Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta
et al., International Journal of Molecular Sciences, doi:10.3390/ijms222413202, Dec 2021
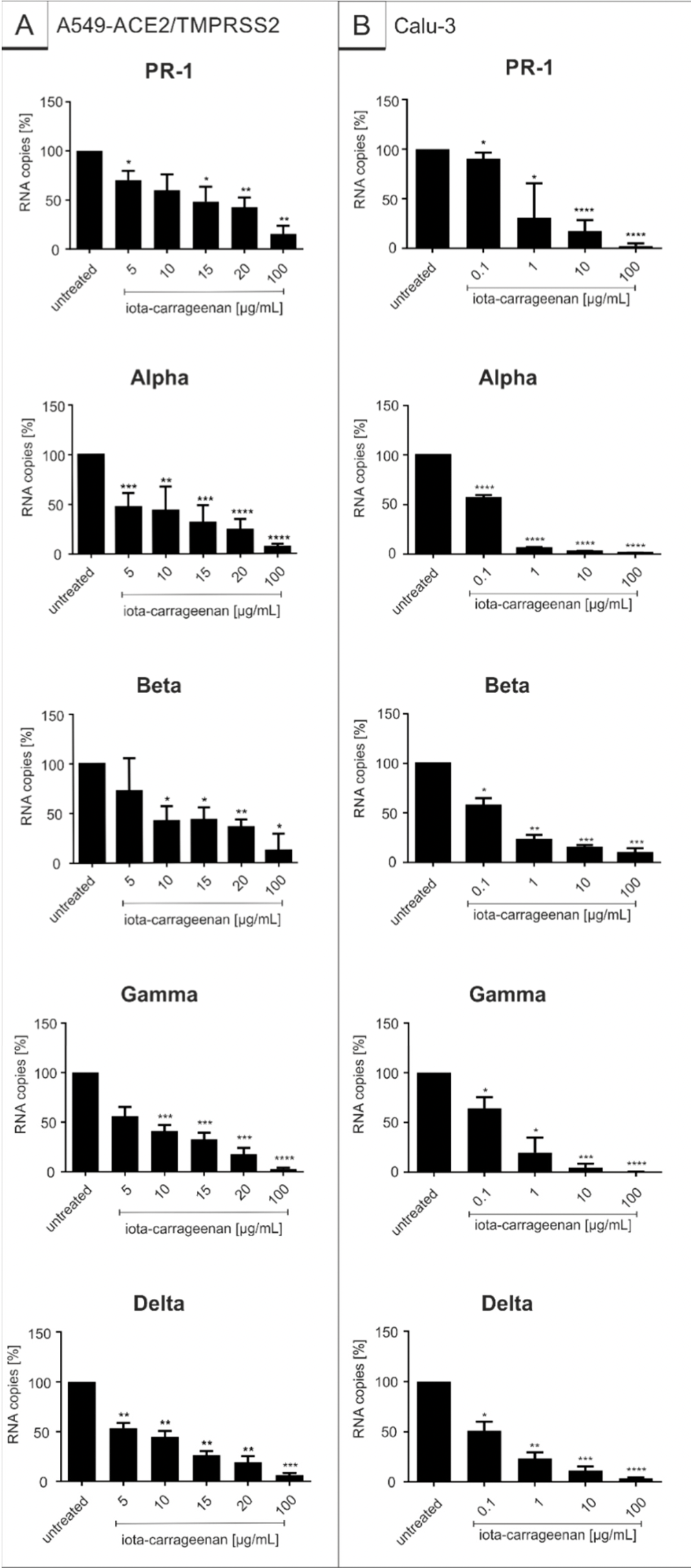
In vitro study of iota-, lambda-, and kappa-carrageenan sulfated polysaccharides extracted from red seaweed on SARS-CoV-2 Wuhan type and variants Alpha, Beta, Gamma and Delta, showing that all three carrageenan types had antiviral activity. Iota-carrageenan had comparable IC50 values against all variants. Authors conclude that iota-carrageenan might be effective for prophylaxis and treatment of SARS-CoV-2 for existing and potentially future variants.
19 preclinical studies support the efficacy of iota-carrageenan for COVID-19:
1.
Herida et al., Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC155.071.
2.
Krylova et al., Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents, International Journal of Molecular Sciences, doi:10.3390/ijms26136175.
3.
