Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity
et al., Biomacromolecules, doi:10.1021/acs.biomac.5c00576, Jul 2025
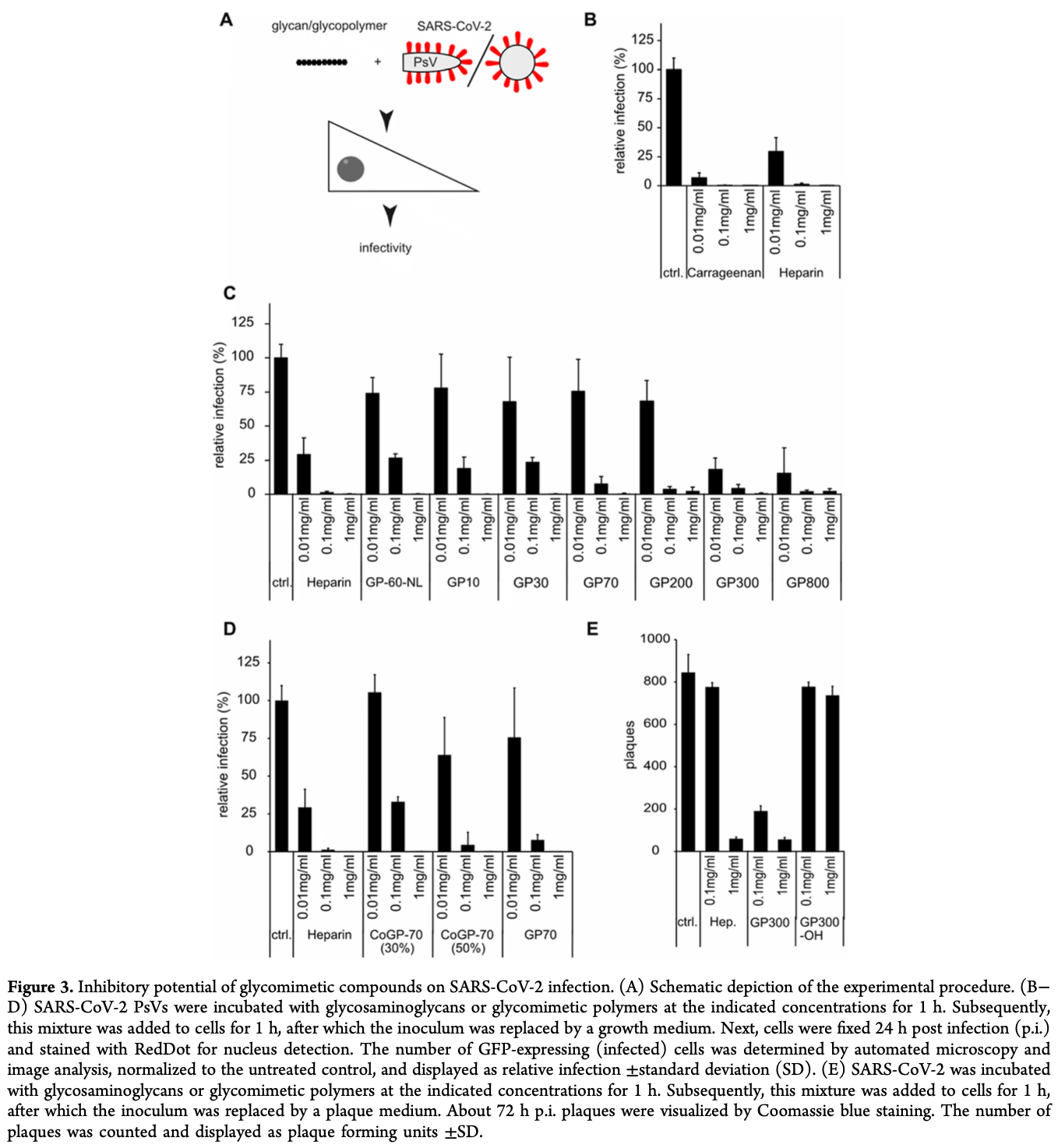
In vitro study showing the efficacy of carrageenan (used as a positive control) in inhibiting SARS-CoV-2 infection. Authors preincubated SARS-CoV-2 pseudovirus (PsV) with carrageenan before infecting Vero cells. Carrageenan reduced viral infection in a concentration-dependent manner, with significant inhibition at concentrations as low as 0.01 mg/mL, outperforming heparin. The study also highlights the importance of sulfation in enhancing antiviral effects, as non-sulfated polymers failed to show significant antiviral activity. The study focuses on sulfated glycosaminoglycan (sGAG) glycopolymer mimetics with controlled sulfation density, which showed high antiviral activity against SARS-CoV-2 while reducing anticoagulant activity.
