Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: Re-analysis of randomized trial data
et al., Pharmacology Research and Perspectives, doi:10.1002/prp2.810, Jun 2021
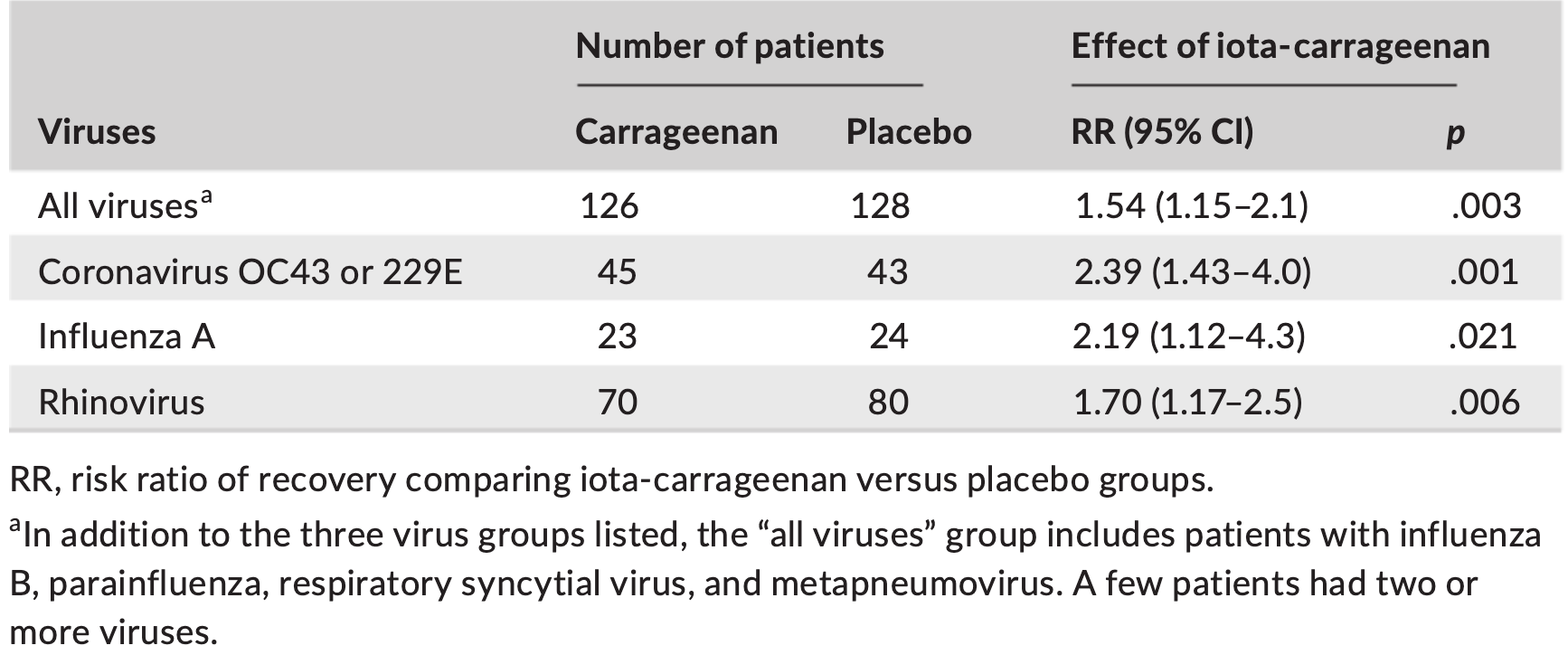
Individual patient data meta analysis of two RCTs showing nasal iota-carrageenan improved recovery rate and shortened the duration of long colds. There was a 71% reduction in the risk of long colds (>20 days). The increase in recovery rate was 139% for coronavirus infections, 119% for influenza A infections, and 70% for rhinovirus infections.
Hemilä et al., 14 Jun 2021, peer-reviewed, 2 authors.
Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: Re‐analysis of randomized trial data
Pharmacology Research & Perspectives, doi:10.1002/prp2.810
In this individual patient data meta-analysis we examined datasets of two randomized placebo-controlled trials which investigated the effect of nasal carrageenan separately on children and adults. In both trials, iota-carrageenan was administered nasally three times per day for 7 days for patients with the common cold and follow-up lasted for 21 days. We used Cox regression to estimate the effect of carrageenan on recovery rate. We also used quantile regression to calculate the effect of carrageenan on colds of differing lengths. Nasal carrageenan increased the recovery rate from all colds by 54% (95% CI 15%-105%; p = .003). The increase in recovery rate was 139% for coronavirus infections, 119% for influenza A infections, and 70% for rhinovirus infections. The mean duration of all colds in the placebo groups of the first four quintiles were 4.0, 6.8, 8.8, and 13.7 days, respectively. The fifth quintile contained patients with censored data. The 13.7-day colds were shortened by 3.8 days (28% reduction), and 8.8-day colds by 1.3 days (15% reduction). Carrageenan had no meaningful effect on shorter colds. In the placebo group, 21 patients had colds lasting over 20 days, compared with six patients in the carrageenan group, which corresponds to a 71% (p = .003) reduction in the risk of longer colds. Given that carrageenan has an effect on diverse virus groups, and effects at the clinical level on two old coronaviruses, it seems plausible that carrageenan may have an effect on COVID-19. Further research on nasal iota-carrageenan is warranted.
AUTH O R CO NTR I B UTI O N S HH planned the study, measured the published survival curves, 28 entered the data into a spreadsheet and carried out the statistical analysis, and wrote the draft manuscript. EC checked that the entered data were consistent with the published survival curves and participated in the critical revision of the manuscript. Both authors read and approved the final manuscript.
References
Baba, Snoeck, Pauwels, De Clercq, Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus, Antimicrob Agents Chemother, doi:10.1128/aac.32.11.1742
Bansal, Jonsson, Taylor, Iota-carrageenan and xylitol inhibit SARS-CoV-2 in cell culture, bioRxiv, doi:10.1101/2020.08.19.225854
Buck, Thompson, Roberts, Müller, Lowy et al., Carrageenan is a potent inhibitor of papillomavirus infection, PLoS Pathog, doi:10.1371/journal.ppat.0020069
Carfì, Bernabei, Landi, Gemelli, Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19, JAMA, doi:10.1001/jama.2020.12603
Carlucci, Scolaro, Noseda, Cerezo, Damonte, Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice, Antiviral Res, doi:10.1016/j.antiviral.2004.07.001
Core, R: a language and environment for statistical computing
Davis, Assaf, Mccorkell, Characterizing Long COVID in an international cohort: 7 months of symptoms and their impact, doi:10.1101/2020.12.24.20248802
Eby, Zinc lozenges as cure for the common cold -a review and hypothesis, Med Hypotheses, doi:10.1016/j.mehy.2009.10.017
Eccles, Iota-carrageenan as an antiviral treatment for the common cold, Open Virol J, doi:10.2174/1874357902014010009
Eccles, Is the common cold a clinical entity or a cultural concept?, Rhinology, doi:10.4193/rhino12.123
Eccles, Meier, Jawad, Weinmüllner, Grassauer et al., Efficacy and safety of an antiviral iota-carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold, Respir Res, doi:10.1186/1465-9921-11-108
Eccles, Winther, Johnston, Robinson, Trampisch et al., Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial, Respir Res, doi:10.1186/s12931-015-0281-8
Fazekas, Eickhoff, Pruckner, Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold, BMC Complement Altern Med, doi:10.1186/1472-6882-12-147
Fergusson, Aaron, Guyatt, Hébert, Demets et al., Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis, JAMA, doi:10.1001/jama.2018.19192
Figueroa, Lombardo, Dogliotti, Trial, Efficacy of a nasal spray containing iota-carrageenan in the prophylaxis of COVID-19 in hospital personnel dedicated to patients care with COVID-19 disease: a pragmatic multicenter, randomized, double-blind, placebo-controlled trial (CARR-COV-02, doi:10.1101/2021.04.13.21255409
Girond, Crance, Van Cuyck-Gandre, Renaudet, Deloince, Antiviral activity of carrageenan on hepatitis A virus replication in cell culture, Res Virol, doi:10.1016/0923-2516(91)90011-q
González, Alarcón, Carrasco, Polysaccharides as antiviral agents: antiviral activity of carrageenan, Antimicrob Agents Chemother, doi:10.1128/aac.31.9.1388
Graf, Bernkop-Schnürch, Egyed, Koller, Prieschl-Grassauer et al., Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis, Int J Gen Med, doi:10.2147/ijgm.s167123
Grassauer, Weinmuellner, Meier, Pretsch, Prieschl-Grassauer et al., Iota-Carrageenan is a potent inhibitor of rhinovirus infection, Virol J, doi:10.1186/1743-422x-5-107
Hebar, Koller, Seifert, Non-clinical safety evaluation of intranasal iota-carrageenan, PLoS One, doi:10.1371/journal.pone.0122911
Hemilä, Carr, Chalker, Vitamin C may increase the recovery rate of outpatient cases of SARS-CoV-2 infection by 70%: Reanalysis of the COVID A to Z randomized clinical trial, Frontiers in Immunology, doi:10.3389/fimmu.2021.674681
Hemilä, Chalker, Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: reanalysis of randomized trial data, doi:10.21203/rs.3.rs-108775/v1
Hemilä, Chalker, The effectiveness of high dose zinc acetate lozenges on various common cold symptoms: a meta-analysis, BMC Family Pract, doi:10.1186/s12875-015-0237-6
Hemilä, Chalker, Vitamin C for preventing and treating the common cold, Cochrane Database Syst Rev, doi:10.1002/14651858.CD000980.pub4
Hemilä, Do vitamins C and E affect respiratory infections?