Rohilla et al., Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV-2, Stresses, doi:10.3390/stresses3030039.
4.
Thet, H., The potential of carrageenan for the drug discovery of COVID-19 via molecular docking with angiotensin-converting enzyme 2 (ACE2) and the main protease (Mpro) of SARS-CoV-2, Journal of Bioinformatics and Genomics, doi:10.18454/jbg.2022.18.2.001.
5.
Alsaidi et al., Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model, Marine Drugs, doi:10.3390/md19080418.
6.
Sattari et al., Repositioning Therapeutics for COVID-19: Virtual Screening of the Potent Synthetic and Natural Compounds as SARS-CoV-2 3CLpro Inhibitors, Research Square, doi:10.21203/rs.3.rs-37994/v1.
7.
Hoffmann et al., Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity, Biomacromolecules, doi:10.1021/acs.biomac.5c00576.
8.
Yathindranath et al., Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection, International Journal of Nanomedicine, doi:10.2147/IJN.S448005.
9.
Setz et al., Iota-Carrageenan Inhibits Replication of the SARS-CoV-2 Variants of Concern Omicron BA.1, BA.2 and BA.5, Nutraceuticals, doi:10.3390/nutraceuticals3030025.
10.
Meister et al., Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2, Journal of Hospital Infection, doi:10.1016/j.jhin.2021.10.019.
11.
Bovard et al., Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochemistry and Biophysics Reports, doi:10.1016/j.bbrep.2021.101187.
12.
Fröba et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, International Journal of Molecular Sciences, doi:10.3390/ijms222413202.
13.
Varese et al., Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model, bioRxiv, doi:10.1101/2021.04.27.441512.
14.
Morokutti-Kurz et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE, doi:10.1371/journal.pone.0237480.
15.
Song et al., Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food & Function, doi:10.1039/D0FO02017F.
Fröba et al., 8 Dec 2021, peer-reviewed, 14 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta
International Journal of Molecular Sciences, doi:10.3390/ijms222413202
The COVID-19 pandemic continues to spread around the world and remains a major public health threat. Vaccine inefficiency, vaccination breakthroughs and lack of supply, especially in developing countries, as well as the fact that a non-negligible part of the population either refuse vaccination or cannot be vaccinated due to age, pre-existing illness or non-response to existing vaccines intensify this issue. This might also contribute to the emergence of new variants, being more efficiently transmitted, more virulent and more capable of escaping naturally acquired and vaccine-induced immunity. Hence, the need of effective and viable prevention options to reduce viral transmission is of outmost importance. In this study, we investigated the antiviral effect of iota-, lambda-and kappa-carrageenan, sulfated polysaccharides extracted from red seaweed, on SARS-CoV-2 Wuhan type and the spreading variants of concern (VOCs) Alpha, Beta, Gamma and Delta. Carrageenans as part of broadly used nasal and mouth sprays as well as lozenges have the potential of first line defense to inhibit the infection and transmission of SARS-CoV-2. Here, we demonstrate by using a SARS-CoV-2 spike pseudotyped lentivirus particles (SSPL) system and patient-isolated SARS-CoV-2 VOCs to infect transgenic A549ACE2/TMPRSS2 and Calu-3 human lung cells that all three carrageenan types exert antiviral activity. Iota-carrageenan exhibits antiviral activity with comparable IC 50 values against the SARS-CoV-2 Wuhan type and the VOCs. Altogether, these results indicate that iota-carrageenan might be effective for prophylaxis and treatment of SARS-CoV-2 infections independent of the present and potentially future variants.
Supplementary Materials: The following are available online at https://www.mdpi.com/article/ 10.3390/ijms222413202/s1. Author Contributions: Conceptualization, M.F., M.G., C.S., A.G. and U.S.; methodology, M.F., M.G., C.S., P.R., J.A., L.S., J.M., N.R., M.S., M.M.-K., P.G., E.P.-G. and U.S.; validation, M.F., M.G., C.S., M.S., M.M.-K., E.P.-G., A.G. and U.S.; investigation, M.F., M.G., C.S., M.S., P.R., J.A., L.S., J.M., N.R. and M.M.-K., P.G.; data curation, U.S.; writing-original draft preparation, M.F., M.G., C.S, J.M., M.M.-K. and U.S.; writing-review and editing, M.F., M.G., C.S., P.R., J.A., L.S., J.M., N.R., M.S., M.
