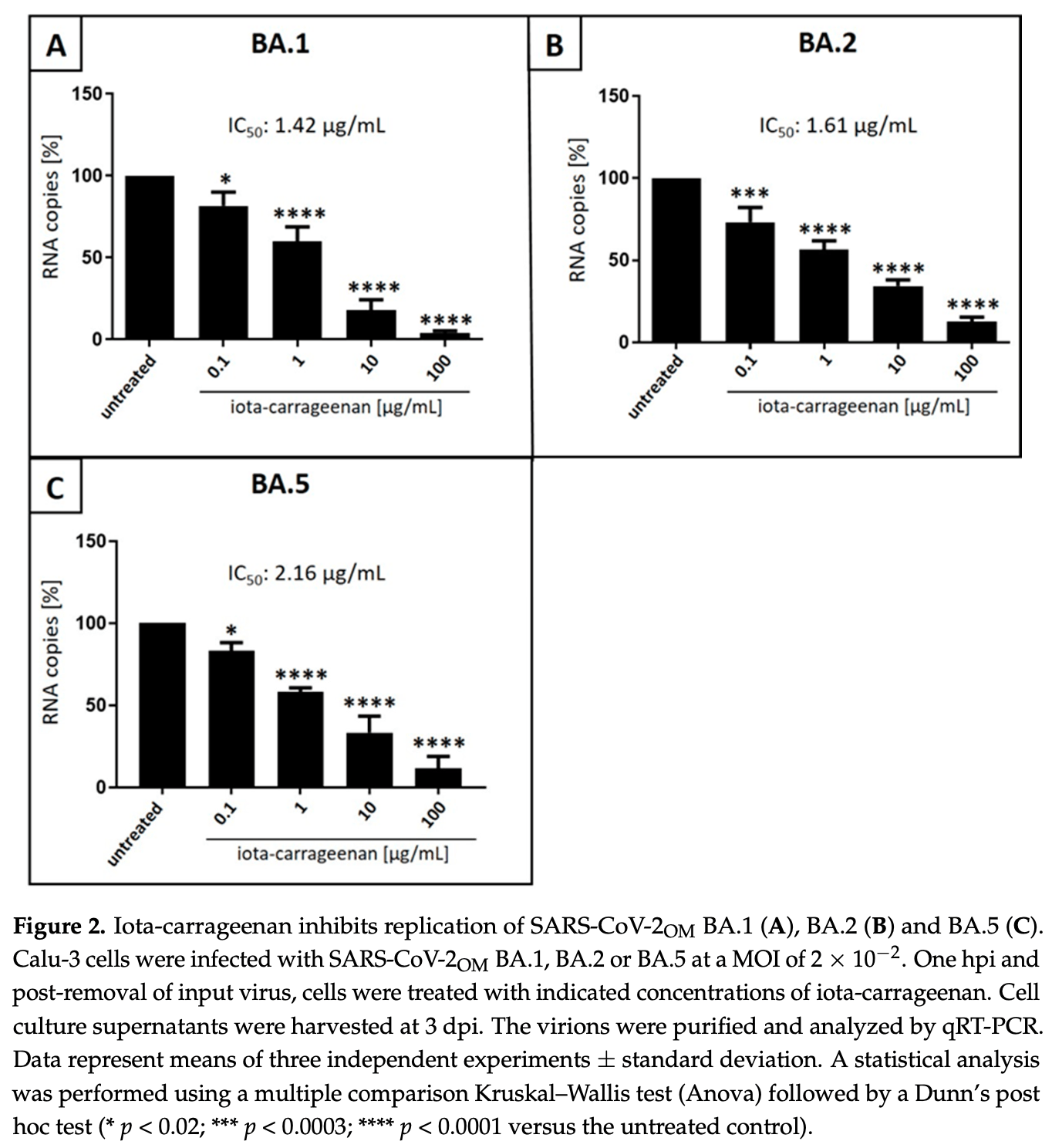
Iota-Carrageenan Inhibits Replication of the SARS-CoV-2 Variants of Concern Omicron BA.1, BA.2 and BA.5
et al., Nutraceuticals, doi:10.3390/nutraceuticals3030025, Jul 2023
In vitro study showing that iota-carrageenan inhibits SARS-CoV-2 replication for omicron variants BA.1, BA.2 and BA.5. Iota-carrageenan was more effective than kappa- and lambda-carrageenan.
19 preclinical studies support the efficacy of iota-carrageenan for COVID-19:
1.
