Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials
et al., Multidisciplinary Respiratory Medicine, doi:10.1186/2049-6958-9-57, Nov 2014
Analysis of 2 RCTs concluding that carrageenan nasal spray reduced duration of virus-confirmed common colds, increased viral clearance, and reduced relapses.
Koenighofer et al., 12 Nov 2014, peer-reviewed, 8 authors.
Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials
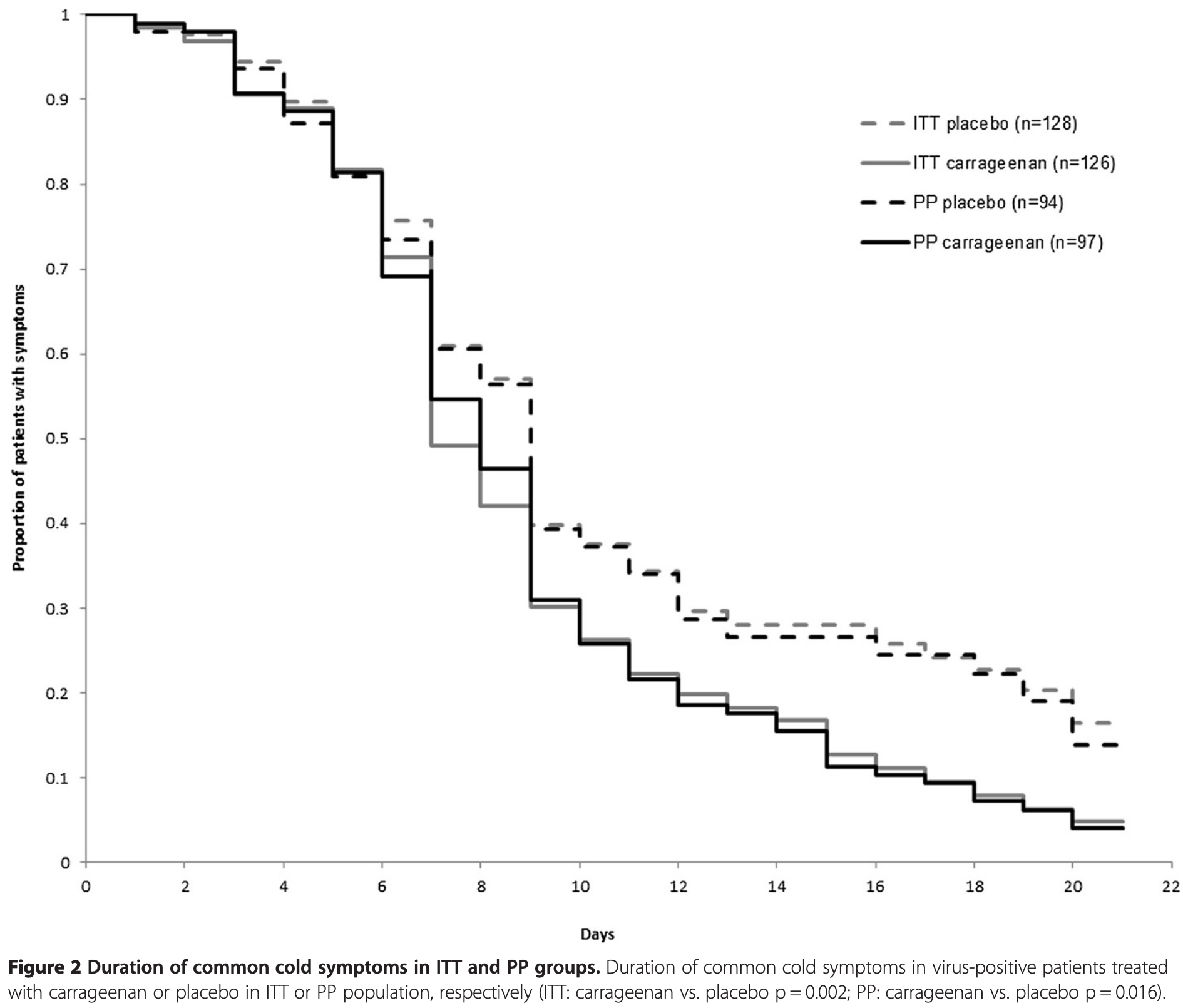
Background: Clinical trials applying iota-carrageenan nasal spray have previously shown to reduce duration of virus-confirmed common cold. The present study pooled data of two similar clinical trials to provide further evidence for the antiviral effectiveness of carrageenan. Methods: Individual patient data were analyzed from two randomized double blind placebo controlled trials assessing the therapeutic effectiveness of carrageenan nasal spray in acute common cold. Patients with virus-confirmed common cold (n = 254, verum 126, placebo 128) were included and the following parameters were appraised: duration of disease, number of patients with relapses, number of respiratory viruses and viral titers at inclusion (visit 1) compared to days 3-5 (visit 2). Results: Carrageenan treated patients showed a significant reduction in duration of disease of almost 2 days (p < 0.05) as well as significantly fewer relapses during 21 days of observation period (p < 0.05). The virus clearance between visit 1 and visit 2 was significantly more pronounced in the carrageenan group (p < 0.05). In both studies, virus-confirmed common cold was caused by three main virus subtypes: human rhinovirus (46%), human coronavirus (25%) and influenza A (14%) virus. Carrageenan nasal spray showed significant antiviral efficacy in all three virus subgroups, the highest effectiveness was observed in human corona virus-infected patients. The reduced duration of disease was 3 days (p < 0.01) and the number of relapses was three times less (p < 0.01) in carrageenan treated corona-virus-infected patients compared to control patients. Conclusions: Administration of carrageenan nasal spray in children as well as in adults suffering from virus-confirmed common cold reduced duration of disease, increased viral clearance and reduced relapses of symptoms. Carrageenan nasal spray appeared as an effective treatment of common cold in children and adults.
Competing interests The authors declare that they have no competing interests.
Authors' contributions All authors read and approved the final manuscript.
References
Ahanchian, Jones, Chen, Sly, Respiratory viral infections in children with asthma: do they matter and can we prevent them?, BMC Pediatr
Bertino, Cost burden of viral respiratory infections: issues for formulary decision makers, Am J Med
Buck, Thompson, Roberts, Muller, Lowy et al., Carrageenan is a potent inhibitor of papilloma virus infection, PLoS Pathog
Chiu, Chan, Tsai, Li, Wu, Prevention of human enterovirus 71 infection by kappa carrageenan, Antiviral Res