Conflicts of Interest: The authors have read the journal's policy and declare that the authors Andreas Grassauer, Eva Prieschl-Grassauer, Philipp Graf, and Martina Morokutti-Kurz are employed by Marinomed Biotech AG. Andreas Grassauer and Eva Prieschl-Grassauer are co-founders of Marinomed Biotech AG. Andreas Grassauer, Eva Prieschl-Grassauer and Martina Morokutti-Kurz are inventors of a patent submission related to the content of the manuscript; the number of this patent application is EP20186334. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
Aguiar, Tremblay, Mansfield, Woody, Lobb et al., Gene expression and in situ protein profiling of candidate SARS-CoV-2 receptors in human airway epithelial cells and lung tissue, Eur. Respir. J, doi:10.1183/13993003.01123-2020
Alsaidi, Cornejal, Mahoney, Melo, Verma et al., Griffithsin and carrageenan combination results in antiviral synergy against SARS-CoV-1 and 2 in a pseudoviral model, Mar. Drugs, doi:10.3390/md19080418
Andrei, Snoeck, Goubau, Desmyter, De Clercq, Comparative activity of various compounds against clinical strains of herpes simplex virus, Eur. J. Clin. Microbiol. Infect. Dis, doi:10.1007/BF01967066
Aune, Plant foods, antioxidant biomarkers, and the risk of cardiovascular disease, cancer, and mortality: A review of the evidence, Adv. Nutr, doi:10.1093/advances/nmz042
Auth, Fröba, Große, Rauch, Ruetalo et al., Lectin from Triticum vulgaris (WGA) inhibits infection with SARS-CoV-2 and its variants of concern alpha and beta, Int. J. Mol. Sci, doi:10.3390/ijms221910205
Becker, Dulovic, Junker, Ruetalo, Kaiser et al., Immune response to SARS-CoV-2 variants of concern in vaccinated individuals, Nat. Commun, doi:10.1038/s41467-021-23473-6
Bhattacharyya, Liu, Zhang, Jam, Dudeja et al., Carrageenan-induced innate immune response is modified by enzymes that hydrolyze distinct galactosidic bonds, J. Nutr. Biochem
Buck, Thompson, Roberts, Müller, Lowy et al., Carrageenan is a potent inhibitor of papillomavirus infection, PLoS Pathog, doi:10.1371/journal.ppat.0020069
Campo, Kawano, Silva, Carvalho, Carrageenans: Biological properties, chemical modifications and structural analysis-A review, Carbohydr. Polym, doi:10.1016/j.carbpol.2009.01.020
Chahla, Medina Ruiz, Ortega, Morales, Barreiro et al., A Randomized trial-Intensive treatment based in ivermectin and iota-carrageenan as pre-exposure prophylaxis for COVID-19 in healthcare agents, medRxiv
Cherian, Potdar, Jadhav, Yadav, Gupta et al., Convergent evolution of SARS-CoV-2 spike mutations, L452R, E484Q and P681R
Collier, De Marco, Ferreira, Meng, Datir et al., Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies, Nature, doi:10.1038/s41586-021-03412-7
Corman, Landt, Kaiser, Molenkamp, Meijer et al., Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull, doi:10.2807/1560-7917.ES.2020.25.3.2000045
Davies, Abbott, Barnard, Jarvis, Kucharski et al., Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England, Science
Eccles, Iota-carrageenan as an antiviral treatment for the common cold, Open Virol. J, doi:10.2174/1874357902014010009
Eccles, Meier, Jawad, Weinmullner, Grassauer et al., Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: A randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold, Respir. Res, doi:10.1186/1465-9921-11-108
Eccles, Winther, Johnston, Robinson, Trampisch et al., Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: The ICICC trial, Respir. Res, doi:10.1186/s12931-015-0281-8
Fazekas, Eickhoff, Pruckner, Vollnhofer, Fischmeister et al., Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold, BMC Complement. Altern. Med, doi:10.1186/1472-6882-12-147
Figueroa, Lombardo, Dogliotti, Flynn, Giugliano et al., Efficacy of a nasal spray containing iota-carrageenan in the postexposure prophylaxis of COVID-19 in hospital personnel dedicated to patients care with COVID-19 disease, Int. J. Gen. Med, doi:10.2147/IJGM.S328486
Frediansyah, The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: A critical review, Clin. Epidemiol. Glob. Health, doi:10.1016/j.cegh.2021.100826
Galloway, Paul, Maccannell, Johansson, Brooks et al., Emergence of SARS-CoV-2 B.1.1.7 lineage-United States, 29, MMWR Morb. Mortal. Wkly. Rep, doi:10.15585/mmwr.mm7003e2
