Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection
et al., International Journal of Nanomedicine, doi:10.2147/IJN.S448005, Mar 2024
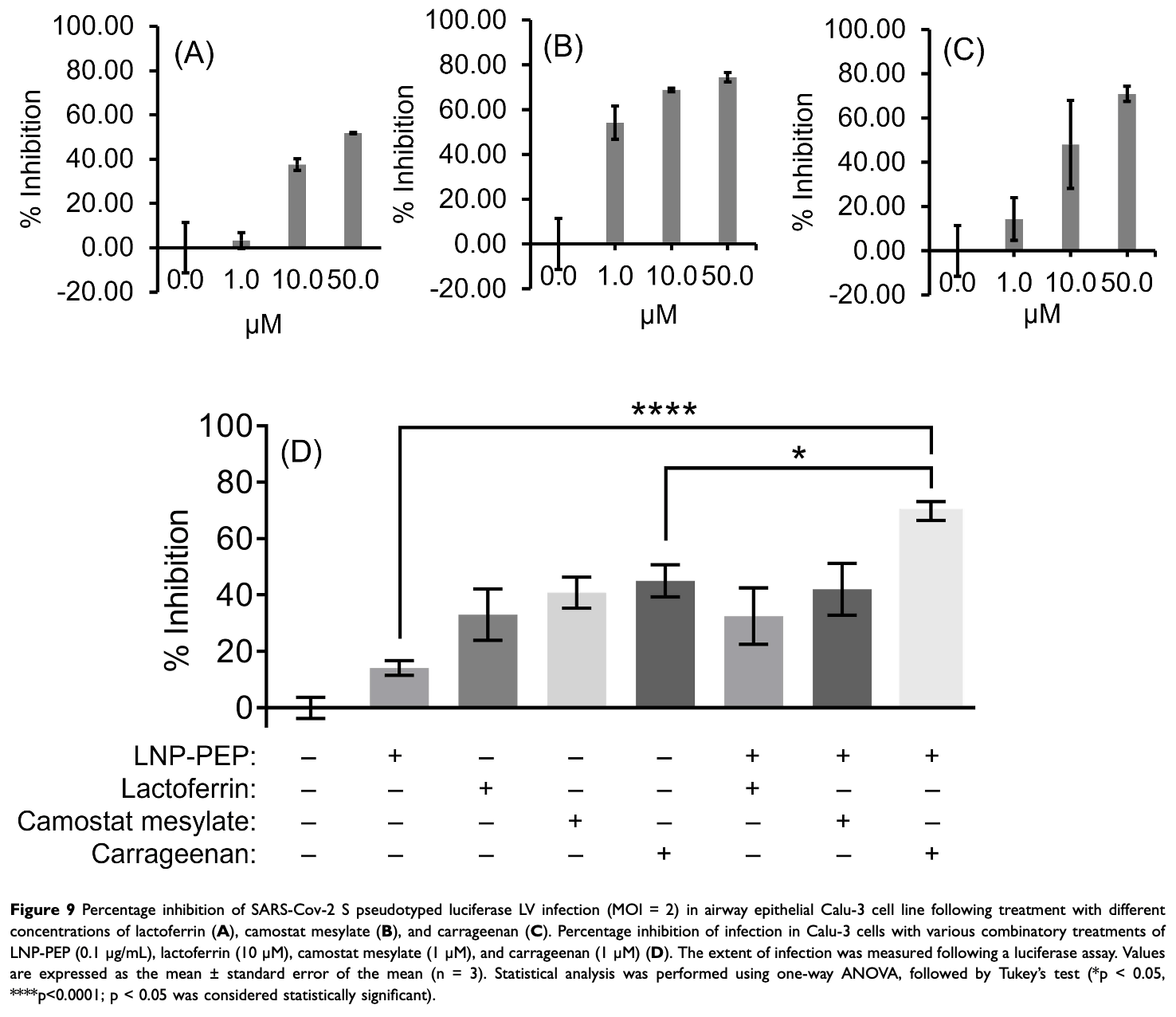
In vitro study showing that lactoferrin, camostat mesylate, and carrageenan inhibit SARS-CoV-2 pseudovirus infection in airway epithelial Calu-3 cells. All show dose-dependent inhibition. The study focuses on novel LNP formulations and the combination of carrageenan with the authors' LNP-PEP formulation containing ACE2 peptide showed significantly higher inhibition compared to carrageenan alone.