Hemilä, Duration of the common cold and similar continuous outcomes should be analyzed on the relative scale: a case study of two zinc lozenge trials, BMC Med Res Methodol, doi:10.1186/s12874-017-0356-y
Hemilä, Fitzgerald, Petrus, Prasad, Zinc acetate lozenges may improve the recovery rate of common cold patients: an individual patient data meta-analysis, Open Forum Infect Dis, doi:10.1093/ofid/ofx059
Hemilä, Vitamin C and infections, Nutrients, doi:10.3390/nu9040339
Hemilä, Vitamin C supplementation and common cold symptoms: problems with inaccurate reviews, Nutrition, doi:10.1016/S0899-9007(96)00223-7
Hemilä, Vitamin, the placebo effect, and the common cold: a case study of how preconceptions influence the analysis of results, J Clin Epidemiol, doi:10.1016/0895-4356(96)00189-8
Hemilä, Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage, JRSM Open, doi:10.1177/2054270417694291
Hong, Christiani, Li, Quantile regression for survival data in modern cancer research: expanding statistical tools for precision medicine, Precis Clin Med, doi:10.1093/pcmedi/pbz007
Jang, Shin, Lee, Antiviral activity of lambdacarrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2, Sci Rep, doi:10.1038/s41598-020-80896-9
Koenighofer, Lion, Bodenteich, Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials, Multidiscip Respir Med, doi:10.1186/2049-6958-9-57
Koenker, Quantile treatment effects. Quantile Regression
Koenker, Quantreg, Quantile regression
Lee, Carrageenans as broad-spectrum microbicides: current status and challenges, Mar Drugs, doi:10.3390/md18090435
Leibbrandt, Meier, König-Schuster, Iota-carrageenan is a potent inhibitor of influenza A virus infection, PLoS One, doi:10.1371/journal.pone.0014320
Louhiala, Hemilä, Can CAM treatments be evidence-based? Focus, Altern Complement Ther, doi:10.1111/fct.12110
Ludwig, Enzenhofer, Schneider, Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial, Respir Res, doi:10.1186/1465-9921-14-124
Mckim, Willoughby, Sr, Blakemore, Weiner, Clarifying the confusion between poligeenan, degraded carrageenan, and carrageenan: a review of the chemistry, nomenclature, and in vivo toxicology by the oral route, Crit Rev Food Sci Nutr, doi:10.1080/10408398.2018.1481822
Morokutti-Kurz, Fröba, Graf, Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS One, doi:10.1371/journal.pone.0237480
Morokutti-Kurz, Graf, Prieschl-Grassauer, Amylmetacresol/2,4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat, Int J Gen Med, doi:10.2147/ijgm.s120665
Morokutti-Kurz, König-Schuster, Koller, The intranasal application of Zanamivir and carrageenan is synergistically active against influenza A virus in the murine model, PLoS One, doi:10.1371/journal.pone.0128794
Nakazawa, Fmsb, Functions for medical statistics Book
Schütz, Conzelmann, Fois, Carrageenan containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00552.2020
Shao, Guo, Wp, Li, Tt, Specific inhibitory effect of κ-carrageenan polysaccharide on swine pandemic 2009 H1N1 influenza virus, PLoS One, doi:10.1371/journal.pone.0126577
Shi, Wang, Lu, Qin, Hu et al., Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds, Carbohydr Res, doi:10.1016/j.carres.2017.10.020
Therneau, A package for survival analysis in R
Veiga, Martins, Riediger, Mazetto, Debur et al., More than just a common cold: endemic coronaviruses OC43, HKU1, NL63, and 229E associated with severe acute respiratory infection and fatality cases among healthy adults, J Med Virol, doi:10.1002/jmv.26362
Weiner, Food additive carrageenan: Part II: a critical review of carrageenan in vivo safety studies, Crit Rev Toxicol, doi:10.3109/10408444.2013.861798
Weiner, Parameters and pitfalls to consider in the conduct of food additive research, Carrageenan as a case study, Food Chem Toxicol, doi:10.1016/j.fct.2015.11.014
DOI record:
{
"DOI": "10.1002/prp2.810",
"ISSN": [
"2052-1707",
"2052-1707"
],
"URL": "http://dx.doi.org/10.1002/prp2.810",
"abstract": "<jats:title>Abstract</jats:title><jats:p>In this individual patient data meta‐analysis we examined datasets of two randomized placebo‐controlled trials which investigated the effect of nasal carrageenan separately on children and adults. In both trials, iota‐carrageenan was administered nasally three times per day for 7 days for patients with the common cold and follow‐up lasted for 21 days. We used Cox regression to estimate the effect of carrageenan on recovery rate. We also used quantile regression to calculate the effect of carrageenan on colds of differing lengths. Nasal carrageenan increased the recovery rate from all colds by 54% (95% CI 15%–105%; <jats:italic>p</jats:italic> = .003). The