921-14-124",
"author": "Ludwig",
"doi-asserted-by": "publisher",
"first-page": "124",
"journal-title": "Respir Res",
"key": "ref16",
"volume": "14",
"year": "2013"
},
{
"DOI": "10.1186/s12931-015-0281-8",
"author": "Eccles",
"doi-asserted-by": "publisher",
"first-page": "121",
"journal-title": "Respir Res",
"key": "ref17",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1152/ajplung.00552.2020",
"author": "Schütz",
"doi-asserted-by": "publisher",
"first-page": "L750",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "ref18",
"volume": "320",
"year": "2021"
},
{
"DOI": "10.1039/d0fo02017f",
"author": "Song",
"doi-asserted-by": "publisher",
"first-page": "7415",
"journal-title": "Food Funct",
"key": "ref19",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1101/2020.07.28.224733",
"author": "Morokutti-Kurz",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "ref20",
"year": "2020"
},
{
"DOI": "10.1101/2020.08.19.225854",
"author": "Bansal",
"doi-asserted-by": "publisher",
"journal-title": "BioRxiv",
"key": "ref21",
"year": "2020"
},
{
"DOI": "10.1101/2021.04.27.441512",
"author": "Varese",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "ref22",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2001737",
"author": "Zou",
"doi-asserted-by": "publisher",
"first-page": "1177",
"journal-title": "N Engl J Med",
"key": "ref23",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.2147/IJGM.S167123",
"author": "Graf",
"doi-asserted-by": "publisher",
"first-page": "275",
"journal-title": "Int J Gen Med",
"key": "ref24",
"volume": "11",
"year": "2018"
},
{
"DOI": "10.7326/M20-0504",
"author": "Lauer",
"doi-asserted-by": "publisher",
"first-page": "577",
"journal-title": "Ann Intern Med",
"key": "ref25",
"volume": "172",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciab501",
"author": "Xin",
"doi-asserted-by": "publisher",
"first-page": "ciab501",
"journal-title": "Clin Infect Dis",
"key": "ref26",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2016638",
"author": "Boulware",
"doi-asserted-by": "publisher",
"first-page": "517",
"journal-title": "N Engl J Med",
"key": "ref27",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.7326/M20-6519",
"author": "Barnabas",
"doi-asserted-by": "publisher",
"first-page": "344",
"journal-title": "Ann Intern Med",
"key": "ref28",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021801",
"author": "Mitjà",
"doi-asserted-by": "publisher",
"first-page": "417",
"journal-title": "N Engl J Med",
"key": "ref29",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1186/s13063-020-04659-7",
"author": "Niriella",
"doi-asserted-by": "publisher",
"first-page": "748",
"journal-title": "Trials",
"key": "ref30",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.24875/AIDSRev.200001261",
"author": "Griffin",
"doi-asserted-by": "publisher",
"first-page": "40",
"journal-title": "AIDS Rev",
"key": "ref31",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.3109/10408444.2013.861798",
"author": "Weiner",
"doi-asserted-by": "publisher",
"first-page": "244",
"journal-title": "Crit Rev Toxicol",
"key": "ref32",
"volume": "44",
"year": "2014"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2021.04.13.21255409",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/efficacy-of-a-nasal-spray-containing-iota-carrageenan-in-the-postexpos-peer-reviewed-fulltext-article-IJGM"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease",
"type": "journal-article",
"volume": "Volume 14"
}