Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model
et al., Marine Drugs, doi:10.3390/md19080418, Jul 2021
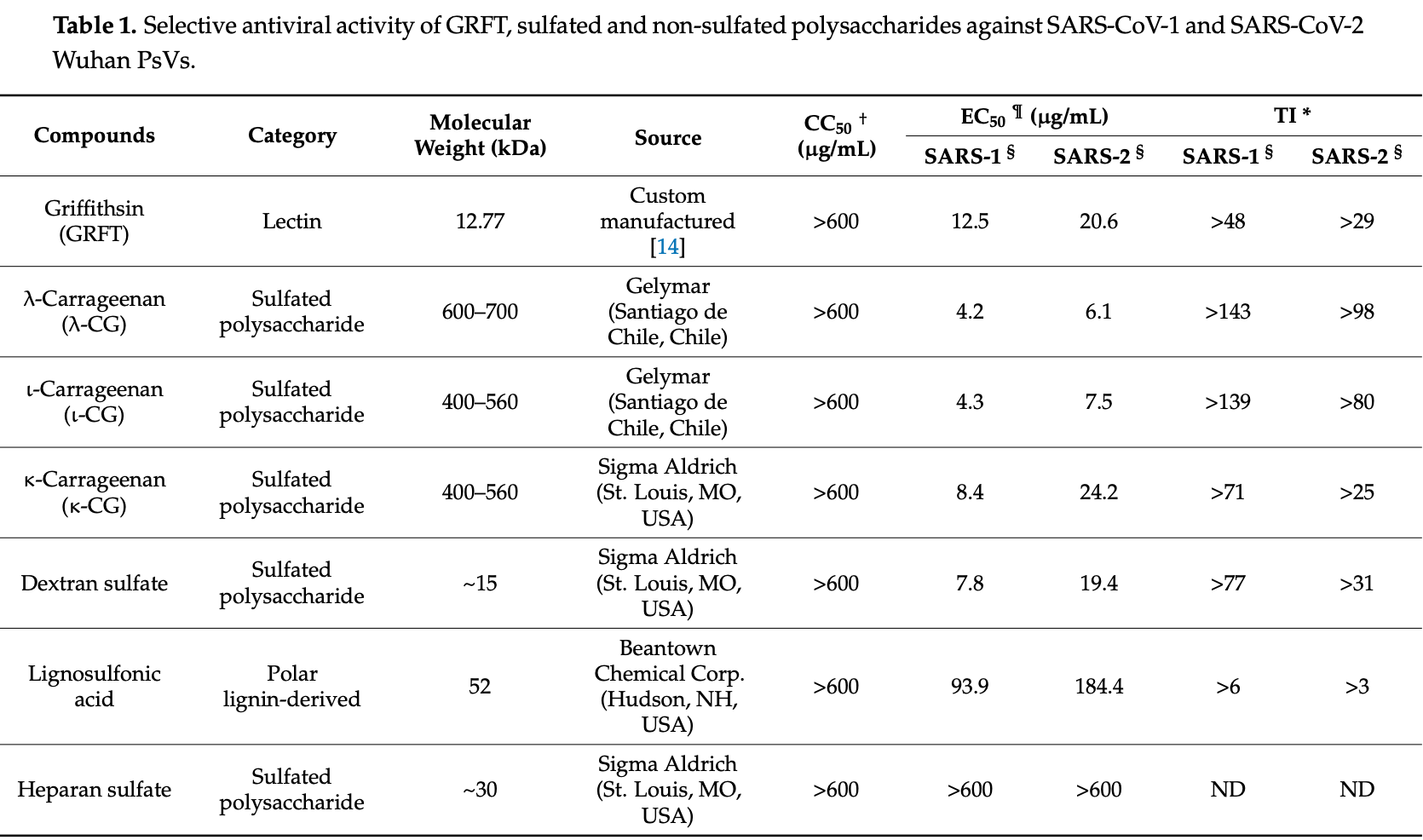
In vitro and in silico analysis showing SARS-CoV-2 antiviral activity of carageenan, and synergistic activity from the combination of carrageenan and griffithsin.
19 preclinical studies support the efficacy of iota-carrageenan for COVID-19:
1.
Herida et al., Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC155.071.
2.
Krylova et al., Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents, International Journal of Molecular Sciences, doi:10.3390/ijms26136175.
3.
Rohilla et al., Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV-2, Stresses, doi:10.3390/stresses3030039.