19 preclinical studies support the efficacy of iota-carrageenan for COVID-19:
Study covers iota-carrageenan, lactoferrin, and TMPRSS2 inhibitors.
1.
Herida et al., Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC155.071.
2.
Krylova et al., Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents, International Journal of Molecular Sciences, doi:10.3390/ijms26136175.
3.
Rohilla et al., Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV-2, Stresses, doi:10.3390/stresses3030039.
4.
Thet, H., The potential of carrageenan for the drug discovery of COVID-19 via molecular docking with angiotensin-converting enzyme 2 (ACE2) and the main protease (Mpro) of SARS-CoV-2, Journal of Bioinformatics and Genomics, doi:10.18454/jbg.2022.18.2.001.
5.
Alsaidi et al., Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model, Marine Drugs, doi:10.3390/md19080418.
6.
Sattari et al., Repositioning Therapeutics for COVID-19: Virtual Screening of the Potent Synthetic and Natural Compounds as SARS-CoV-2 3CLpro Inhibitors, Research Square, doi:10.21203/rs.3.rs-37994/v1.
7.
Hoffmann et al., Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity, Biomacromolecules, doi:10.1021/acs.biomac.5c00576.
8.
Yathindranath et al., Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection, International Journal of Nanomedicine, doi:10.2147/IJN.S448005.
9.
Setz et al., Iota-Carrageenan Inhibits Replication of the SARS-CoV-2 Variants of Concern Omicron BA.1, BA.2 and BA.5, Nutraceuticals, doi:10.3390/nutraceuticals3030025.
10.
Meister et al., Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2, Journal of Hospital Infection, doi:10.1016/j.jhin.2021.10.019.
11.
Bovard et al., Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochemistry and Biophysics Reports, doi:10.1016/j.bbrep.2021.101187.
12.
Fröba et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, International Journal of Molecular Sciences, doi:10.3390/ijms222413202.
13.
Varese et al., Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model, bioRxiv, doi:10.1101/2021.04.27.441512.
14.
Morokutti-Kurz et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE, doi:10.1371/journal.pone.0237480.
15.
Song et al., Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food & Function, doi:10.1039/D0FO02017F.
Yathindranath et al., 28 Mar 2024, peer-reviewed, 5 authors.
Contact: donald.miller@umanitoba.ca.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection
International Journal of Nanomedicine, doi:10.2147/ijn.s448005
Purpose: The global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the lingering threat to public health has fueled the search for effective therapeutics to treat SARS-CoV-2. This study aimed to develop lipid nanoparticle (LNP) inhibitors of SARS-CoV-2 entry to reduce viral infection in the nose and upper airway. Methods: Two types of LNP formulations were prepared following a microfluidic mixing method. The LNP-Trap consisted of DOPC, DSPC, cholesterol, and DSPE-PEG-COOH modified with various spike protein binding ligands, including ACE2 peptide, recombinant human ACE2 (rhACE2) or monoclonal antibody to spike protein (mAb). The LNP-Trim consisted of ionizing cationic DLin-MC3-DMA, DSPC, cholesterol, and DMG-PEG lipids encapsulating siACE2 or siTMPRSS2. Both formulations were assayed for biocompatibility and cell uptake in airway epithelial cells (Calu-3). Functional assessment of activity was performed using SARS-CoV-2 spike protein binding assays (LNP-Trap), host receptor knockdown (LNP-Trim), and SARS-CoV-2 pseudovirus neutralization assay (LNP-Trap and LNP-Trim). Localization and tissue distribution of fluorescently labeled LNP formulations were assessed in mice following intranasal administration. Results: Both LNP formulations were biocompatible based on cell impedance and MTT cytotoxicity studies in Calu-3 cells at concentrations as high as 1 mg/mL. LNP-Trap formulations were able to bind spike protein and inhibit pseudovirus infection by 90% in Calu-3 cells. LNP-Trim formulations reduced ACE2 and TMPRSS2 at the mRNA (70% reduction) and protein level (50% reduction). The suppression of host targets in Calu-3 cells treated with LNP-Trim resulted in over 90% inhibition of pseudovirus infection. In vivo studies demonstrated substantial retention of LNP-Trap and LNP-Trim in the nasal cavity following nasal administration with minimal systemic exposure. Conclusion: Both LNP-Trap and LNP-Trim formulations were able to safely and effectively inhibit SARS-CoV-2 pseudoviral infection in airway epithelial cells. These studies provide proof-of-principle for a localized treatment approach for SARS-CoV-2 in the upper airway.
