Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro
et al., PLoS ONE, doi:10.1371/journal.pone.0237480, Feb 2021
In vitro study showing that SARS-CoV-2 is inhibited by iota-carrageenan in a dose dependent manner and to the same extent as other respiratory viruses for which a clinical benefit has been proven. Authors note that clinical data and post market surveillance data show that iota-carrageenan is well-tolerated with very low reported adverse events.
19 preclinical studies support the efficacy of iota-carrageenan for COVID-19:
1.
Herida et al., Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC155.071.
2.
Krylova et al., Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents, International Journal of Molecular Sciences, doi:10.3390/ijms26136175.
3.
Rohilla et al., Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV-2, Stresses, doi:10.3390/stresses3030039.
4.
Thet, H., The potential of carrageenan for the drug discovery of COVID-19 via molecular docking with angiotensin-converting enzyme 2 (ACE2) and the main protease (Mpro) of SARS-CoV-2, Journal of Bioinformatics and Genomics, doi:10.18454/jbg.2022.18.2.001.
5.
Alsaidi et al., Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model, Marine Drugs, doi:10.3390/md19080418.
6.
Sattari et al., Repositioning Therapeutics for COVID-19: Virtual Screening of the Potent Synthetic and Natural Compounds as SARS-CoV-2 3CLpro Inhibitors, Research Square, doi:10.21203/rs.3.rs-37994/v1.
7.
Hoffmann et al., Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity, Biomacromolecules, doi:10.1021/acs.biomac.5c00576.
8.
Yathindranath et al., Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection, International Journal of Nanomedicine, doi:10.2147/IJN.S448005.
9.
Setz et al., Iota-Carrageenan Inhibits Replication of the SARS-CoV-2 Variants of Concern Omicron BA.1, BA.2 and BA.5, Nutraceuticals, doi:10.3390/nutraceuticals3030025.
10.
Meister et al., Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2, Journal of Hospital Infection, doi:10.1016/j.jhin.2021.10.019.
11.