Gesellschaft ; Hygiene, Anwendung von Gurgel-Lösungen und Nasensprays-zwei weitere Verbündete in der Abwehr von Viralen Erkältungskrankheitenauch in Covid-19 Zeiten
Girond, Crance, Van Cuyck-Gandre, Renaudet, Deloince, Antiviral activity of carrageenan on hepatitis A virus replication in cell culture, Res. Virol, doi:10.1016/0923-2516(91)90011-Q
Graf, Bernkop-Schnürch, Egyed, Koller, Prieschl-Grassauer et al., Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis, Int. J. Gen. Med, doi:10.2147/IJGM.S167123
Grassauer, Weinmuellner, Meier, Pretsch, Prieschl-Grassauer et al., Iota-carrageenan is a potent inhibitor of rhinovirus infection, Virol. J, doi:10.1186/1743-422X-5-107
Große, Ruetalo, Layer, Hu, Businger et al., Quinine inhibits infection of human cell lines with SARS-CoV-2, Viruses, doi:10.3390/v13040647
Hebar, Koller, Seifert, Chabicovsky, Bodenteich et al., Non-clinical safety evaluation of intranasal iota-carrageenan, PLoS ONE, doi:10.1371/journal.pone.0122911
Hemilä, Chalker, Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: Re-analysis of randomized trial data, Pharmacol. Res. Perspect, doi:10.1002/prp2.810
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hou, Okuda, Edwards, Martinez, Asakura et al., SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, Cell, doi:10.1016/j.cell.2020.05.042
Jang, Shin, Lee, Kwon, Shin et al., Antiviral activity of lambdacarrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2, Sci. Rep, doi:10.1038/s41598-020-80896-9
Kemp, Meng, Ferriera, Datir, Harvey et al., Recurrent emergence and transmission of a SARS-CoV-2 spike deletion H69/V70, bioRxiv
Kim, Jang, Soh, Lee, Lee, The Impact on infectivity and neutralization efficiency of SARS-CoV-2 lineage B.1.351 pseudovirus, Viruses, doi:10.3390/v13040633
Koenighofer, Lion, Bodenteich, Prieschl-Grassauer, Grassauer et al., Carrageenan nasal spray in virus confirmed common cold: Individual patient data analysis of two randomized controlled trials, Multidiscip. Respir. Med, doi:10.1186/2049-6958-9-57
Korber, Fischer, Gnanakaran, Yoon, Theiler et al., Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus, Cell, doi:10.1016/j.cell.2020.06.043
Leibbrandt, Meier, König-Schuster, Weinmüllner, Kalthoff et al., Iota-carrageenan is a potent inhibitor of influenza A virus infection, PLoS ONE, doi:10.1371/journal.pone.0014320
Levendosky, Mizenina, Martinelli, Jean-Pierre, Kizima et al., griffithsin and carrageenan combination to target herpes simplex virus 2 and human papillomavirus, Antimicrob. Agents Chemother, doi:10.1128/AAC.01816-15
Little, Read, Amlôt, Chadborn, Rice et al., Reducing risks from coronavirus transmission in the home-the role of viral load, BMJ, doi:10.1136/bmj.m1728
Liu, Zhan, Wan, Wang, Wang, Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects, Carbohydr. Polym, doi:10.1016/j.carbpol.2014.11.063
Ludwig, Enzenhofer, Schneider, Rauch, Bodenteich et al., Efficacy of a Carrageenan nasal spray in patients with common cold: A randomized controlled trial, Respir. Res, doi:10.1186/1465-9921-14-124
Luo, Shao, Nie, Wei, Li et al., Antitumor and adjuvant activity of λ-carrageenan by stimulating immune response in cancer immunotherapy, Sci. Rep, doi:10.1038/srep11062
Mani, Johnson, Steel, Broszczak, Neilsen et al., Natural product-derived phytochemicals as potential agents against coronaviruses: A review, Virus Res, doi:10.1016/j.virusres.2020.197989
Meselson, Droplets and aerosols in the transmission of SARS-CoV-2, N. Engl. J. Med, doi:10.1056/NEJMc2009324
Mitsuya, Looney, Kuno, Ueno, Wong-Staal et al., Dextran sulfate suppression of viruses in the HIV family: Inhibition of virion binding to CD4+ cells, Science, doi:10.1126/science.2452480
Morokutti-Kurz, Fröba, Graf, Große, Grassauer et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE
Morokutti-Kurz, Graf, Prieschl-Grassauer, Amylmetacresol/2,4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat, Int. J. Gen. Med, doi:10.2147/IJGM.S120665
Morokutti-Kurz, König-Schuster, Koller, Graf, Graf et al., The intranasal application of zanamivir and carrageenan is synergistically active against influenza a virus in the murine model, PLoS ONE, doi:10.1371/journal.pone.0128794
Morokutti-Kurz, Unger-Manhart, Graf, Rauch, Kondar et al., The saliva of probands sucking an iota-carrageenan containing lozenge inhibits viral binding and replication of the most predominant common cold viruses and SARS-CoV-2, Int. J. Gen. Med, doi:10.2147/IJGM.S325861