Disclosure
References
Ahn, Kim, Hong, Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19, J Clin Investig, doi:10.1172/JCI148517
Arias, Oliveros, Lechtig, Bustos, Biologics in COVID-19 so far: systematic review, Pharmaceuticals, doi:10.3390/ph15070783
Baram-Pinto, Shukla, Gedanken, Sarid, Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles, Small, doi:10.1002/smll.200902384
Bayón-Cordero, Alkorta, Arana, Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs, Nanomaterials, doi:10.3390/nano9030474
Belliveau, Huft, Lin, Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA, Mol Ther Nucleic Acids, doi:10.1038/mtna.2012.28
Bowman, Ballard, Ackerson, Feldheim, Margolis et al., Inhibition of HIV fusion with multivalent gold nanoparticles, J Am Chem Soc, doi:10.1021/ja710321g
Cahn, Amosu, Maisel, Duncan, Biomaterials for intranasal and inhaled vaccine delivery, Nat Rev Bioeng, doi:10.1038/s44222-022-00012-6
Chan, Tan, Narayanan, Procko, An engineered decoy receptor for SARS-CoV-2 broadly binds protein S sequence variants, Sci Adv, doi:10.1126/sciadv.abf1738
Chen, Cheng, Roffler, Polyethylene glycol immunogenicity: theoretical, clinical, and practical aspects of anti-polyethylene glycol antibodies, ACS Nano, doi:10.1021/acsnano.1c05922
Chen, Fang, Chen, Targeting and enrichment of viral pathogen by cell membrane cloaked magnetic nanoparticles for enhanced detection, ACS Appl Mater Interfaces, doi:10.1021/acsami.7b09931
El-Shennawy, Hoffmann, Dashzeveg, Circulating ACE2-expressing extracellular vesicles block broad strains of SARS-CoV-2, Nat Commun, doi:10.1038/s41467-021-27893-2
Giovane, Rezai, Henderson, Current pharmacological modalities for management of novel coronavirus disease 2019 (COVID-19) and the rationale for their utilization: a review, Rev Med Virol, doi:10.1002/rmv.2136
Gizurarson, The relevance of nasal physiology to the design of drug absorption studies, Adv Drug Deliv Rev, doi:10.1016/0169-409X(93)90015-V
Gong, -J Y, PTD4-apoptin protein and dacarbazine show a synergistic antitumor effect on B16-F1 melanoma in vitro and in vivo, Eur J Pharmacol, doi:10.1016/j.ejphar.2010.12.004
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.0202
Hak, Helgesen, Hektoen, The effect of nanoparticle polyethylene glycol surface density on ligand-directed tumor targeting studied in vivo by dual modality imaging, ACS Nano, doi:10.1021/nn301630n
Han, Penn-Nicholson, Cho, Identification of critical determinants on ACE2 for SARS-CoV entry and development of a potent entry inhibitor, International Journal of Nanomedicine, doi:10.1016/j.virol.2006.01.029
Haschke, Schuster, Poglitsch, Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects, Clin Pharmacokinet, doi:10.1007/s40262-013-0072-7
Higuchi, Suzuki, Arimori, Engineered ACE2 receptor therapy overcomes mutational escape of SARS-CoV-2, Nat Commun, doi:10.1038/s41467-021-24013-y
Hoffmann, Hofmann-Winkler, Smith, Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity, EBioMedicine, doi:10.1016/j.ebiom.2021.103255
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hou, Zaks, Langer, Dong, Lipid nanoparticles for mRNA delivery, Nat Rev Mater, doi:10.1038/s41578-021-00358-0
Huang, Leobandung, Foss, Peppas, Molecular aspects of muco-and bioadhesion: tethered structures and site-specific surfaces, J Control Release, doi:10.1016/S0168-3659(99)00233-3
Jia, Yue, Lazartigues, ACE2 mouse models: a toolbox for cardiovascular and pulmonary research, Nat Commun, doi:10.1038/s41467-020-18880-0
Kreuzberger, Hirsch, Chai, SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19, Cochrane Database Syst Rev, doi:10.1002/14651858.CD013825.pub2
Kuba, Yamaguchi, Penninger, Angiotensin-Converting Enzyme 2 (ACE2) in the pathogenesis of ARDS in COVID-19, Front Immunol, doi:10.3389/fimmu.2021.732690
Martinez-Avila, Hijazi, Marradi, Gold manno-glyconanoparticies: multivalent systems to block HIV-1 gp120 binding to the lectin DC-SIGN, Chem Eur J, doi:10.1002/chem.200900923