4.
Thet, H., The potential of carrageenan for the drug discovery of COVID-19 via molecular docking with angiotensin-converting enzyme 2 (ACE2) and the main protease (Mpro) of SARS-CoV-2, Journal of Bioinformatics and Genomics, doi:10.18454/jbg.2022.18.2.001.
5.
Alsaidi et al., Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model, Marine Drugs, doi:10.3390/md19080418.
6.
Sattari et al., Repositioning Therapeutics for COVID-19: Virtual Screening of the Potent Synthetic and Natural Compounds as SARS-CoV-2 3CLpro Inhibitors, Research Square, doi:10.21203/rs.3.rs-37994/v1.
7.
Hoffmann et al., Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity, Biomacromolecules, doi:10.1021/acs.biomac.5c00576.
8.
Yathindranath et al., Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection, International Journal of Nanomedicine, doi:10.2147/IJN.S448005.
9.
Setz et al., Iota-Carrageenan Inhibits Replication of the SARS-CoV-2 Variants of Concern Omicron BA.1, BA.2 and BA.5, Nutraceuticals, doi:10.3390/nutraceuticals3030025.
10.
Meister et al., Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2, Journal of Hospital Infection, doi:10.1016/j.jhin.2021.10.019.
11.
Bovard et al., Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochemistry and Biophysics Reports, doi:10.1016/j.bbrep.2021.101187.
12.
Fröba et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, International Journal of Molecular Sciences, doi:10.3390/ijms222413202.
13.
Varese et al., Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model, bioRxiv, doi:10.1101/2021.04.27.441512.
14.
Morokutti-Kurz et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE, doi:10.1371/journal.pone.0237480.
15.
Song et al., Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food & Function, doi:10.1039/D0FO02017F.
Alsaidi et al., 26 Jul 2021, peer-reviewed, 12 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model
Marine Drugs, doi:10.3390/md19080418
Over 182 million confirmed cases of COVID-19 and more than 4 million deaths have been reported to date around the world. It is essential to identify broad-spectrum antiviral agents that may prevent or treat infections by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) but also by other coronaviruses that may jump the species barrier in the future. We evaluated the antiviral selectivity of griffithsin and sulfated and non-sulfated polysaccharides against SARS-CoV-1 and SARS-CoV-2 using a cytotoxicity assay and a cell-based pseudoviral model. The half-maximal cytotoxic concentration (CC 50 ) and half-maximal effective concentration (EC 50 ) were determined for each compound, using a dose-response-inhibition analysis on GraphPad Prism v9.0.2 software (San Diego, CA, USA). The therapeutic index (TI = CC 50 /EC 50 ) was calculated for each compound. The potential synergistic, additive, or antagonistic effect of different compound combinations was determined by CalcuSyn v1 software (Biosoft, Cambridge, UK), which estimated the combination index (CI) values. Iota and lambda carrageenan showed the most potent antiviral activity (EC 50 between 3.2 and 7.5 µg/mL). Carrageenan and griffithsin combinations exhibited synergistic activity (EC 50 between 0.2 and 3.8 µg/mL; combination index <1), including against recent SARS-CoV-2 mutations. The griffithsin and carrageenan combination is a promising candidate to prevent or treat infections by SARS-CoV-1 and SARS-CoV-2.