Dhariwal, Edwards, Johnston, Anti-viral agents: potential utility in exacerbations of asthma, Curr Opin Pharmacol
Eccles, Martensson, Chen, Effects of intranasal xylometazoline, alone or in combination with ipratropium, in patients with common cold, Curr Med Res Opin
Fazekas, Eickhoff, Pruckner, Vollnhofer, Fischmeister et al., Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold, BMC Complement Altern Med
Fendrick, Monto, Nightengale, Sarnes, The economic burden of non-influenza-related viral respiratory tract infection in the United States, Arch Intern Med
Fox, Hall, Cooney, Luce, Kronmal, The Seattle virus watch. II. Objectives, study population and its observation, data processing and summary of illnesses, Am J Epidemiol
Garibaldi, Epidemiology of community-acquired respiratory tract infections in adults. Incidence, etiology, and impact, Am J Med
Goldmann, Epidemiology and prevention of pediatric viral respiratory infections in health-care institutions, Emerg Infect Dis
Gonzalez, Alarcon, Carrasco, Polysaccharides as antiviral agents: antiviral activity of carrageenan, Antimicrob Agents Chemother
Grassauer, Weinmüllner, Meier, Pretsch, Prieschl-Grassauer et al., Iota-Carrageenan is a potent inhibitor of rhinovirus infection, Virol J
Hershenson, Rhinovirus-induced exacerbations of asthma and COPD, Scientifica
Jackson, Dowling, Spiesman, Boand, Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity, AMA Arch Intern Med
Leibbrandt, Meier, König-Schuster, Weinmüllner, Kalthoff et al., Iota-Carrageenan is a potent inhibitor of influenza a virus infection, PLoS One
Ludwig, Enzenhofer, Schneider, Rauch, Bodenteich et al., Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial, Respir Res
Maguire, Zacharopoulos, Phillips, Carrageenan-based nonoxynol-9 spermicides for prevention of sexually transmitted infections, Sex Transm Dis
Makela, Puhakka, Ruuskanen, Leinonen, Saikku et al., Viruses and bacteria in the etiology of the common cold, J Clin Microbiol
Mchugh, A guide to seaweed industry, FAO Fish Tech Pap
Monto, Fendrick, Sarnes, Respiratory illness caused by picornavirus infection: a review of clinical outcomes, Clin Ther
Monto, Sullivan, Acute respiratory illness in the community. Frequency of illness and the agents involved, Epidemiol Infect
Wark, Tooze, Powell, Parsons, Viral and bacterial infection in acute asthma and chronic obstructive pulmonary disease increases the risk of readmission, Respirology
DOI record:
{
"DOI": "10.1186/2049-6958-9-57",
"ISSN": [
"2049-6958"
],
"URL": "http://dx.doi.org/10.1186/2049-6958-9-57",
"alternative-id": [
"2049-6958-9-57"
],
"author": [
{
"affiliation": [],
"family": "Koenighofer",
"given": "Martin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lion",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bodenteich",
"given": "Angelika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prieschl-Grassauer",
"given": "Eva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grassauer",
"given": "Andreas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Unger",