increase in recovery rate was 139% for coronavirus infections, 119% for influenza A infections, and 70% for rhinovirus infections. The mean duration of all colds in the placebo groups of the first four quintiles were 4.0, 6.8, 8.8, and 13.7 days, respectively. The fifth quintile contained patients with censored data. The 13.7‐day colds were shortened by 3.8 days (28% reduction), and 8.8‐day colds by 1.3 days (15% reduction). Carrageenan had no meaningful effect on shorter colds. In the placebo group, 21 patients had colds lasting over 20 days, compared with six patients in the carrageenan group, which corresponds to a 71% (<jats:italic>p</jats:italic> = .003) reduction in the risk of longer colds. Given that carrageenan has an effect on diverse virus groups, and effects at the clinical level on two old coronaviruses, it seems plausible that carrageenan may have an effect on COVID‐19. Further research on nasal iota‐carrageenan is warranted.</jats:p>",
"alternative-id": [
"10.1002/prp2.810"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-04-27"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-05-02"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-06-14"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4710-307X",
"affiliation": [
{
"name": "Department of Public Health University of Helsinki Helsinki Finland"
}
],
"authenticated-orcid": false,
"family": "Hemilä",
"given": "Harri",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-1593-3770",
"affiliation": [
{
"name": "School of Public Health University of Sydney Sydney Australia"
}
],
"authenticated-orcid": false,
"family": "Chalker",
"given": "Elizabeth",
"sequence": "additional"
}
],
"container-title": "Pharmacology Research & Perspectives",
"container-title-short": "Pharmacology Res & Perspec",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bpspubs.onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
6,
15
]
],
"date-time": "2021-06-15T06:36:20Z",
"timestamp": 1623738980000
},
"deposited": {
"date-parts": [
[
2023,
8,
28
]
],
"date-time": "2023-08-28T03:42:45Z",
"timestamp": 1693194165000
},
"indexed": {
"date-parts": [
[
2023,
8,
29
]
],
"date-time": "2023-08-29T11:11:24Z",
"timestamp": 1693307484252
},
"is-referenced-by-count": 7,
"issue": "4",
"issued": {
"date-parts": [
[
2021,
6,
14
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2021,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
6,
14
]
],
"date-time": "2021-06-14T00:00:00Z",
"timestamp": 1623628800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/prp2.810",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/prp2.810",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://bpspubs.onlinelibrary.wiley.com/doi/pdf/10.1002/prp2.810",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2021,
6,
14
]
]
},
"published-online": {
"date-parts": [
[
2021,
6,
14
]
]
},
"published-print": {
"date-parts": [
[
2021,
8
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.carres.2017.10.020",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_2_1"
},
{
"DOI": "10.3390/md18090435",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_3_1"
},
{
"DOI": "10.2174/1874357902014010009",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_1"
},
{
"DOI": "10.1128/aac.31.9.1388",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_1"
},
{
"DOI": "10.1128/aac.32.11.1742",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_6_1"
},
{
"DOI": "10.1016/0923‐2516(91)90011‐q",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_7_1"
},
{
"DOI": "10.1371/journal.ppat.0020069",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_8_1"
},
{
"DOI": "10.1186/1743‐422x‐5‐107",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_9_1"
},
{
"DOI": "10.1371/journal.pone.0126577",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_10_1"
},
{
"DOI": "10.2147/ijgm.s120665",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_11_1"
},
{
"DOI": "10.2147/ijgm.s167123",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_12_1"
},
{
"DOI": "10.1371/journal.pone.0237480",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_13_1"
},
{
"DOI": "10.1152/ajplung.00552.2020",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_14_1"
},
{
"DOI": "10.1101/2020.08.19.225854",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_15_1"
},
{
"DOI": "10.1038/s41598‐020‐80896‐9",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_16_1"
},
{
"DOI": "10.1371/journal.pone.0014320",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_17_1"
},
{
"DOI": "10.1371/journal.pone.0128794",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_18_1"