References
Buck, Thompson, Roberts, Muller, Lowy et al., Carrageenan is a potent inhibitor of papillomavirus infection, PLoS Pathog, doi:10.1371/journal.ppat.0020069
Derby, Lal, Aravantinou, Kizima, Barnable et al., Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo, Nat. Commun, doi:10.1038/s41467-018-06349-0
Fernandez-Romero, Abraham, Rodriguez, Kizima, Jean-Pierre et al., Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge, Antimicrob. Agents Chemother, doi:10.1128/AAC.05461-11
Friedland, Hoesley, Plagianos, Hoskin, Zhang et al., First-in-Human Trial of MIV-150 and Zinc Acetate Coformulated in a Carrageenan Gel: Safety, Pharmacokinetics, Acceptability, Adherence, and Pharmacodynamics, JAIDS J. Acquir. Immune Defic. Syndr, doi:10.1097/QAI.0000000000001136
Guarner, Three Emerging Coronaviruses in Two Decades, Am. J. Clin. Pathol, doi:10.1093/ajcp/aqaa029
Hebar, Koller, Seifert, Chabicovsky, Bodenteich et al., Non-clinical safety evaluation of intranasal iota-carrageenan, PLoS ONE, doi:10.1371/journal.pone.0122911
Hoffmann, Kleine-Weber, Schroeder, Kruger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Koenighofer, Lion, Bodenteich, Prieschl-Grassauer, Grassauer et al., Carrageenan nasal spray in virus confirmed common cold: Individual patient data analysis of two randomized controlled trials, Multidiscip. Respir. Med, doi:10.1186/2049-6958-9-57
Kwon, Oh, Kwon, Jin, Zhang et al., Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro, Cell Discov, doi:10.1038/s41421-020-00192-8
Lee, Griffithsin, a Highly Potent Broad-Spectrum Antiviral Lectin from Red Algae: From Discovery to Clinical Application, Mar. Drugs, doi:10.3390/md17100567
Leibbrandt, Meier, Konig-Schuster, Weinmullner, Kalthoff et al., Iota-carrageenan is a potent inhibitor of influenza A virus infection, PLoS ONE, doi:10.1371/journal.pone.0014320
Leung, Shum, Leung, Lam, Wu, Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to, Eurosurveillance, doi:10.2807/1560-7917.ES.2020.26.1.2002106
Levendosky, Mizenina, Martinelli, Jean-Pierre, Kizima et al., Griffithsin and Carrageenan Combination To Target Herpes Simplex Virus 2 and Human Papillomavirus, Antimicrob. Agents Chemother, doi:10.1128/AAC.01816-15
Lusvarghi, Bewley, Griffithsin: An Antiviral Lectin with Outstanding Therapeutic Potential, Viruses, doi:10.3390/v8100296
Morokutti-Kurz, Froba, Graf, Grosse, Grassauer et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE
Moulaei, Alexandre, Shenoy, Meyerson, Krumpe et al., Griffithsin tandemers: Flexible and potent lectin inhibitors of the human immunodeficiency virus, Retrovirology, doi:10.1186/s12977-014-0127-3
O'keefe, Giomarelli, Barnard, Shenoy, Chan et al., Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae, J. Virol, doi:10.1128/JVI.02322-09
O'keefe, Vojdani, Buffa, Shattock, Montefiori et al., Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component, Proc. Natl. Acad. Sci, doi:10.1073/pnas.0901506106
Parker, Shrotri, Kampmann, Keeping track of the SARS-CoV-2 vaccine pipeline, Nat. Rev. Immunol, doi:10.1038/s41577-020-00455-1
Plante, Liu, Liu, Xia, Johnson et al., Spike mutation D614G alters SARS-CoV-2 fitness, Nature, doi:10.1038/s41586-020-2895-3
Rodriguez, Kleinbeck, Mizenina, Kizima, Levendosky et al., In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition, Antivir. Res, doi:10.1016/j.antiviral.2014.05.018
Schmidt, Weisblum, Muecksch, Hoffmann, Michailidis et al., Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses, J. Exp. Med, doi:10.1084/jem.20201181
Shikov, Flisyuk, Obluchinskaya, Pozharitskaya, Pharmacokinetics of Marine-Derived Drugs, Mar. Drugs, doi:10.3390/md18110557
Sun, Remdesivir for Treatment of COVID-19: Combination of Pulmonary and IV Administration May Offer Aditional Benefit, AAPS J, doi:10.1208/s12248-020-00459-8
Tegally, Wilkinson, Giovanetti, Iranzadeh, Fonseca et al., Detection of a SARS-CoV-2 variant of concern in South Africa, Nature, doi:10.1038/s41586-021-03402-9
Who, Coronavirus Disease (COVID-19) Pandemic
DOI record:
{
"DOI": "10.3390/md19080418",
"ISSN": [
"1660-3397"
],
"URL": "http://dx.doi.org/10.3390/md19080418",
"abstract": "<jats:p>Over 182 million confirmed cases of COVID-19 and more than 4 million deaths have been reported to date around the world. It is essential to identify broad-spectrum antiviral agents that may prevent or treat infections by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) but also by other coronaviruses that may jump the species barrier in the future. We evaluated the antiviral selectivity of griffithsin and sulfated and non-sulfated polysaccharides against SARS-CoV-1 and SARS-CoV-2 using a cytotoxicity assay and a cell-based pseudoviral model. The half-maximal cytotoxic concentration (CC50) and half-maximal effective concentration (EC50) were determined for each compound, using a dose-response-inhibition analysis on GraphPad Prism v9.0.2 software (San Diego, CA, USA). The therapeutic index (TI = CC50/EC50) was calculated for each compound. The potential synergistic, additive, or antagonistic effect of different compound combinations was determined by CalcuSyn v1 software (Biosoft, Cambridge, UK), which estimated the combination index (CI) values. Iota and lambda carrageenan showed the most potent antiviral activity (EC50 between 3.2 and 7.5 µg/mL). Carrageenan and griffithsin combinations exhibited synergistic activity (EC50 between 0.2 and 3.8 µg/mL; combination index <1), including against recent SARS-CoV-2 mutations. The griffithsin and carrageenan combination is a promising candidate to prevent or treat infections by SARS-CoV-1 and SARS-CoV-2.</jats:p>",