Herida et al., Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC155.071.
2.
Krylova et al., Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents, International Journal of Molecular Sciences, doi:10.3390/ijms26136175.
3.
Rohilla et al., Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV-2, Stresses, doi:10.3390/stresses3030039.
4.
Thet, H., The potential of carrageenan for the drug discovery of COVID-19 via molecular docking with angiotensin-converting enzyme 2 (ACE2) and the main protease (Mpro) of SARS-CoV-2, Journal of Bioinformatics and Genomics, doi:10.18454/jbg.2022.18.2.001.
5.
Alsaidi et al., Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model, Marine Drugs, doi:10.3390/md19080418.
6.
Sattari et al., Repositioning Therapeutics for COVID-19: Virtual Screening of the Potent Synthetic and Natural Compounds as SARS-CoV-2 3CLpro Inhibitors, Research Square, doi:10.21203/rs.3.rs-37994/v1.
7.
Hoffmann et al., Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity, Biomacromolecules, doi:10.1021/acs.biomac.5c00576.
8.
Yathindranath et al., Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection, International Journal of Nanomedicine, doi:10.2147/IJN.S448005.
9.
Setz et al., Iota-Carrageenan Inhibits Replication of the SARS-CoV-2 Variants of Concern Omicron BA.1, BA.2 and BA.5, Nutraceuticals, doi:10.3390/nutraceuticals3030025.
10.
Meister et al., Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2, Journal of Hospital Infection, doi:10.1016/j.jhin.2021.10.019.
11.
Bovard et al., Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochemistry and Biophysics Reports, doi:10.1016/j.bbrep.2021.101187.
12.
Fröba et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, International Journal of Molecular Sciences, doi:10.3390/ijms222413202.
13.
Varese et al., Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model, bioRxiv, doi:10.1101/2021.04.27.441512.
14.
Morokutti-Kurz et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE, doi:10.1371/journal.pone.0237480.
15.
Song et al., Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food & Function, doi:10.1039/D0FO02017F.
Setz et al., 5 Jul 2023, peer-reviewed, 15 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Iota-Carrageenan Inhibits Replication of the SARS-CoV-2 Variants of Concern Omicron BA.1, BA.2 and BA.5
Nutraceuticals, doi:10.3390/nutraceuticals3030025
Even with its endemic transition, the COVID-19 pandemic remains a public health threat, particularly in the light of emerging variants of concern (VoCs) and the need for pandemic preparedness in the future. In November 2021, the SARS-CoV-2 VoC Omicron emerged and its subvariants BA.1, BA.2 and BA.5 became predominant. Although the protease inhibitor Paxlovid ® and the polymerase inhibitors Molnupiravir and Remdesivir were approved as specific antiviral treatment options for COVID-19 patients in the early stages after infection, effective prophylactically acting substances without adverse effects are not available yet. In a recent study, we demonstrated that iotacarrageenan, a sulfated polysaccharide extracted from red seaweed, efficiently inhibits the replication of the SARS-CoV-2 Wuhan Type and the VoCs Alpha, Beta, Gamma and Delta. Now, we extended this study by investigating the antiviral effects of iota-, lambda-and kappa-carrageenans on the VoC Omicron subvariants BA.1, BA.2 and BA.5. Using a VoC Omicron BA.1 spike pseudotyped murine leukemia virus (BA.1 MLV OM VLP) as well as patient-derived SARS-CoV-2 Omicron isolates BA.1, BA.2 and BA.5 (SARS-CoV-2 OM BA.1 , SARS-CoV-2 OM BA.2 and SARS-CoV-2 OM BA.5 ), we demonstrate that iota-carrageenan exhibits similar antiviral activity against all analyzed Omicron subvariants. As with other VoCs shown before, the biologically inert iota-carrageenan was more efficient than kappa-and lambda-carrageenan. Altogether, these results confirm that, independent of the current and potential future variants, the physical barrier provided by iota-carrageenan might be applicable for prophylaxis and early treatment of SARS-CoV-2 infections.