Mesias, Zhu, Tang, Effective ACE2 peptide-nanoparticle conjugation and its binding with the SARS-Cov-2 RBD quantified by dynamic light scattering, Chem Comm, doi:10.1039/D1CC02267A
Nathan, Shawa, De, Torre, A narrative review of the clinical practicalities of bamlanivimab and etesevimab antibody therapies for SARS-CoV-2, Infect Dis Ther, doi:10.1007/s40121-021-00515-6
Nikiforuk, Kuchinski, Twa, The contrasting role of nasopharyngeal angiotensin converting enzyme 2 (ACE2) transcription in SARS-CoV-2 infection: a cross-sectional study of people tested for COVID-19 in British Columbia, Canada, International Journal of Nanomedicine, doi:10.1016/j.ebiom.2021.103316
Niknam, Jafari, Golchin, Potential therapeutic options for COVID-19: an update on current evidence, Eur J Med Res, doi:10.1186/s40001-021-00626-3
Panahi, Gorabi, Talaei, An overview on the treatments and prevention against COVID-19, Virol J, doi:10.1186/s12985-023-01973-9
Papp, Sieben, Ludwig, Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles, Small, doi:10.1002/smll.201001349
Planas, Veyer, Baidaliuk, Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization, Nature, doi:10.1038/s41586-021-03777-9
Polack, Thomas, Kitchin, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577
Politch, Cu-Uvin, Moench, Safety, acceptability, and pharmacokinetics of a monoclonal antibody-based vaginal multipurpose prevention film (MB66): a Phase I randomized trial, PLoS Med, doi:10.1371/journal.pmed.1003495
Pustake, Tambolkar, Giri, Gandhi, SARS, MERS and CoVID-19: an overview and comparison of clinical, laboratory and radiological features, J Family Med Prim Care, doi:10.4103/jfmpc.jfmpc_839_21
Rao, Xia, Xu, Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines, Proc Natl Acad Sci, doi:10.1073/pnas.2014352117
Ren, Wang, Gao, Zhou, Omicron variant (B.1.1.529) of SARS-CoV-2: mutation, infectivity, transmission, and vaccine resistance, World J Clin Cases, doi:10.12998/wjcc.v10.i1.1
Schütz, Ruiz-Blanco, Münch, Kirchhoff, Sanchez-Garcia et al., Peptide and peptide-based inhibitors of SARS-CoV-2 entry, Adv Drug Deliv Rev, doi:10.1016/j.addr.2020.11.007
Scopes, Measurement of protein by spectrophotometry at 205 nm, Analy Biochem, doi:10.1016/0003-2697(74)90034-7
Semple, Klimuk, Harasym, Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures, Biochim Biophys Acta, doi:10.1016/S0005-2736(00)00343-6
Serra, Doménech, Peppas, Design of poly(ethylene glycol)-tethered copolymers as novel mucoadhesive drug delivery systems, CAS, SciSearch ® , Current Contents ® /Clinical Medicine, Journal Citation Reports/Science Edition, doi:10.1016/j.ejpb.2005.10.011
Sneller, Blazkova, Justement, Combination anti-HIV antibodies provide sustained virological suppression, Nature, doi:10.1038/s41586-022-04797-9
Sullivan, Gebo, Shoham, Early outpatient treatment for Covid-19 with convalescent plasma, N Engl J Med, doi:10.1056/NEJMoa2119657
Sungnak, Huang, Bécavin, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med, doi:10.1038/s41591-020-0868-6
Tam, Chen, Cullis, Advances in lipid nanoparticles for siRNA delivery, Pharmaceutics, doi:10.3390/pharmaceutics5030498
Tam, Kulkarni, An, Lipid nanoparticle formulations for optimal RNA-based topical delivery to murine airways, Eur J Pharm Sci, doi:10.1016/j.ejps.2022.106234
V'kovski, Kratzel, Steiner, Stalder, Thiel, Coronavirus biology and replication: implications for SARS-CoV-2, Nat Rev Microbiol, doi:10.1038/s41579-020-00468-6
Valenti, Antonini, Lactoferrin: an important host defence against microbial and viral attack. Cellular and molecular life sciences, Cell Mol Life Sci, doi:10.1007/s00018-005-5372-0
Van Der Meel, Chen, Zaifman, Modular lipid nanoparticle platform technology for siRNA and lipophilic prodrug delivery, Small, doi:10.1002/smll.202103025
Walsh, Seaman, Broadly neutralizing antibodies for HIV-1 prevention, Front Immunol, doi:10.3389/fimmu.2021.712122
Wei, Zhang, Ran, T-cell-mimicking nanoparticles can neutralize HIV infectivity, Adv Mater, doi:10.1002/adma.201802233