"alternative-id": [
"md19080418"
],
"author": [
{
"affiliation": [],
"family": "Alsaidi",
"given": "Sahar",
"sequence": "first"
},
{
"affiliation": [],
"family": "Cornejal",
"given": "Nadjet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahoney",
"given": "Oneil",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2663-1196",
"affiliation": [],
"authenticated-orcid": false,
"family": "Melo",
"given": "Claudia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Verma",
"given": "Neeharika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bonnaire",
"given": "Thierry",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0696-9755",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chang",
"given": "Theresa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0772-4856",
"affiliation": [],
"authenticated-orcid": false,
"family": "O’Keefe",
"given": "Barry R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sailer",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zydowsky",
"given": "Thomas M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teleshova",
"given": "Natalia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2864-8293",
"affiliation": [],
"authenticated-orcid": false,
"family": "Romero",
"given": "José A. Fernández",
"sequence": "additional"
}
],
"container-title": "Marine Drugs",
"container-title-short": "Marine Drugs",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
7,
27
]
],
"date-time": "2021-07-27T02:22:46Z",
"timestamp": 1627352566000
},
"deposited": {
"date-parts": [
[
2021,
7,
27
]
],
"date-time": "2021-07-27T16:51:26Z",
"timestamp": 1627404686000
},
"indexed": {
"date-parts": [
[
2023,
12,
18
]
],
"date-time": "2023-12-18T14:29:55Z",
"timestamp": 1702909795264
},
"is-referenced-by-count": 28,
"issue": "8",
"issued": {
"date-parts": [
[
2021,
7,
26
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2021,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
26
]
],
"date-time": "2021-07-26T00:00:00Z",
"timestamp": 1627257600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1660-3397/19/8/418/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "418",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
7,
26
]
]
},
"published-online": {
"date-parts": [
[
2021,
7,
26
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref1",
"unstructured": "Coronavirus Disease (COVID-19) Pandemic\nhttps://www.who.int/emergencies/diseases/novel-coronavirus-2019"
},
{
"DOI": "10.1093/ajcp/aqaa029",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.2807/1560-7917.ES.2020.26.1.2002106",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1038/s41577-020-00455-1",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1038/s41586-020-2895-3",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1038/s41586-021-03402-9",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1128/AAC.01816-15",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1128/JVI.02322-09",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1038/s41421-020-00192-8",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1371/journal.pone.0237480",
"article-title": "Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro",
"author": "Morokutti-Kurz",
"doi-asserted-by": "crossref",
"journal-title": "PLoS ONE",
"key": "ref11",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1186/s12977-014-0127-3",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.3390/md17100567",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1073/pnas.0901506106",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.3390/v8100296",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1371/journal.ppat.0020069",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/j.antiviral.2014.05.018",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1097/QAI.0000000000001136",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1371/journal.pone.0122911",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"key": "ref20",
"series-title": "CFR—Code of Federal Regulations. Title 21: Food and Drugs. Electronic Code Federal Regulation",
"year": "2014"
},
{
"DOI": "10.1186/2049-6958-9-57",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1371/journal.pone.0014320",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.3390/md18110557",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1208/s12248-020-00459-8",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1038/s41467-018-06349-0",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1128/AAC.05461-11",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1084/jem.20201181",
"doi-asserted-by": "publisher",
"key": "ref27"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1660-3397/19/8/418"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Drug Discovery",
"Pharmacology, Toxicology and Pharmaceutics (miscellaneous)",
"Pharmaceutical Science"
],
"subtitle": [],
"title": "Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model",
"type": "journal-article",
"volume": "19"
}