19 preclinical studies support the efficacy of iota-carrageenan for COVID-19:
1.
Herida et al., Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC155.071.
2.
Krylova et al., Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents, International Journal of Molecular Sciences, doi:10.3390/ijms26136175.
3.
Rohilla et al., Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV-2, Stresses, doi:10.3390/stresses3030039.
4.
Thet, H., The potential of carrageenan for the drug discovery of COVID-19 via molecular docking with angiotensin-converting enzyme 2 (ACE2) and the main protease (Mpro) of SARS-CoV-2, Journal of Bioinformatics and Genomics, doi:10.18454/jbg.2022.18.2.001.
5.
Alsaidi et al., Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model, Marine Drugs, doi:10.3390/md19080418.
6.
Sattari et al., Repositioning Therapeutics for COVID-19: Virtual Screening of the Potent Synthetic and Natural Compounds as SARS-CoV-2 3CLpro Inhibitors, Research Square, doi:10.21203/rs.3.rs-37994/v1.
7.
Hoffmann et al., Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity, Biomacromolecules, doi:10.1021/acs.biomac.5c00576.
8.
Yathindranath et al., Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection, International Journal of Nanomedicine, doi:10.2147/IJN.S448005.
9.
Setz et al., Iota-Carrageenan Inhibits Replication of the SARS-CoV-2 Variants of Concern Omicron BA.1, BA.2 and BA.5, Nutraceuticals, doi:10.3390/nutraceuticals3030025.
10.
Meister et al., Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2, Journal of Hospital Infection, doi:10.1016/j.jhin.2021.10.019.
11.
Bovard et al., Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochemistry and Biophysics Reports, doi:10.1016/j.bbrep.2021.101187.
12.
Fröba et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, International Journal of Molecular Sciences, doi:10.3390/ijms222413202.
13.
Varese et al., Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model, bioRxiv, doi:10.1101/2021.04.27.441512.
14.
Morokutti-Kurz et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE, doi:10.1371/journal.pone.0237480.
15.
Song et al., Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food & Function, doi:10.1039/D0FO02017F.
Hoffmann et al., 6 Jul 2025, peer-reviewed, 10 authors.
Contact: schelhaas@unimuenster.de, laura.hartmann@makro.uni-freiburg.de.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity
Biomacromolecules, doi:10.1021/acs.biomac.5c00576
Sulfated glycosaminoglycans (sGAGs) make up a class of cell-surface glycans known to mediate pathogen engagement. Glycopolymers mimicking sGAGs can reduce or prevent pathogen attachment. However, their high anticoagulant activity limits their biomedical applications. Here, we report the synthesis and evaluation of synthetic glycopolymers mimicking sGAGs with high antiviral activity but low anticoagulant activity. The key lies in the control of the density of carbohydrates presented along the polymeric backbone. This was accomplished via copolymerization of carbohydrate with noncarbohydrate monomers. We reveal that the polymer chain length affects inhibition of SARS-CoV-2 pseudovirus (PsV) and authentic virus infections, and that above a critical chain length, density of carbohydrate and sulfate groups can be reduced, maintaining high antiviral activity while minimizing anticoagulant activity. This demonstrates, for the first time, how specific structural parameters of glycopolymers can be used to maximize inhibition while minimizing anticoagulative properties unlocking the full potential of sGAG mimetics in fighting infections.
Authors Miriam Hoffmann -Department of Organic
Notes The authors declare no competing financial interest.