Moulard, Lortat-Jacob, Mondor, Roca, Wyatt et al., Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120, J. Virol, doi:10.1128/JVI.74.4.1948-1960.2000
Mwenda, Saasa, Sinyange, Busby, Chipimo et al., Detection of B.1.351 SARS-CoV-2 variant strain-Zambia, december 2020, MMWR Morb. Mortal. Wkly. Rep
Nyberg, Ekblad, Bergström, Freeman, Parish et al., The low molecular weight heparan sulfate-mimetic, PI-88, inhibits cell-to-cell spread of herpes simplex virus, Antivir. Res, doi:10.1016/j.antiviral.2004.01.001
Ou, Liu, Lei, Li, Mi et al., Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV, Nat. Commun, doi:10.1038/s41467-020-15562-9
Planas, Veyer, Baidaliuk, Staropoli, Guivel-Benhassine et al., Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization, Nature, doi:10.1038/s41586-021-03777-9
Reed, Muench, A simple method of estimating fifty per cent endpoints12, Am. J. Epidemiol, doi:10.1093/oxfordjournals.aje.a118408
Sanda, Morrison, Goldman, N-and O-glycosylation of the SARS-CoV-2 spike protein, Anal. Chem
Schütz, Conzelmann, Fois, Groß, Weil et al., Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures, Am. J. Physiol. Lung Cell. Mol. Physiol, doi:10.1152/ajplung.00552.2020
Seyedpour, Khodaei, Loghman, Seyedpour, Kisomi et al., Targeted therapy strategies against SARS-CoV-2 cell entry mechanisms: A systematic review of in vitro and in vivo studies, J. Cell. Physiol, doi:10.1002/jcp.30032
Shrestha, Marco Canosa, Nowacki, Procop, Vogel et al., Distribution of transmission potential during nonsevere COVID-19 illness, Clin. Infect. Dis, doi:10.1093/cid/ciaa886
Talarico, Damonte, Interference in dengue virus adsorption and uncoating by carrageenans, Virology
Tegally, Wilkinson, Giovanetti, Iranzadeh, Fonseca et al., Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa, medRxiv
Uemura, Sasaki, Sanaki, Toba, Takahashi et al., MRC5 cells engineered to express ACE2 serve as a model system for the discovery of antivirals targeting SARS-CoV-2, Sci. Rep, doi:10.1038/s41598-021-84882-7
Usfda, Part 172-Food Additives Permitted for Direct Addition to Food for Human Consumption
Volz, Mishra, Chand, Barrett, Johnson et al., Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England, Nature, doi:10.1038/s41586-021-03470-x
Wan, Shang, Graham, Baric, Li, Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus, J. Virol, doi:10.1128/JVI.00127-20
Wang, Casner, Nair, Wang, Yu et al., Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization, bioRxiv, doi:10.1016/j.chom.2021.04.007
Watanabe, Allen, Wrapp, Mclellan, Crispin, Site-specific glycan analysis of the SARS-CoV-2 spike, Science, doi:10.1126/science.abb9983
Wibmer, Ayres, Hermanus, Madzivhandila, Kgagudi et al., SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma, Nat. Med, doi:10.1038/s41591-021-01285-x
Yeh, Chen, Immunomodulation by carrageenans in the white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus, Aquaculture, doi:10.1016/j.aquaculture.2008.01.034
Yermak, Barabanova, Aminin, Davydova, Sokolova et al., Effects of structural peculiarities of carrageenans on their immunomodulatory and anticoagulant activities, Carbohydr. Polym, doi:10.1016/j.carbpol.2011.08.053
Younes, Aggett, Aguilar, Crebelli, Filipič et al., Re-evaluation of carrageenan (E • 407) and processed Eucheuma seaweed (E • 407a) as food additives, EFSA J
Yuan, Song, Li, Li, Dai, Immunomodulation and antitumor activity of kappa-carrageenan oligosaccharides, Cancer Lett, doi:10.1016/j.canlet.2005.11.032
Zhao, Zhang, Xu, Huang, Zhong et al., Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China, J. Med. Microbiol, doi:10.1099/jmm.0.05320-0
Zhou, Sheng, Yao, Wang, Effect of low molecular lambda-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu, Pharmacol. Res, doi:10.1016/j.phrs.2005.09.009
DOI record:
{
"DOI": "10.3390/ijms222413202",
"ISSN": [
"1422-0067"
],
"URL": "http://dx.doi.org/10.3390/ijms222413202",