Conflicts of Interest: The authors have read the journal's policy and declare that the authors Andreas Grassauer, Eva Prieschl-Grassauer, Philipp Graf and Martina Morokutti-Kurz are employed by Marinomed Biotech AG. Andreas Grassauer and Eva Prieschl-Grassauer are co-founders of Marinomed Biotech AG. Andreas Grassauer, Eva Prieschl-Grassauer and Martina Morokutti-Kurz are the inventors of a patent submission related to the content of the manuscript; the number of this patent application is EP20186334. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
Aguiar, Tremblay, Mansfield, Woody, Lobb et al., Gene expression and in situ protein profiling of candidate SARS-CoV-2 receptors in human airway epithelial cells and lung tissue, Eur. Respir. J, doi:10.1183/13993003.01123-2020
Arora, Kempf, Nehlmeier, Schulz, Jäck et al., Omicron sublineage BQ.1.1 resistance to monoclonal antibodies, Lancet Infect. Dis, doi:10.1016/S1473-3099(22)00733-2
Auth, Fröba, Große, Rauch, Ruetalo et al., Lectin from Triticum vulgaris (WGA) Inhibits Infection with SARS-CoV-2 and Its Variants of Concern Alpha and Beta, Int. J. Mol. Sci, doi:10.3390/ijms221910205
Boswell, Verga, Mackle, Guerrero-Vazquez, Thomas et al., Silico Approaches for the Screening and Discovery of Broad-Spectrum Marine Natural Product Antiviral Agents Against Coronaviruses, doi:10.2147/IDR.S395203
Campo, Kawano, Da Silva, Carvalho, Carrageenans, Biological properties, chemical modifications and structural analysis-A review, Carbohydr. Polym, doi:10.1016/j.carbpol.2009.01.020
Cao, Wang, Jian, Xiao, Song et al., Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature, doi:10.1038/s41586-021-04385-3
Capron, Yvon, Muller, In-vitro gastric stability of carrageenan. Food Hydrocoll, doi:10.1016/S0268-005X(96)80040-3
Chahla, Medina Ruiz, Ortega, Morales, Barreiro et al., Intensive Treatment with Ivermectin and Iota-Carrageenan as Pre-exposure Prophylaxis for COVID-19 in Health Care Workers from Tucuman, Am. J. Ther, doi:10.1097/MJT.0000000000001433
Cherian, Potdar, Jadhav, Yadav, Gupta et al., SARS-CoV-2 Spike Mutations, L452R, T478K, E484Q and P681R, in the Second Wave of COVID-19 in Maharashtra, doi:10.3390/microorganisms9071542
Collier, De Marco, Ferreira, Meng, Datir et al., Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies, Nature, doi:10.1038/s41586-021-03412-7
Corman, Landt, Kaiser, Molenkamp, Meijer et al., Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Euro Surveill. Bull. Eur. Sur Les Mal. Transm. = Eur. Commun. Dis. Bull, doi:10.2807/1560-7917.ES.2020.25.3.2000045
Creech, Walker, Samuels, SARS-CoV-2 Vaccines, JAMA, doi:10.1001/jama.2021.3199
Douma, Boualy, Manaut, Hammal, Byadi et al., Sulphated polysaccharides from seaweeds as potential entry inhibitors and vaccine adjuvants against SARS-CoV-2 RBD spike protein: A computational approach, J. Taibah Univ. Sci, doi:10.1080/16583655.2021.1999068
Eccles, Iota-Carrageenan as an antiviral treatment for the Common Cold, Open Virol. J. 2020, doi:10.2174/1874357902014010009
Eccles, Meier, Jawad, Weinmüllner, Grassauer et al., Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: A randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold, Respir. Res, doi:10.1186/1465-9921-11-108
Eccles, Winther, Johnston, Robinson, Trampisch et al., Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: The ICICC trial, Respir. Res, doi:10.1186/s12931-015-0281-8
Ema, Issues Advice on Use of Paxlovid (PF-07321332 and Ritonavir) for the Treatment of COVID-19: Rolling Review Starts in Parallel
Fazekas, Eickhoff, Pruckner, Vollnhofer, Fischmeister et al., Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold, BMC Complement. Altern. Med, doi:10.1186/1472-6882-12-147
Figueroa, Lombardo, Dogliotti, Flynn, Giugliano et al., Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease, Int. J. Gen. Med, doi:10.2147/IJGM.S328486