References
Abdelfadiel, Gunta, Villuri, Afosah, Sankaranarayanan et al., Designing Smaller, Synthetic, Functional Mimetics of Sulfated Glycosaminoglycans as Allosteric Modulators of Coagulation Factors, J. Med. Chem, doi:10.1021/acs.biomac.5c00576?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Abdulsalam, Li, Loka, Sletten, Nguyen, Heparan Sulfate-Mimicking Glycopolymers Bind SARS-CoV-2 Spike Protein in a Length-and Sulfation Pattern-Dependent Manner, ACS Med. Chem. Lett, doi:10.1021/acsmedchemlett.3c00319?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Ackermann, Verleden, Kuehnel, Haverich, Welte et al., Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2015432
Acosta-Gutiérrez, Buckley, Battaglia, The Role of Host Cell Glycans on Virus Infectivity: The SARS-CoV-2 Case, Adv. Sci, doi:10.1002/advs.202201853
Baier, Giesler, Hartmann, Split-and-Combine Approach Towards Branched Precision Glycomacromolecules and Their Lectin Binding Behavior, Chem.�Eur. J, doi:10.1002/chem.201704179
Barrett, Moore, Yaffe, Moore, ISTH Interim Guidance on Recognition and Management of Coagulopathy in COVID-19: A Comment, J. Thromb. Haemost, doi:10.1111/jth.14860
Bathe, Rutledge, Grodzinsky, Tidor, Osmotic Pressure of Aqueous Chondroitin Sulfate Solution: A Molecular Modeling Investigation, Biophys. J, doi:10.1529/biophysj.105.067918
Bermejo-Jambrina, Eder, Kaptein, Van Hamme, Helgers et al., Infection and Transmission of SARS-CoV-2 Depend on Heparan Sulfate Proteoglycans, EMBO J, doi:10.15252/embj.2020106765
Cerezo-Maganã, Bang-Rudenstam, Belting, Proteoglycans: A Common Portal for SARS-CoV-2 and Extracellular Vesicle Uptake, Am. J. Physiol. Cell Physiol, doi:10.1152/ajpcell.00453.2022
Chittum, Sankaranarayanan, O'hara, Desai, On the Selectivity of Heparan Sulfate Recognition by SARS-CoV-2 Spike Glycoprotein, ACS Med. Chem. Lett, doi:10.1021/acsmedchemlett.1c00343?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Clausen, Sandoval, Spliid, Pihl, Perrett et al., SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2, Cell, doi:10.1016/j.cell.2020.09.033
De Pasquale, Quiccione, Tafuri, Avallone, Pavone, Heparan Sulfate Proteoglycans in Viral Infection and Treatment: A Special Focus on SARS-CoV-2, Int. J. Mol. Sci, doi:10.3390/ijms22126574
Demeter, Peleskei, Kutvölgyi, Rusznyák, Fenyvesi et al., Synthesis and Biological Profiling of Seven Heparin and Heparan Sulphate Analogue Trisaccharides, Biomolecules, doi:10.3390/biom14091052
Fröba, Große, Setz, Rauch, Auth et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, Int. J. Mol. Sci, doi:10.3390/ijms222413202
Gandhi, Mancera, The Structure of Glycosaminoglycans and Their Interactions with Proteins, Chem. Biol. Drug Des, doi:10.1111/j.1747-0285.2008.00741.x
Gerke, Ebbesen, Jansen, Boden, Freichel et al., Sequence-Controlled Glycopolymers via Step-Growth Polymerization of Precision Glycomacromolecules for Lectin Receptor Clustering, Biomacromolecules, doi:10.1021/acs.biomac.6b01657?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Gerling-Driessen, Hoffmann, Schmidt, Snyder, Hartmann, Glycopolymers against Pathogen Infection, Chem. Soc. Rev, doi:10.1039/D2CS00912A
Ghosh, Chattopadhyay, Marschall, Karmakar, Mandal et al., Focus on Antivirally Active Sulfated Polysaccharides: From Structure-Activity Analysis to Clinical Evaluation, Glycobiology, doi:10.1093/glycob/cwn092
Godula, Bertozzi, Density Variant Glycan Microarray for Evaluating Cross-Linking of Mucin-Like Glycoconjugates by Lectins, J. Am. Chem. Soc, doi:10.1021/ja302193u?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Gou, Geng, Richards, Burns, Remzi Becer et al., A Detailed Study on Understanding Glycopolymer Library and Con A Interactions, J. Polym. Sci., Part A: Polym. Chem, doi:10.1002/pola.26646