"abstract": "<jats:p>The COVID-19 pandemic continues to spread around the world and remains a major public health threat. Vaccine inefficiency, vaccination breakthroughs and lack of supply, especially in developing countries, as well as the fact that a non-negligible part of the population either refuse vaccination or cannot be vaccinated due to age, pre-existing illness or non-response to existing vaccines intensify this issue. This might also contribute to the emergence of new variants, being more efficiently transmitted, more virulent and more capable of escaping naturally acquired and vaccine-induced immunity. Hence, the need of effective and viable prevention options to reduce viral transmission is of outmost importance. In this study, we investigated the antiviral effect of iota-, lambda- and kappa-carrageenan, sulfated polysaccharides extracted from red seaweed, on SARS-CoV-2 Wuhan type and the spreading variants of concern (VOCs) Alpha, Beta, Gamma and Delta. Carrageenans as part of broadly used nasal and mouth sprays as well as lozenges have the potential of first line defense to inhibit the infection and transmission of SARS-CoV-2. Here, we demonstrate by using a SARS-CoV-2 spike pseudotyped lentivirus particles (SSPL) system and patient-isolated SARS-CoV-2 VOCs to infect transgenic A549ACE2/TMPRSS2 and Calu-3 human lung cells that all three carrageenan types exert antiviral activity. Iota-carrageenan exhibits antiviral activity with comparable IC50 values against the SARS-CoV-2 Wuhan type and the VOCs. Altogether, these results indicate that iota-carrageenan might be effective for prophylaxis and treatment of SARS-CoV-2 infections independent of the present and potentially future variants.</jats:p>",
"alternative-id": [
"ijms222413202"
],
"author": [
{
"affiliation": [],
"family": "Fröba",
"given": "Maria",
"sequence": "first"
},
{
"affiliation": [],
"family": "Große",
"given": "Maximilian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Setz",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rauch",
"given": "Pia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Auth",
"given": "Janina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spanaus",
"given": "Lucas",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7316-7141",
"affiliation": [],
"authenticated-orcid": false,
"family": "Münch",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruetalo",
"given": "Natalia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8989-5813",
"affiliation": [],
"authenticated-orcid": false,
"family": "Schindler",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morokutti-Kurz",
"given": "Martina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Graf",
"given": "Philipp",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0118-4297",
"affiliation": [],
"authenticated-orcid": false,
"family": "Prieschl-Grassauer",
"given": "Eva",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3715-2545",
"affiliation": [],
"authenticated-orcid": false,
"family": "Grassauer",
"given": "Andreas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schubert",
"given": "Ulrich",
"sequence": "additional"
}
],
"container-title": [
"International Journal of Molecular Sciences"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
10
]
],
"date-time": "2021-12-10T07:07:18Z",
"timestamp": 1639120038000
},
"deposited": {
"date-parts": [
[
2021,
12,
10
]
],
"date-time": "2021-12-10T07:37:13Z",
"timestamp": 1639121833000
},
"funder": [
{
"DOI": "10.13039/501100001659",
"award": [
"401821119/GRK2504"
],
"doi-asserted-by": "publisher",
"name": "Deutsche Forschungsgemeinschaft"
},
{
"award": [
"MD-Thesis Scholarship Programme"
],
"name": "Interdisciplinary Center for Clinical Research (IZKF) at the University Hospital of the University of Erlangen-Nuremberg"
},
{
"DOI": "10.13039/501100004955",
"award": [
"880687"
],
"doi-asserted-by": "publisher",
"name": "Austrian Research Promotion Agency"
}
],
"indexed": {
"date-parts": [
[
2021,
12,
11
]
],
"date-time": "2021-12-11T06:17:00Z",
"timestamp": 1639203420319
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1422-0067"
}
],
"issue": "24",
"issued": {
"date-parts": [
[
2021,
12,
8
]
]
},
"journal-issue": {
"issue": "24",
"published-online": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
8
]
],
"date-time": "2021-12-08T00:00:00Z",
"timestamp": 1638921600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1422-0067/22/24/13202/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "13202",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
12,
8
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
8
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref1",