},
{
"DOI": "10.1016/j.antiviral.2004.07.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_19_1"
},
{
"DOI": "10.3109/10408444.2013.861798",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_20_1"
},
{
"DOI": "10.1016/j.fct.2015.11.014",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_21_1"
},
{
"DOI": "10.1080/10408398.2018.1481822",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_22_1"
},
{
"DOI": "10.2903/j.efsa.2018.5238",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_23_1"
},
{
"DOI": "10.1371/journal.pone.0122911",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_24_1"
},
{
"DOI": "10.1186/1465‐9921‐11‐108",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_25_1"
},
{
"DOI": "10.1186/1465‐9921‐14‐124",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_26_1"
},
{
"DOI": "10.1186/s12931‐015‐0281‐8",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_27_1"
},
{
"DOI": "10.1186/1472‐6882‐12‐147",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_28_1"
},
{
"DOI": "10.1186/2049‐6958‐9‐57",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_29_1"
},
{
"DOI": "10.1186/s12874‐017‐0356‐y",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_30_1"
},
{
"DOI": "10.1136/bmj.325.7365.652",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_31_1"
},
{
"DOI": "10.1001/jama.2018.19192",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_32_1"
},
{
"key": "e_1_2_11_33_1",
"unstructured": "R Core Team 2020.R: a language and environment for statistical computing.https://www.R‐project.org/. Accessed April 22 2021."
},
{
"key": "e_1_2_11_34_1",
"unstructured": "TherneauT.A package for survival analysis in R.https://CRAN.R‐project.org/package=survival. Accessed April 22 2021."
},
{
"DOI": "10.1017/CBO9780511754098.003",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_35_1"
},
{
"DOI": "10.1093/pcmedi/pbz007",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_36_1"
},
{
"key": "e_1_2_11_37_1",
"unstructured": "KoenkerR.quantreg: Quantile regression.https://CRAN.R‐project.org/package=quantreg. Accessed April 22 2021."
},
{
"DOI": "10.3389/fimmu.2021.674681",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_38_1"
},
{
"key": "e_1_2_11_39_1",
"unstructured": "NakazawaM.fmsb: Functions for medical statistics Book.https://CRAN.R‐project.org/package=fmsb. Accessed April 22 2021."
},
{
"DOI": "10.1016/j.mehy.2009.10.017",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_40_1"
},
{
"DOI": "10.1093/ofid/ofx059",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_41_1"
},
{
"DOI": "10.1177/2054270417694291",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_42_1"
},
{
"DOI": "10.1186/s12875‐015‐0237‐6",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_43_1"
},
{
"DOI": "10.1002/14651858.CD000980.pub4",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_44_1"
},
{
"DOI": "10.1016/S0899‐9007(96)00223‐7",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_45_1"
},
{
"DOI": "10.1016/0895‐4356(96)00189‐8",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_46_1"
},
{
"DOI": "10.1111/fct.12110",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_47_1"
},
{
"key": "e_1_2_11_48_1",
"unstructured": "HemiläH.Do vitamins C and E affect respiratory infections?Ph.D. Thesis University of Helsinki Helsinki Finland.2006;21‐45 61‐66. Available at:https://helda.helsinki.fi/handle/10138/20335"
},
{
"DOI": "10.3390/nu9040339",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_49_1"
},
{
"DOI": "10.1002/jmv.26362",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_50_1"
},
{
"DOI": "10.1101/2021.04.13.21255409",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_51_1"
},
{
"DOI": "10.1001/jama.2020.12603",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_52_1"
},
{
"DOI": "10.1101/2020.12.24.20248802",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_53_1"
},
{
"DOI": "10.4193/rhino12.123",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_54_1"
},
{
"DOI": "10.1186/1477‐7525‐4‐79",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_55_1"
},
{
"DOI": "10.21203/rs.3.rs‐108775/v1",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_56_1"
}
],
"reference-count": 55,
"references-count": 55,
"relation": {},
"resource": {
"primary": {
"URL": "https://bpspubs.onlinelibrary.wiley.com/doi/10.1002/prp2.810"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Pharmacology, Toxicology and Pharmaceutics",
"Neurology"
],
"subtitle": [],
"title": "Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: Re‐analysis of randomized trial data",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "9"
}