Guerrini, Beccati, Shriver, Naggi, Viswanathan et al., Oversulfated Chondroitin Sulfate Is a Contaminant in Heparin Associated with Adverse Clinical Events, Nat. Biotechnol, doi:10.1038/nbt1407
Guimond, Mycroft-West, Gandhi, Tree, Le et al., Synthetic Heparan Sulfate Mimetic Pixatimod (PG545) Potently Inhibits SARS-CoV-2 by Disrupting the Spike-ACE2 Interaction, ACS Cent. Sci, doi:10.1021/acscentsci.1c01293?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Hartmann, Department of Organic and Macromolecular Chemistry
He, Song, Jin, Li, Xia et al., Marine Sulfated Glycans Inhibit the Interaction of Heparin with S-Protein of SARS-CoV-2 Omicron XBB Variant, Glycoconj. J, doi:10.1007/s10719-024-10150-1
Hoffmann, Snyder, Hartmann, Polymers Inspired by Heparin and Heparan Sulfate for Viral Targeting, Macromolecules, doi:10.1021/acs.macromol.2c00675?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Holmes, Nagarajan, Desai, 3-O-Sulfation Induces Sequence-Specific Compact Topologies in Heparan Sulfate That Encode a Dynamic Sulfation Code, Comput. Struct. Biotechnol. J, doi:10.1016/j.csbj.2022.07.013
Ignjatovic, Activated Partial Thromboplastin Time
Ignjatovic, Thrombin Clotting Time
Jiang, Zhang, Lu, Li, Lv et al., Heparin Mimetics as Potential Intervention for COVID-19 and Their Bio-Manufacturing, Synth. Syst. Biotechnol, doi:10.1016/j.synbio.2022.10.002
Johnson, Li, Adams, Huntington, Antithrombin-S195A Factor Xa-Heparin Structure Reveals the Allosteric Mechanism of Antithrombin Activation, EMBO J, doi:10.1038/sj.emboj.7601089
Jono, Nagao, Oh, Sonoda, Hoshino et al., Controlling the Lectin Recognition of Glycopolymers via Distance Arrangement of Sugar Blocks, Chem. Commun, doi:10.1039/C7CC07107H
Kearns, Sandoval, Casalino, Clausen, Rosenfeld et al., Spike-Heparan Sulfate Interactions in SARS-CoV-2 Infection, Curr. Opin. Struct. Biol, doi:10.1016/j.sbi.2022.102439
Kjellén, Lindahl, Specificity of Glycosaminoglycan-Protein Interactions, Curr. Opin. Struct. Biol, doi:10.1016/j.sbi.2017.12.011
Konecki, Lesaffre, Guillou, Feliu, Dubuisson et al., Population Pharmacokinetics of Unfractionated Heparin and Multivariable Analysis of Activated Clotting Time in Patients Undergoing Radiofrequency Ablation of Atrial Fibrillation, Biomed. Pharmacother, doi:10.1016/j.biopha.2024.117700
Kreuger, Kjellén, Heparan Sulfate Biosynthesis: Regulation and Variability, J. Histochem. Cytochem, doi:10.1369/0022155412464972
Kreuger, Kjellén, Heparan Sulfate Biosynthesis: Regulation and Variability, J. Histochem. Cytochem, doi:10.1369/0022155412464972
Lai, Rizzato, Aydin, Villalonga-Planells, Drexler et al., A Ran-Binding Protein Facilitates Nuclear Import of Human Papillomavirus Type 16, PLoS Pathog, doi:10.1371/journal.ppat.1009580
Lamuraglia, Houbballah, Laposata, The Identification and Management of Heparin-Induced Thrombocytopenia in the Vascular Patient, J. Vasc. Surg, doi:10.1016/j.jvs.2011.10.082
Li, Johnson, Esmon, Huntington, 00576 Biomacromolecules XXXX, XXX, XXX-XXX L plex Reveals the Antithrombotic Mechanism of Heparin, Nat. Struct. Mol. Biol, doi:10.1038/nsmb811
Lindahl, Kusche-Gullberg, Kjellén, Regulated Diversity of Heparan Sulfate, J. Biol. Chem, doi:10.1074/jbc.273.39.24979
Liu, Chen, Chen, Chemical Synthesis of Glycosaminoglycan-Mimetic Polymers, Polym. Chem, doi:10.1039/c8py01338a
Liu, Chopra, Li, Bouwman, Tompkins et al., Heparan Sulfate Proteoglycans as Attachment Factor for SARS-CoV-2, ACS Cent. Sci, doi:10.1021/acscentsci.1c00010?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Liu, Zhang, Linhardt, Lessons Learned from the Contamination of Heparin, Nat. Prod. Rep, doi:10.1039/b819896a