"unstructured": "COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins Universityhttps://coronavirus.jhu.edu/map.html"
},
{
"DOI": "10.1038/s41586-021-03412-7",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1038/s41591-021-01285-x",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1099/jmm.0.05320-0",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1128/JVI.00127-20",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1021/acs.analchem.0c03173",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1126/science.abb9983",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1002/jcp.30032",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1038/s41467-020-15562-9",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1016/j.cell.2020.06.043",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.15585/mmwr.mm7003e2",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.2139/ssrn.3780277",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"article-title": "Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa",
"author": "Tegally",
"journal-title": "medRxiv",
"key": "ref14",
"year": "2020"
},
{
"key": "ref15"
},
{
"key": "ref16",
"unstructured": "World Health Organization Tracking SARS-CoV-2 Variantshttps://www.who.int/en/activities/tracking-SARS-CoV-2-variants"
},
{
"DOI": "10.15585/mmwr.mm7008e2",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"key": "ref18"
},
{
"article-title": "Convergent evolution of SARS-CoV-2 spike mutations, L452R, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India",
"author": "Cherian",
"journal-title": "bioRxiv",
"key": "ref19",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03470-x",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1126/science.abg3055",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.3390/v13040633",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1038/s41586-021-03777-9",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1016/j.chom.2021.04.007",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"key": "ref25"
},
{
"key": "ref26",
"unstructured": "European Centre for Disease Prevention and Control Risk of SARS-CoV-2 Transmission from Newly-Infected Individuals with Documented Previous Infection or Vaccinationhttps://www.ecdc.europa.eu/en/publications-data/sars-cov-2-transmission-newly-infected-individuals-previous-infection#copy-to-clipboard"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1056/NEJMc2009324",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1136/bmj.m1728",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1093/cid/ciaa886",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.3390/v13040647",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1016/j.virusres.2020.197989",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.3390/ijms221910205",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1016/j.carbpol.2014.11.063",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1186/1743-422X-5-107",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1371/journal.pone.0014320",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.2147/IJGM.S120665",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1371/journal.pone.0237480",
"article-title": "Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro",
"author": "Morokutti-Kurz",
"doi-asserted-by": "crossref",
"journal-title": "PLoS ONE",
"key": "ref38",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1152/ajplung.00552.2020",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1038/s41598-020-80896-9",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1186/1472-6882-12-147",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1186/2049-6958-9-57",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1186/1465-9921-14-124",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1186/1465-9921-11-108",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1186/s12931-015-0281-8",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.2147/IJGM.S328486",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"article-title": "A Randomized trial—Intensive treatment based in ivermectin and iota-carrageenan as pre-exposure prophylaxis for COVID-19 in healthcare agents",
"author": "Chahla",
"journal-title": "medRxiv",
"key": "ref47",
"year": "2021"
},
{