Bovard et al., Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochemistry and Biophysics Reports, doi:10.1016/j.bbrep.2021.101187.
12.
Fröba et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, International Journal of Molecular Sciences, doi:10.3390/ijms222413202.
13.
Varese et al., Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model, bioRxiv, doi:10.1101/2021.04.27.441512.
14.
Morokutti-Kurz et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE, doi:10.1371/journal.pone.0237480.
15.
Song et al., Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food & Function, doi:10.1039/D0FO02017F.
Morokutti-Kurz et al., 17 Feb 2021, peer-reviewed, 8 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro
PLOS ONE, doi:10.1371/journal.pone.0237480
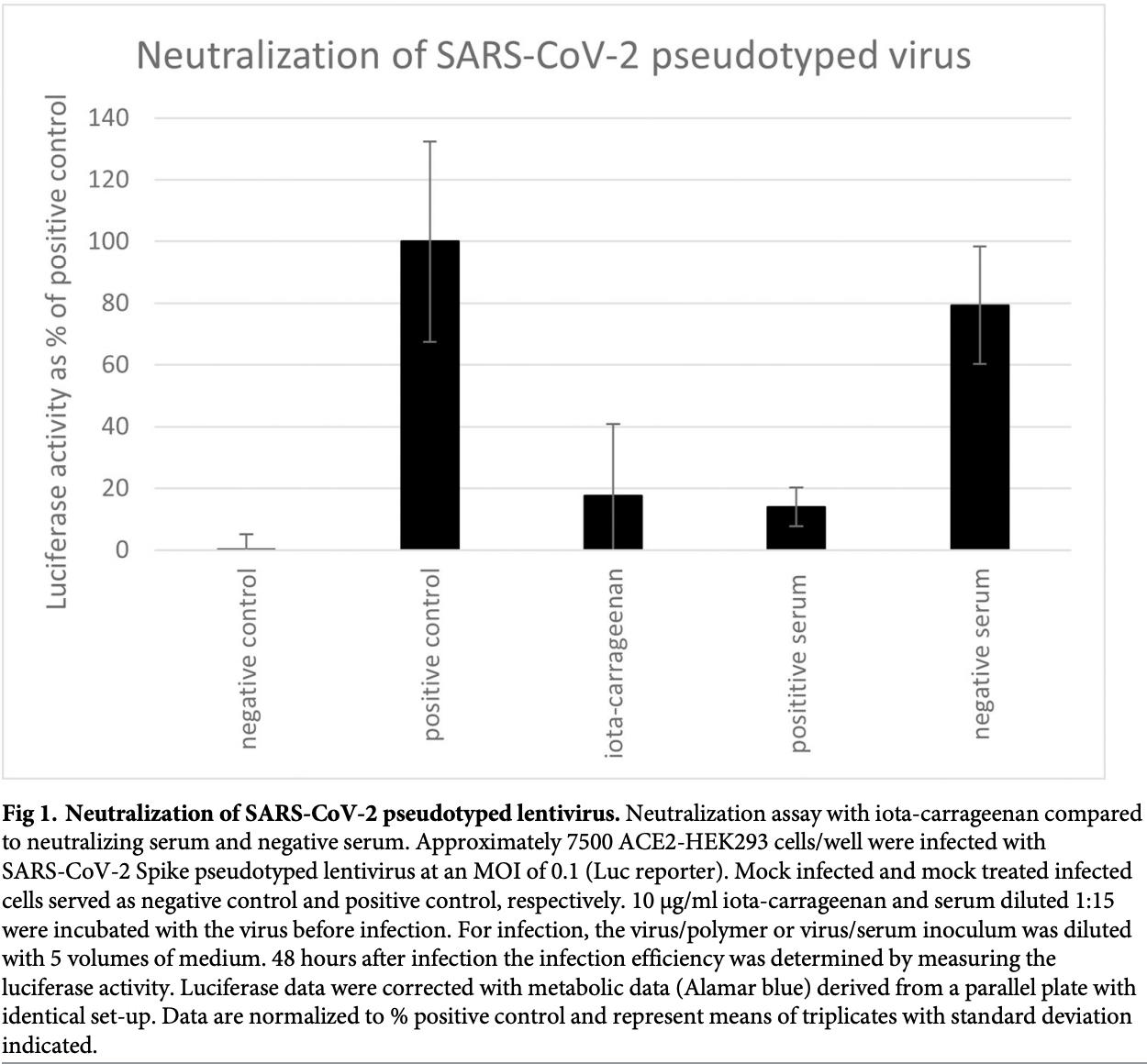
In the absence of a vaccine and other effective prophylactic or therapeutic countermeasures the severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) remains a significant public health threat. Attachment and entry of coronaviruses including SARS-CoV-2 is mainly mediated by the spike glycoprotein. Here, we show that iota-carrageenan can inhibit the cell entry of the SARS-CoV-2 spike pseudotyped lentivirus in a dose dependent manner. SARS-CoV-2 spike pseudotyped lentivirus particles were efficiently neutralized with an IC 50 value of 2.6 μg/ml iota-carrageenan. Experiments with patient isolated wild type SARS-CoV-2 virus showed an inhibition of replication in a similar range. In vitro data on iotacarrageenan against various Rhino-and endemic Coronaviruses showed similar IC 50 values and translated readily into clinical effectiveness when a nasal spray containing iota-carrageenan demonstrated a reduction of severity and duration of symptoms of common cold caused by various respiratory viruses. Accordingly, our in vitro data on SARS-CoV-2 spike pseudotyped lentivirus and replication competent SARS-CoV-2 suggest that administration of iota-carrageenan may be an effective and safe prophylaxis or treatment for SARS-CoV-2 infections.