Liu, Zhang, Linhardt, Lessons Learned from the Contamination of Heparin, Nat. Prod. Rep, doi:10.1039/b819896a
Miura, Fukuda, Seto, Hoshino, Development of Glycosaminoglycan Mimetics Using Glycopolymers, Polym. J, doi:10.1038/pj.2015.110
Mohamed, Anhlan, Schöfbänker, Schreiber, Classen et al., Hypericum perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2, Pharmaceuticals, doi:10.3390/ph15050530
Mohamed, Coombe, Heparin Mimetics: Their Therapeutic Potential, Pharmaceuticals, doi:10.3390/ph10040078
Mulloy, Hogwood, Gray, Lever, Page, Pharmacology of Heparin and Related Drugs, Pharmacol. Rev, doi:10.1124/pr.115.011247
Nahain, Ignjatovic, Monagle, Tsanaktsidis, Vamvounis et al., Anticoagulant Heparin Mimetics via RAFT Polymerization, Biomacromolecules, doi:10.1021/acs.biomac.9b01688?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Nahain, Ignjatovic, Monagle, Tsanaktsidis, Vamvounis et al., Sulfonated RAFT Copolymers as Heparin Mimetics: Synthesis, Reactivity Ratios, and Anticoagulant Activity, Macromol. Biosci, doi:10.1002/mabi.202000110
Nie, Pouyan, Lauster, Trimpert, Kerkhoff et al., Polysulfates Block SARS-CoV-2 Uptake through Electrostatic Interactions, Angew. Chem., Int. Ed, doi:10.1002/anie.202102717
Olson, Richard, Izaguirre, Schedin-Weiss, Gettins, Molecular Mechanisms of Antithrombin-Heparin Regulation of Blood Clotting Proteinases: A Paradigm for Understanding Proteinase Regulation by Serpin Family Protein Proteinase Inhibitors, Biochimie, doi:10.1016/j.biochi.2010.05.011
Paluck, Nguyen, Maynard, Heparin-Mimicking Polymers: Synthesis and Biological Applications, Biomacromolecules, doi:10.1021/acs.biomac.6b01147?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Paluck, Nguyen, Maynard, Heparin-Mimicking Polymers: Synthesis and Biological Applications, Biomacromolecules, doi:10.1021/acs.biomac.6b01147?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Pongener, O'shea, Wootton, Watkinson, Miller, Developments in the Chemical Synthesis of Heparin and Heparan Sulfate, Chem. Rec, doi:10.1002/tcr.202100173
Ray, Ali, Jana, Mukherjee, Pal et al., Antiviral Strategies Using Natural Source-Derived Sulfated Polysaccharides in the Light of the COVID-19 Pandemic and Major Human Pathogenic Viruses, Viruses, doi:10.3390/v14010035
Sasisekharan, Venkataraman, Heparin and Heparan Sulfate: Biosynthesis, Structure and Function, Curr. Opin. Chem. Biol, doi:10.1016/S1367-5931(00)00145-9
Soria-Martinez, Bauer, Giesler, Schelhaas, Materlik et al., Prophylactic Antiviral Activity of Sulfated Glycomimetic Oligomers and Polymers, J. Am. Chem. Soc, doi:10.1021/jacs.9b13484?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Sun, Chopra, Tomris, Van Der Woude, Liu et al., Well-Defined Heparin Mimetics Can Inhibit Binding of the Trimeric Spike of SARS-CoV-2 in a Length-Dependent Manner, JACS Au, doi:10.1021/jacsau.3c00042?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Valles, Naeem, Rozenfeld, Aldasooky, Wong et al., Multivalent Binding of Concanavalin A on Variable-Density Mannoside Microarrays, Faraday Discuss, doi:10.1039/C9FD00028C
Wang, Hsieh, Xu, Thieker, Chai et al., Synthesis of 3-O-Sulfated Oligosaccharides to Understand the Relationship between Structures and Functions of Heparan Sulfate, J. Am. Chem. Soc, doi:10.1021/jacs.7b01923?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Wilkins, Phillips, Deller, Davies, Gibson, Synthesis and Characterisation of Glucose-Functional Glycopolymers and Gold Nanoparticles: Study of Their Potential Interactions with Ovine Red Blood Cells, Carbohydr. Res, doi:10.1016/j.carres.2014.09.009