"given": "Hermann",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mueller",
"given": "Christian A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fazekas",
"given": "Tamás",
"sequence": "additional"
}
],
"container-title": "Multidisciplinary Respiratory Medicine",
"container-title-short": "Multidisciplinary Respiratory Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2014,
11,
12
]
],
"date-time": "2014-11-12T10:01:34Z",
"timestamp": 1415786494000
},
"deposited": {
"date-parts": [
[
2024,
1,
19
]
],
"date-time": "2024-01-19T16:17:54Z",
"timestamp": 1705681074000
},
"indexed": {
"date-parts": [
[
2024,
5,
8
]
],
"date-time": "2024-05-08T13:51:54Z",
"timestamp": 1715176314143
},
"is-referenced-by-count": 69,
"issue": "1",
"issued": {
"date-parts": [
[
2014
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2014
]
]
}
},
"language": "en",
"member": "27750",
"original-title": [],
"page": "57",
"prefix": "10.5826",
"published": {
"date-parts": [
[
2014
]
]
},
"published-print": {
"date-parts": [
[
2014
]
]
},
"publisher": "Mattioli1885",
"reference": [
{
"DOI": "10.3201/eid0702.010220",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B1"
},
{
"DOI": "10.1016/S0149-2918(01)80133-8",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B2"
},
{
"DOI": "10.1017/S0950268800050779",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B3"
},
{
"DOI": "10.1001/archinte.163.4.487",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B5"
},
{
"DOI": "10.1128/AAC.31.9.1388",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B7"
},
{
"DOI": "10.1097/00007435-199810000-00010",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B8"
},
{
"DOI": "10.1371/journal.ppat.0020069",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B9"
},
{
"DOI": "10.1186/1743-422X-5-107",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B10"
},
{
"DOI": "10.1016/j.antiviral.2012.05.009",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B12"
},
{
"DOI": "10.1185/03007991003648015",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B13"
},
{
"DOI": "10.1186/1472-6882-12-147",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B14"
},
{
"DOI": "10.1186/1465-9921-14-124",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B15"
},
{
"DOI": "10.1001/archinte.1958.00260140099015",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B16"
},
{
"DOI": "10.1016/0002-9343(85)90361-4",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B17"
},
{
"DOI": "10.1111/resp.12099",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B21"
},
{
"DOI": "10.1016/j.coph.2013.04.010",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B22"
},
{
"DOI": "10.1186/1471-2431-12-147",
"doi-asserted-by": "publisher",
"key": "10.1186/2049-6958-9-57-B23"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {},
"resource": {
"primary": {
"URL": "http://mrmjournal.biomedcentral.com/articles/10.1186/2049-6958-9-57"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials",
"type": "journal-article",
"volume": "9"
}