Supporting information S1
Author Contributions Conceptualization: Martina Morokutti-Kurz, Andreas Grassauer, Ulrich Schubert, Eva Prieschl-Grassauer. Data curation: Martina Morokutti-Kurz, Maria Fro ¨ba, Philipp Graf, Maximilian Große, Janina Auth, Ulrich Schubert, Eva Prieschl-Grassauer. Formal analysis: Martina Morokutti-Kurz, Maria Fro ¨ba, Philipp Graf, Maximilian Große, Andreas Grassauer, Janina Auth. Funding acquisition: Andreas Grassauer, Ulrich Schubert, Eva Prieschl-Grassauer. Investigation: Maria Fro ¨ba, Philipp Graf, Maximilian Große, Janina Auth. Methodology: Martina Morokutti-Kurz, Maria Fro ¨ba, Janina Auth, Ulrich Schubert. Project administration: Janina Auth, Eva Prieschl-Grassauer. Resources: Andreas Grassauer, Janina Auth, Ulrich Schubert, Eva Prieschl-Grassauer. Software: Eva Prieschl-Grassauer. Supervision: Ulrich Schubert. Validation: Eva Prieschl-Grassauer. Visualization: Eva Prieschl-Grassauer. Writing -original draft: Martina Morokutti-Kurz, Andreas Grassauer, Ulrich Schubert, Eva Prieschl-Grassauer. Writing -review & editing: Martina Morokutti-Kurz, Maria Fro ¨ba, Maximilian Große, Andreas Grassauer, Ulrich Schubert, Eva Prieschl-Grassauer.
References
Corman, Landt, Kaiser, Molenkamp, Meijer et al., Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Euro Surveill, doi:10.2807/1560-7917.ES.2020.25.3.2000045
Dogan, Kozhaya, Placek, Gunter, Yigit et al., Novel SARS-CoV-2 specific antibody and neutralization assays reveal wide range of humoral immune response during COVID-19, medRxiv, doi:10.1101/2020.07.07.20148106
Eccles, Iota-Carrageenan as an Antiviral Treatment for the Common Cold, The Open Virology Journal
Eccles, Meier, Jawad, Weinmu ¨llner R, Grassauer et al., Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold, Respir Res, doi:10.1186/1465-9921-11-108
Eccles, Winther, Johnston, Robinson, Trampisch et al., Efficacy and safety of iotacarrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial, Respir Res, doi:10.1186/s12931-015-0281-8
Fazekas, Eickhoff, Pruckner, Vollnhofer, Fischmeister et al., Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold, BMC Complement Altern Med, doi:10.1186/1472-6882-12-147
Gates, Responding to Covid-19-A Once-in-a-Century Pandemic, N Engl J Med, doi:10.1056/NEJMp2003762
Grassauer, Weinmu ¨llner R, Meier, Pretsch, Prieschl-Grassauer et al., Iota-Carrageenan is a potent inhibitor of rhinovirus infection, Virol J, doi:10.1186/1743-422X-5-107
Große, Ruetalo, Businger, Rheber, Setz et al., Evidence That Quinine Exhibits Antiviral Activity against SARS-CoV-2 Infection In Vitro, Preprints
Hoffmann, Kleine-Weber, Schroeder, Kru ¨ger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Koenighofer, Lion, Bodenteich, Prieschl-Grassauer, Grassauer et al., Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials, Multidiscip Respir Med, doi:10.1186/2049-6958-9-57
Leibbrandt, Meier, Ko ¨nig-Schuster, Weinmu ¨llner R, Kalthoff et al., Iota-Carrageenan Is a Potent Inhibitor of Influenza A Virus Infection, PLoS One, doi:10.1371/journal.pone.0014320
Long, Tang, Shi, Li, Deng et al., Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections, Nat Med, doi:10.1038/s41591-020-0965-6
Ludwig, Enzenhofer, Schneider, Rauch, Bodenteich et al., Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial, Respir Res, doi:10.1186/1465-9921-14-124
Mcintosh, Dees, Becker, Kapikian, Chanock, Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease, Proc Natl Acad Sci U.S.A, doi:10.1073/pnas.57.4.933
Morokutti-Kurz, Konig-Schuster, Koller, Graf, Graf et al., The Intranasal Application of Zanamivir and Carrageenan Is Synergistically Active against Influenza A Virus in the Murine Model 4944, PLoS ONE, doi:10.1371/journal.pone.0128794
Perlman, Another Decade, Another Coronavirus, N Engl J Med, doi:10.1056/NEJMe2001126
Reed, Muench, A simple method of estimating fifty percent endpoints, Am J Hyg
Wu, Zhao, Yu, Chen, Song, A new coronavirus associated with human respiratory disease in China, Nature, doi:10.1038/s41586-020-2008-3
Zhu, Zhang, Li, Yang, Song, A Novel Coronavirus from Patients with Pneumonia in China, 2019, N Engl J Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.1371/journal.pone.0237480",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0237480",