Zettl, Meister, Vollmer, Fischer, Steinmann et al., Rapid Quantification of SARS-CoV-2-Neutralizing Antibodies Using Propagation-Defective Vesicular Stomatitis Virus Pseudotypes, Vaccines, doi:10.3390/vaccines8030386
DOI record:
{
"DOI": "10.1021/acs.biomac.5c00576",
"ISSN": [
"1525-7797",
"1526-4602"
],
"URL": "http://dx.doi.org/10.1021/acs.biomac.5c00576",
"alternative-id": [
"10.1021/acs.biomac.5c00576"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0001-7709-5886",
"affiliation": [
{
"name": "Department of Organic and Macromolecular Chemistry, Heinrich-Heine-University Düsseldorf, Universitätsstraße 1, Düsseldorf 40225, Germany"
}
],
"authenticated-orcid": true,
"family": "Hoffmann",
"given": "Miriam",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Organic and Macromolecular Chemistry, Heinrich-Heine-University Düsseldorf, Universitätsstraße 1, Düsseldorf 40225, Germany"
}
],
"family": "Bonda",
"given": "Lorand",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Cellular Virology, ZMBE and Cells in Motion Interfaculty Centre CiMIC",
"place": [
"Münster, Germany"
]
},
{
"name": "University of Münster",
"place": [
"Münster, Germany"
]
}
],
"family": "Fels",
"given": "Ines",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Molecular Virology, ZMBE",
"place": [
"Münster, Germany"
]
},
{
"name": "University of Münster",
"place": [
"Münster, Germany"
]
}
],
"family": "Anhlan",
"given": "Darisuran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Molecular Virology, ZMBE",
"place": [
"Münster, Germany"
]
},
{
"name": "University of Münster",
"place": [
"Münster, Germany"
]
}
],
"family": "Hrincius",
"given": "Eike",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Central Institute of Laboratory Medicine, Medical Faculty, University Hospital Düsseldorf",
"place": [
"Düsseldorf, Germany"
]
},
{
"name": "Heinrich-Heine-University",
"place": [
"Düsseldorf, Germany"
]
}
],
"family": "Hermsen",
"given": "Derik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Molecular Virology, ZMBE",
"place": [
"Münster, Germany"
]
},
{
"name": "University of Münster",
"place": [
"Münster, Germany"
]
}
],
"family": "Ludwig",
"given": "Stephan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Cellular Virology, ZMBE and Cells in Motion Interfaculty Centre CiMIC",
"place": [
"Münster, Germany"
]
},
{
"name": "University of Münster",
"place": [
"Münster, Germany"
]
}
],
"family": "Schelhaas",
"given": "Mario",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2508-4090",
"affiliation": [
{
"name": "Department of Chemistry",
"place": [
"Davidson, United States"
]
},
{
"name": "Davidson College",
"place": [
"Davidson, United States"
]
}
],
"authenticated-orcid": true,
"family": "Snyder",
"given": "Nicole L.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0115-6405",
"affiliation": [
{
"name": "Department of Organic and Macromolecular Chemistry, Heinrich-Heine-University Düsseldorf, Universitätsstraße 1, Düsseldorf 40225, Germany"
},
{
"name": "Institute for Macromolecular Chemistry, University of Freiburg, Stefan-Meier-Street 31, Freiburg i.Br. 79104, Germany"
}
],
"authenticated-orcid": true,
"family": "Hartmann",
"given": "Laura",
"sequence": "additional"
}
],
"container-title": "Biomacromolecules",
"container-title-short": "Biomacromolecules",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
7,
7
]
],
"date-time": "2025-07-07T04:33:41Z",
"timestamp": 1751862821000
},
"deposited": {
"date-parts": [
[
2025,
7,
7
]
],
"date-time": "2025-07-07T08:09:13Z",
"timestamp": 1751875753000
},
"funder": [
{
"DOI": "10.13039/100001309",
"award": [
"27368"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100001309",