Frediansyah, The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: A critical review, Clin. Epidemiol. Glob. Health, doi:10.1016/j.cegh.2021.100826
Fröba, Große, Setz, Rauch, Auth et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, Int. J. Mol. Sci, doi:10.3390/ijms222413202
Galloway, Paul, Maccannell, Johansson, Brooks et al., Emergence of SARS-CoV-2 B.1.1.7 Lineage-United States, MMWR Morb. Mortal. Wkly. Rep, doi:10.15585/mmwr.mm7003e2
Gao, Guo, Luo, Omicron variant (B.1.1.529) of SARS-CoV-2, a global urgent public health alert!, J. Med. Virol, doi:10.1002/jmv.27491
Gesellschaft Für Hygiene, Und Präventivmedizin, Anwendung von Gurgel-Lösungen und Nasensprays-Zwei Weitere Verbündete in der Abwehr von Viralen Erkältungskrankheitenauch in COVID-19 Zeiten
Gesellschaft Für Krankenhaushygiene, Empfehlung der DGKH: Viruzides Gurgeln und Viruzider Nasenspray
Girond, Crance, Van Cuyck-Gandre, Renaudet, Deloince, Antiviral activity of carrageenan on hepatitis A virus replication in cell culture, Res. Virol, doi:10.1016/0923-2516(91)90011-Q
Grassauer, Carragelose®Containing Products Launched. Available online
Grassauer, Weinmuellner, Meier, Pretsch, Prieschl-Grassauer et al., Iota-Carrageenan is a potent inhibitor of rhinovirus infection, Virol. J, doi:10.1186/1743-422X-5-107
Große, Ruetalo, Layer, Hu, Businger et al., Quinine Inhibits Infection of Human Cell Lines with SARS-CoV-2. Viruses, doi:10.3390/v13040647
Health, Investigation of SARS-CoV-2 Variants of Concern: Technical Briefings
Hebar, Koller, Seifert, Chabicovsky, Bodenteich et al., Non-clinical safety evaluation of intranasal iota-carrageenan, PLoS ONE, doi:10.1371/journal.pone.0122911
Hemilä, Chalker, Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: Re-analysis of randomized trial data, Pharmacol. Res. Perspect, doi:10.1002/prp2.810
Hjerde, Smidsrød, Christensen, Analysis of the conformational properties of κ-and ι-carrageenan by size-exclusion chromatography combined with low-angle laser light scattering, Biopolymers, doi:10.1002/(SICI)1097-0282(199901)49:1<71::AID-BIP7>3.0.CO;2-H
Jang, Shin, Lee, Kwon, Shin et al., Antiviral activity of lambdacarrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2, Sci. Rep, doi:10.1038/s41598-020-80896-9
Karim, Karim, Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic, Lancet
Ke, Chang, Marasco, Immune Evasion of SARS-CoV-2 Omicron Subvariants. Vaccines, doi:10.3390/vaccines10091545
Kitazato, Wang, Kobayashi, Viral infectious disease and natural products with antiviral activity, Drug Discov. Ther
Koenighofer, Lion, Bodenteich, Prieschl-Grassauer, Grassauer et al., Carrageenan nasal spray in virus confirmed common cold: Individual patient data analysis of two randomized controlled trials, Multidiscip. Respir. Med, doi:10.1186/2049-6958-9-57
Korber, Fischer, Gnanakaran, Yoon, Theiler et al., Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus, Cell, doi:10.1016/j.cell.2020.06.043
Leibbrandt, Meier, König-Schuster, Weinmüllner, Kalthoff et al., Iota-carrageenan is a potent inhibitor of influenza A virus infection, PLoS ONE, doi:10.1371/journal.pone.0014320
Levendosky, Mizenina, Martinelli, Jean-Pierre, Kizima et al., Griffithsin and Carrageenan Combination to Target Herpes Simplex Virus 2 and Human Papillomavirus, Antimicrob. Agents Chemother, doi:10.1128/AAC.01816-15
Liu, Zhan, Wan, Wang, Wang, Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects, Carbohydr. Polym, doi:10.1016/j.carbpol.2014.11.063
Ludwig, Enzenhofer, Schneider, Rauch, Bodenteich et al., Efficacy of a carrageenan nasal spray in patients with common cold: A randomized controlled trial, Respir. Res, doi:10.1186/1465-9921-14-124
Luo, Shao, Nie, Wei, Li et al., Antitumor and Adjuvant Activity of λ-carrageenan by Stimulating Immune Response in Cancer Immunotherapy, Sci. Rep, doi:10.1038/srep11062