"key": "ref48"
},
{
"key": "ref49"
},
{
"key": "ref50"
},
{
"DOI": "10.1016/j.carbpol.2009.01.020",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.1016/j.cegh.2021.100826",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1183/13993003.01123-2020",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.3390/md19080418",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.1056/NEJMoa2022926",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.1093/advances/nmz042",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.1371/journal.pone.0128794",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.1038/srep11062",
"doi-asserted-by": "publisher",
"key": "ref58"
},
{
"DOI": "10.1128/AAC.01816-15",
"doi-asserted-by": "publisher",
"key": "ref59"
},
{
"DOI": "10.1002/prp2.810",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.2147/IJGM.S325861",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"key": "ref62",
"unstructured": "Deutsche Gesellschaft für Krankenhaushygiene e.V.: Empfehlung der DGKH: Viruzides Gurgeln und Viruzider Nasensprayhttps://www.krankenhaushygiene.de/pdfdata/2020_12_02_Empfehlung-viruzides-gurgeln-nasenspray.pdf"
},
{
"key": "ref63",
"unstructured": "Österreichische Gesellschaft für Hygiene, M.u.P. Anwendung von Gurgel-Lösungen und Nasensprays–zwei weitere Verbündete in der Abwehr von Viralen Erkältungskrankheitenauch in Covid-19 Zeitenhttps://www.oeghmp.at/media/anwendung_von_gurgel-loesungen_und_nasensprays.pdf"
},
{
"key": "ref64",
"unstructured": "Marinomed Biotech AG: Carragelose® Containing Products Launchedhttps://www.carragelose.com/en/portfolio/launched-products"
},
{
"DOI": "10.1016/0923-2516(91)90011-Q",
"doi-asserted-by": "publisher",
"key": "ref65"
},
{
"DOI": "10.1038/s41598-021-84882-7",
"doi-asserted-by": "publisher",
"key": "ref66"
},
{
"DOI": "10.1016/j.carbpol.2011.08.053",
"doi-asserted-by": "publisher",
"key": "ref67"
},
{
"DOI": "10.1016/j.jnutbio.2009.07.002",
"doi-asserted-by": "publisher",
"key": "ref68"
},
{
"DOI": "10.1016/j.phrs.2005.09.009",
"doi-asserted-by": "publisher",
"key": "ref69"
},
{
"DOI": "10.1016/j.canlet.2005.11.032",
"doi-asserted-by": "publisher",
"key": "ref70"
},
{
"DOI": "10.1016/j.aquaculture.2008.01.034",
"doi-asserted-by": "publisher",
"key": "ref71"
},
{
"article-title": "Re-evaluation of carrageenan (E°407) and processed Eucheuma seaweed (E°407a) as food additives",
"author": "Younes",
"first-page": "e05238",
"journal-title": "EFSA J.",
"key": "ref72",
"volume": "16",
"year": "2018"
},
{
"article-title": "Part 172—Food Additives Permitted for Direct Addition to Food for Human Consumption, Sec. 172.620 Carrageenan",
"key": "ref73",
"series-title": "Title 21 of the Code of Federal Regulation",
"volume": "Volume 3",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0122911",
"doi-asserted-by": "publisher",
"key": "ref74"
},
{
"DOI": "10.1016/j.antiviral.2004.01.001",
"doi-asserted-by": "publisher",
"key": "ref75"
},
{
"DOI": "10.1126/science.2452480",
"doi-asserted-by": "publisher",
"key": "ref76"
},
{
"DOI": "10.1007/BF01967066",
"doi-asserted-by": "publisher",
"key": "ref77"
},
{
"DOI": "10.1371/journal.ppat.0020069",
"doi-asserted-by": "publisher",
"key": "ref78"
},
{
"DOI": "10.1016/j.virol.2007.01.043",
"doi-asserted-by": "publisher",
"key": "ref79"
},
{
"DOI": "10.2174/1874357902014010009",
"doi-asserted-by": "publisher",
"key": "ref80"
},
{
"DOI": "10.1128/JVI.74.4.1948-1960.2000",
"doi-asserted-by": "publisher",
"key": "ref81"
},
{
"DOI": "10.2147/IJGM.S167123",
"doi-asserted-by": "publisher",
"key": "ref82"
},
{
"DOI": "10.1038/s41467-021-23473-6",
"doi-asserted-by": "publisher",
"key": "ref83"
},
{
"DOI": "10.1093/oxfordjournals.aje.a118408",
"doi-asserted-by": "publisher",
"key": "ref84"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"doi-asserted-by": "publisher",
"key": "ref85"
}
],
"reference-count": 85,
"references-count": 85,
"relation": {},
"score": 1,
"short-container-title": [
"IJMS"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Inorganic Chemistry",
"Organic Chemistry",
"Physical and Theoretical Chemistry",
"Computer Science Applications",
"Spectroscopy",
"Molecular Biology",
"General Medicine",
"Catalysis"
],
"subtitle": [],
"title": [
"Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta"
],
"type": "journal-article",
"volume": "22"
}