"id-type": "DOI"
}
],
"name": "Research Corporation for Science Advancement"
},
{
"DOI": "10.13039/501100001659",
"award": [
"HA5950/5-2",
"SCHE1552/3-2"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100001659",
"id-type": "DOI"
}
],
"name": "Deutsche Forschungsgemeinschaft"
},
{
"DOI": "10.13039/100000089",
"award": [
"1854028"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000089",
"id-type": "DOI"
}
],
"name": "Office of International Science and Engineering"
}
],
"indexed": {
"date-parts": [
[
2025,
7,
7
]
],
"date-time": "2025-07-07T08:40:11Z",
"timestamp": 1751877611751,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
7,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 1,
"start": {
"date-parts": [
[
2025,
7,
7
]
],
"date-time": "2025-07-07T00:00:00Z",
"timestamp": 1751846400000
}
}
],
"link": [
{
"URL": "https://pubs.acs.org/doi/pdf/10.1021/acs.biomac.5c00576",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "unspecified"
},
{
"URL": "https://pubs.acs.org/doi/pdf/10.1021/acs.biomac.5c00576",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "316",
"original-title": [],
"prefix": "10.1021",
"published": {
"date-parts": [
[
2025,
7,
6
]
]
},
"published-online": {
"date-parts": [
[
2025,
7,
6
]
]
},
"publisher": "American Chemical Society (ACS)",
"reference": [
{
"DOI": "10.1152/ajpcell.00453.2022",
"doi-asserted-by": "publisher",
"key": "ref1/cit1"
},
{
"DOI": "10.1002/advs.202201853",
"doi-asserted-by": "publisher",
"key": "ref2/cit2"
},
{
"DOI": "10.15252/embj.2020106765",
"doi-asserted-by": "publisher",
"key": "ref3/cit3"
},
{
"DOI": "10.1016/j.cell.2020.09.033",
"doi-asserted-by": "publisher",
"key": "ref4/cit4"
},
{
"DOI": "10.1021/acsmedchemlett.1c00343",
"doi-asserted-by": "publisher",
"key": "ref5/cit5"
},
{
"DOI": "10.1021/acscentsci.1c00010",
"doi-asserted-by": "publisher",
"key": "ref6/cit6"
},
{
"DOI": "10.1016/j.sbi.2022.102439",
"doi-asserted-by": "publisher",
"key": "ref7/cit7"
},
{
"DOI": "10.1111/j.1747-0285.2008.00741.x",
"doi-asserted-by": "publisher",
"key": "ref8/cit8"
},
{
"DOI": "10.1369/0022155412464972",
"doi-asserted-by": "publisher",
"key": "ref9/cit9"
},
{
"DOI": "10.3390/ijms22126574",
"doi-asserted-by": "publisher",
"key": "ref10/cit10"
},
{
"DOI": "10.3390/v14010035",
"doi-asserted-by": "publisher",
"key": "ref11/cit11"
},
{
"DOI": "10.1056/NEJMoa2015432",
"doi-asserted-by": "publisher",
"key": "ref12/cit12"
},
{
"DOI": "10.1111/jth.14860",
"doi-asserted-by": "publisher",
"key": "ref13/cit13"
},
{
"DOI": "10.3390/ph10040078",
"doi-asserted-by": "publisher",
"key": "ref14/cit14"
},
{
"DOI": "10.1038/nbt1407",
"doi-asserted-by": "publisher",
"key": "ref15/cit15"
},
{
"DOI": "10.1039/b819896a",
"doi-asserted-by": "publisher",
"key": "ref16/cit16"
},
{
"DOI": "10.1016/j.sbi.2017.12.011",
"doi-asserted-by": "publisher",
"key": "ref17/cit17"
},
{
"DOI": "10.1038/pj.2015.110",
"doi-asserted-by": "publisher",
"key": "ref18/cit18"
},
{
"DOI": "10.1021/acs.biomac.6b01147",
"doi-asserted-by": "publisher",
"key": "ref19/cit19"
},
{
"DOI": "10.1039/c8py01338a",
"doi-asserted-by": "publisher",
"key": "ref20/cit20"
},
{
"DOI": "10.1021/jacs.7b01923",
"doi-asserted-by": "publisher",
"key": "ref21/cit21"
},
{
"DOI": "10.1002/tcr.202100173",
"doi-asserted-by": "publisher",
"key": "ref22/cit22"
},
{
"DOI": "10.1021/acscentsci.1c01293",
"doi-asserted-by": "publisher",
"key": "ref23/cit23"
},
{
"DOI": "10.1016/j.synbio.2022.10.002",
"doi-asserted-by": "publisher",
"key": "ref24/cit24"
},
{
"DOI": "10.1021/jacsau.3c00042",
"doi-asserted-by": "publisher",
"key": "ref25/cit25"
},
{
"DOI": "10.3390/biom14091052",
"doi-asserted-by": "publisher",
"key": "ref26/cit26"
},
{