"abstract": "<jats:p>In the absence of a vaccine and other effective prophylactic or therapeutic countermeasures the severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) remains a significant public health threat. Attachment and entry of coronaviruses including SARS-CoV-2 is mainly mediated by the spike glycoprotein. Here, we show that iota-carrageenan can inhibit the cell entry of the SARS-CoV-2 spike pseudotyped lentivirus in a dose dependent manner. SARS-CoV-2 spike pseudotyped lentivirus particles were efficiently neutralized with an IC<jats:sub>50</jats:sub> value of 2.6 μg/ml iota-carrageenan. Experiments with patient isolated wild type SARS-CoV-2 virus showed an inhibition of replication in a similar range. In vitro data on iota-carrageenan against various Rhino- and endemic Coronaviruses showed similar IC<jats:sub>50</jats:sub> values and translated readily into clinical effectiveness when a nasal spray containing iota-carrageenan demonstrated a reduction of severity and duration of symptoms of common cold caused by various respiratory viruses. Accordingly, our in vitro data on SARS-CoV-2 spike pseudotyped lentivirus and replication competent SARS-CoV-2 suggest that administration of iota-carrageenan may be an effective and safe prophylaxis or treatment for SARS-CoV-2 infections.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Morokutti-Kurz",
"given": "Martina",
"sequence": "first"
},
{
"affiliation": [],
"family": "Fröba",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Graf",
"given": "Philipp",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Große",
"given": "Maximilian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grassauer",
"given": "Andreas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Auth",
"given": "Janina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schubert",
"given": "Ulrich",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prieschl-Grassauer",
"given": "Eva",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2021,
2,
17
]
],
"date-time": "2021-02-17T18:34:13Z",
"timestamp": 1613586853000
},
"deposited": {
"date-parts": [
[
2021,
2,
17
]
],
"date-time": "2021-02-17T18:34:39Z",
"timestamp": 1613586879000
},
"editor": [
{
"affiliation": [],
"family": "Polyak",
"given": "Stephen J.",
"sequence": "first"
}
],
"funder": [
{
"DOI": "10.13039/501100004955",
"award": [
"880687"
],
"doi-asserted-by": "publisher",
"name": "Österreichische Forschungsförderungsgesellschaft"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
8
]
],
"date-time": "2024-05-08T13:53:49Z",
"timestamp": 1715176429581
},
"is-referenced-by-count": 74,
"issue": "2",
"issued": {
"date-parts": [
[
2021,
2,
17
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2021,
2,
17
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
17
]
],
"date-time": "2021-02-17T00:00:00Z",
"timestamp": 1613520000000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0237480",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0237480",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2021,
2,
17
]
]
},
"published-online": {
"date-parts": [
[
2021,
2,
17
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A Novel Coronavirus from Patients with Pneumonia in China, 2019",
"author": "N Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N Engl J Med",
"key": "pone.0237480.ref001",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2008-3",
"article-title": "A new coronavirus associated with human respiratory disease in China",
"author": "F Wu",
"doi-asserted-by": "crossref",
"first-page": "265",
"journal-title": "Nature",
"key": "pone.0237480.ref002",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1056/NEJMe2001126",
"article-title": "Another Decade, Another Coronavirus",
"author": "S. Perlman",
"doi-asserted-by": "crossref",
"first-page": "760",
"journal-title": "N Engl J Med",
"key": "pone.0237480.ref003",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMp2003762",
"article-title": "Responding to Covid-19—A Once-in-a-Century Pandemic",
"author": "B. Gates",
"doi-asserted-by": "crossref",
"first-page": "1677",
"journal-title": "N Engl J Med",
"key": "pone.0237480.ref004",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor",