Lyngse, Kirkeby, Denwood, Christiansen, Mølbak et al., Household transmission of SARS-CoV-2 Omicron variant of concern subvariants BA.1 and BA.2 in Denmark, Nat. Commun, doi:10.1038/s41467-022-33498-0
Mani, Johnson, Steel, Broszczak, Neilsen et al., Natural product-derived phytochemicals as potential agents against coronaviruses: A review, Virus Res, doi:10.1016/j.virusres.2020.197989
Medicines, COVID-19 Vaccines: Authorised. Available online
Mei, Li, Wang, Zhu, Huang et al., An inhaled bioadhesive hydrogel to shield non-human primates from SARS-CoV-2 infection, Nat. Mater, doi:10.1038/s41563-023-01475-7
Meng, Kemp, Papa, Datir, Ferreira et al., Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the Alpha variant B.1.1.7. Cell Rep, doi:10.1016/j.celrep.2021.109292
Morokutti-Kurz, Fröba, Graf, Große, Grassauer et al., Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE, doi:10.1371/journal.pone.0237480
Morokutti-Kurz, Graf, Prieschl-Grassauer, Amylmetacresol/2,4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat, Int. J. Gen. Med, doi:10.2147/IJGM.S120665
Morokutti-Kurz, König-Schuster, Koller, Graf, Graf et al., The Intranasal Application of Zanamivir and Carrageenan Is Synergistically Active against Influenza A Virus in the Murine Model, PLoS ONE, doi:10.1371/journal.pone.0128794
Morokutti-Kurz, Unger-Manhart, Graf, Rauch, Kodnar et al., The Saliva of Probands Sucking an Iota-Carrageenan Containing Lozenge Inhibits Viral Binding and Replication of the Most Predominant Common Cold Viruses and SARS-CoV-2, Int. J. Gen. Med, doi:10.2147/IJGM.S325861
Mwenda, Saasa, Sinyange, Busby, Chipimo et al., Detection of B.1.351 SARS-CoV-2 Variant Strain-Zambia, MMWR Morb. Mortal. Wkly. Rep
Reed, Muench, A simple method of estimating fifty per cent endpoints, Am. J. Epidemiol, doi:10.1093/oxfordjournals.aje.a118408
Salih, Thissera, Yaseen, Hassane, El-Seedi et al., Sulfated Polysaccharides as Promising Antiviral Agents: A Comprehensive Report and Modeling Study Focusing on SARS CoV-2. Mar. Drugs, doi:10.3390/md19080406
Schütz, Conzelmann, Fois, Groß, Weil et al., Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures, Am. J. Physiol.-Lung Cell. Mol. Physiol, doi:10.1152/ajplung.00552.2020
Setz, Fröba, Große, Rauch, Auth et al., European Black Elderberry Fruit Extract Inhibits Replication of SARS-CoV-2 In Vitro, Nutraceuticals 2023, doi:10.3390/nutraceuticals3010007
Singh, Singh, Singh, Misra, Molnupiravir in COVID-19: A systematic review of literature, Diabetes Metab. Syndr, doi:10.1016/j.dsx.2021.102329
Spichtig, Austin, Determination of the low molecular weight fraction of food-grade carrageenans, J. Chromatogr. B Anal. Technol. Biomed. Life Sci, doi:10.1016/j.jchromb.2007.11.012
Sworn, Marrs, Hart, Characterisation of carrageenans by high-performance size-exclusion chromatography using a LiChrospher 1000 DIOL column, J. Chromatogr, doi:10.1016/S0021-9673(00)96368-4
Tegally, Wilkinson, Giovanetti, Iranzadeh, Fonseca et al., Detection of a SARS-CoV-2 variant of concern in South Africa, Nature, doi:10.1038/s41586-021-03402-9
Uraki, Halfmann, Iida, Yamayoshi, Furusawa et al., Characterization of SARS-CoV-2 Omicron BA.4 and BA.5 isolates in rodents, Nature, doi:10.1038/s41586-022-05482-7
Weiner, Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies, Crit. Rev. Toxicol, doi:10.3109/10408444.2013.861798
Who, Therapeutics and COVID-19: Living Guideline
Wibmer, Ayres, Hermanus, Madzivhandila, Kgagudi et al., SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma, Nat. Med, doi:10.1038/s41591-021-01285-x
Younes, Aggett, Aguilar, Crebelli, Filipič et al., Re-evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives
Yuan, Song, Li, Li, Dai, Immunomodulation and antitumor activity of kappa-carrageenan oligosaccharides, Cancer Lett, doi:10.1016/j.canlet.2005.11.032
Zhou, Sheng, Yao, Wang, Effect of low molecular lambda-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu, Pharmacol. Res, doi:10.1016/j.phrs.2005.09.009