"DOI": "10.1007/s10719-024-10150-1",
"doi-asserted-by": "publisher",
"key": "ref27/cit27"
},
{
"DOI": "10.1021/acs.macromol.2c00675",
"doi-asserted-by": "publisher",
"key": "ref28/cit28"
},
{
"DOI": "10.1039/D2CS00912A",
"doi-asserted-by": "publisher",
"key": "ref29/cit29"
},
{
"DOI": "10.1002/anie.202102717",
"doi-asserted-by": "publisher",
"key": "ref30/cit30"
},
{
"DOI": "10.1021/acsmedchemlett.3c00319",
"doi-asserted-by": "publisher",
"key": "ref31/cit31"
},
{
"DOI": "10.1021/jacs.9b13484",
"doi-asserted-by": "publisher",
"key": "ref32/cit32"
},
{
"DOI": "10.1016/j.carres.2014.09.009",
"doi-asserted-by": "publisher",
"key": "ref33/cit33"
},
{
"DOI": "10.3390/vaccines8030386",
"doi-asserted-by": "publisher",
"key": "ref34/cit34"
},
{
"DOI": "10.3390/ph15050530",
"doi-asserted-by": "publisher",
"key": "ref35/cit35"
},
{
"DOI": "10.1371/journal.ppat.1009580",
"doi-asserted-by": "publisher",
"key": "ref36/cit36"
},
{
"DOI": "10.1093/glycob/cwn092",
"doi-asserted-by": "publisher",
"key": "ref37/cit37"
},
{
"DOI": "10.1016/j.biopha.2024.117700",
"doi-asserted-by": "publisher",
"key": "ref38/cit38"
},
{
"DOI": "10.1016/j.jvs.2011.10.082",
"doi-asserted-by": "publisher",
"key": "ref39/cit39"
},
{
"DOI": "10.1074/jbc.273.39.24979",
"doi-asserted-by": "publisher",
"key": "ref40/cit40"
},
{
"DOI": "10.1369/0022155412464972",
"doi-asserted-by": "publisher",
"key": "ref41/cit41"
},
{
"DOI": "10.1021/ja302193u",
"doi-asserted-by": "publisher",
"key": "ref42/cit42"
},
{
"DOI": "10.1002/pola.26646",
"doi-asserted-by": "publisher",
"key": "ref43/cit43"
},
{
"DOI": "10.1021/acs.biomac.6b01657",
"doi-asserted-by": "publisher",
"key": "ref44/cit44"
},
{
"DOI": "10.1039/C7CC07107H",
"doi-asserted-by": "publisher",
"key": "ref45/cit45"
},
{
"DOI": "10.1002/chem.201704179",
"doi-asserted-by": "publisher",
"key": "ref46/cit46"
},
{
"DOI": "10.1039/C9FD00028C",
"doi-asserted-by": "publisher",
"key": "ref47/cit47"
},
{
"DOI": "10.1529/biophysj.105.067918",
"doi-asserted-by": "publisher",
"key": "ref48/cit48"
},
{
"DOI": "10.1016/j.csbj.2022.07.013",
"doi-asserted-by": "publisher",
"key": "ref49/cit49"
},
{
"DOI": "10.3390/ijms222413202",
"doi-asserted-by": "publisher",
"key": "ref50/cit50"
},
{
"DOI": "10.1124/pr.115.011247",
"doi-asserted-by": "publisher",
"key": "ref51/cit51"
},
{
"DOI": "10.1039/b819896a",
"doi-asserted-by": "publisher",
"key": "ref52/cit52"
},
{
"DOI": "10.1016/S1367-5931(00)00145-9",
"doi-asserted-by": "publisher",
"key": "ref53/cit53"
},
{
"DOI": "10.1016/j.biochi.2010.05.011",
"doi-asserted-by": "publisher",
"key": "ref54/cit54"
},
{
"DOI": "10.1038/sj.emboj.7601089",
"doi-asserted-by": "publisher",
"key": "ref55/cit55"
},
{
"DOI": "10.1038/nsmb811",
"doi-asserted-by": "publisher",
"key": "ref56/cit56"
},
{
"author": "Ignjatovic V.",
"first-page": "111",
"key": "ref57/cit57",
"volume": "992",
"volume-title": "Methods in Molecular Biology",
"year": "2013"
},
{
"author": "Ignjatovic V.",
"first-page": "131",
"key": "ref58/cit58",
"volume": "992",
"volume-title": "Methods in Molecular Biology",
"year": "2013"
},
{
"DOI": "10.1021/acs.biomac.6b01147",
"doi-asserted-by": "publisher",
"key": "ref59/cit59"
},
{
"DOI": "10.1021/acs.biomac.9b01688",
"doi-asserted-by": "publisher",
"key": "ref60/cit60"
},
{
"DOI": "10.1002/mabi.202000110",
"doi-asserted-by": "publisher",
"key": "ref61/cit61"
},
{
"DOI": "10.1021/acs.jmedchem.3c00132",
"doi-asserted-by": "publisher",
"key": "ref62/cit62"
}
],
"reference-count": 62,
"references-count": 62,
"relation": {},
"resource": {
"primary": {
"URL": "https://pubs.acs.org/doi/10.1021/acs.biomac.5c00576"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity",
"type": "journal-article"
}