"author": "M Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "pone.0237480.ref005",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.2174/1874357902014010009",
"article-title": "Iota-Carrageenan as an Antiviral Treatment for the Common Cold",
"author": "R. Eccles",
"doi-asserted-by": "crossref",
"first-page": "9",
"issue": "14",
"journal-title": "The Open Virology Journal",
"key": "pone.0237480.ref006",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1186/1743-422X-5-107",
"article-title": "Iota-Carrageenan is a potent inhibitor of rhinovirus infection",
"author": "A Grassauer",
"doi-asserted-by": "crossref",
"journal-title": "Virol J",
"key": "pone.0237480.ref007",
"volume": "5",
"year": "2008"
},
{
"DOI": "10.1371/journal.pone.0014320",
"article-title": "Iota-Carrageenan Is a Potent Inhibitor of Influenza A Virus Infection",
"author": "A Leibbrandt",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "pone.0237480.ref008",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.1371/journal.pone.0128794",
"article-title": "The Intranasal Application of Zanamivir and Carrageenan Is Synergistically Active against Influenza A Virus in the Murine Model 4944",
"author": "M Morokutti-Kurz",
"doi-asserted-by": "crossref",
"first-page": "e0128794",
"journal-title": "PLoS ONE",
"key": "pone.0237480.ref009",
"volume": "10",
"year": "2015"
},
{
"DOI": "10.1186/1465-9921-11-108",
"article-title": "Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold",
"author": "R Eccles",
"doi-asserted-by": "crossref",
"first-page": "108",
"journal-title": "Respir Res",
"key": "pone.0237480.ref010",
"volume": "11",
"year": "2010"
},
{
"DOI": "10.1186/1472-6882-12-147",
"article-title": "Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold",
"author": "T Fazekas",
"doi-asserted-by": "crossref",
"first-page": "147",
"journal-title": "BMC Complement Altern Med",
"key": "pone.0237480.ref011",
"volume": "12",
"year": "2012"
},
{
"DOI": "10.1186/2049-6958-9-57",
"article-title": "Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials",
"author": "M Koenighofer",
"doi-asserted-by": "crossref",
"first-page": "57",
"journal-title": "Multidiscip Respir Med",
"key": "pone.0237480.ref012",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.1186/1465-9921-14-124",
"article-title": "Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial",
"author": "M Ludwig",
"doi-asserted-by": "crossref",
"first-page": "124",
"journal-title": "Respir Res",
"key": "pone.0237480.ref013",
"volume": "14",
"year": "2013"
},
{
"DOI": "10.1186/s12931-015-0281-8",
"article-title": "Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial",
"author": "R Eccles",
"doi-asserted-by": "crossref",
"first-page": "121",
"journal-title": "Respir Res",
"key": "pone.0237480.ref014",
"volume": "16",
"year": "2015"
},
{
"article-title": "Evidence That Quinine Exhibits Antiviral Activity against SARS-CoV-2 Infection In Vitro",
"author": "M Große",
"first-page": "2020070102",
"journal-title": "Preprints",
"key": "pone.0237480.ref015",
"year": "2020"
},
{
"article-title": "A simple method of estimating fifty percent endpoints",
"author": "LJ Reed",
"first-page": "493",
"journal-title": "Am J Hyg",
"key": "pone.0237480.ref016",
"year": "1938"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"article-title": "Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR",
"author": "VM Corman",
"doi-asserted-by": "crossref",
"journal-title": "Euro Surveill",
"key": "pone.0237480.ref017",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1073/pnas.57.4.933",
"article-title": "Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease",
"author": "K McIntosh",
"doi-asserted-by": "crossref",
"first-page": "933",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "pone.0237480.ref018",
"volume": "57",
"year": "1967"
},
{
"article-title": "Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections",
"author": "Q-X Long",
"journal-title": "Nat Med",
"key": "pone.0237480.ref019",
"year": "2020"
},
{
"article-title": "Novel SARS-CoV-2 specific antibody and neutralization assays reveal wide range of humoral immune response during COVID-19",
"author": "M Dogan",
"journal-title": "medRxiv",
"key": "pone.0237480.ref020",
"year": "2020"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0237480"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "16"
}