Wotring, Fursmidt, Ward, Sexton, Evaluating the in vitro efficacy of bovine lactoferrin products against SARS-CoV-2 variants of concern, J Dairy Sci, doi:10.3168/jds.2021-21247
Xu, Ensign, Boylan, Impact of Surface Polyethylene Glycol (PEG) density on biodegradable nanoparticle transport in mucus ex vivo and distribution in vivo, ACS Nano, doi:10.1021/acsnano.5b03876
Yang, Rao, Structural biology of SARS-CoV-2 and implications for therapeutic development, Nat Rev Microbiol, doi:10.1038/s41579-021-00630-8
Yathindranath, Safa, Sajesh, Spermidine/Spermine N1-Acetyltransferase 1 (SAT1)-A potential gene target for selective sensitization of glioblastoma cells using an ionizable lipid nanoparticle to deliver siRNA, Cancers, doi:10.3390/cancers14215179
Zhao, Wu, Heberle, Phase studies of model biomembranes: complex behavior of DSPC/DOPC/cholesterol, Biochim Biophys Acta, doi:10.1016/j.bbamem.2007.07.008
Álvarez-Viñas, Souto, Flórez-Fernández, Torres, Bandín et al., Antiviral activity of carrageenans and processing implications, Mar Drugs, doi:10.3390/md19080437
DOI record:
{
"DOI": "10.2147/ijn.s448005",
"ISSN": [
"1178-2013"
],
"URL": "http://dx.doi.org/10.2147/IJN.S448005",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5053-9704",
"affiliation": [],
"authenticated-orcid": true,
"family": "Yathindranath",
"given": "Vinith",
"sequence": "first"
},
{
"affiliation": [],
"family": "Safa",
"given": "Nura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tomczyk",
"given": "Mateusz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dolinsky",
"given": "Vernon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miller",
"given": "Donald",
"sequence": "additional"
}
],
"container-title": "International Journal of Nanomedicine",
"container-title-short": "IJN",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T06:50:15Z",
"timestamp": 1711608615000
},
"deposited": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T06:50:22Z",
"timestamp": 1711608622000
},
"indexed": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T01:40:31Z",
"timestamp": 1711676431908
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T00:00:00Z",
"timestamp": 1709251200000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/getfile.php?fileID=97961",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/getfile.php?fileID=97961",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "3087-3108",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2024,
3
]
]
},
"published-online": {
"date-parts": [
[
2024,
3
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"key": "ref1",
"unstructured": "Available from: https://covid19.who.int/. Accessed March 7, 2023. WHO COVID-19 dashboard"
},
{
"DOI": "10.4103/jfmpc.jfmpc_839_21",
"author": "Pustake",
"doi-asserted-by": "publisher",
"first-page": "10",
"journal-title": "J Family Med Prim Care",
"key": "ref2",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.12998/wjcc.v10.i1.1",
"author": "Ren",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "World J Clin Cases",
"key": "ref3",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1002/rmv.2136",
"author": "Giovane",
"doi-asserted-by": "publisher",
"first-page": "e2136",
"journal-title": "Rev Med Virol",
"key": "ref4",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"journal-title": "Cell",
"key": "ref5",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.3390/ph15070783",
"author": "Arias",
"doi-asserted-by": "publisher",
"first-page": "783",
"journal-title": "Pharmaceuticals",
"key": "ref6",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1186/s40001-021-00626-3",
"author": "Niknam",
"doi-asserted-by": "publisher",
"first-page": "6",
"journal-title": "Eur J Med Res",
"key": "ref7",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1186/s12985-023-01973-9",
"author": "Panahi",
"doi-asserted-by": "publisher",
"first-page": "23",
"journal-title": "Virol J",
"key": "ref8",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2119657",
"author": "Sullivan",
"doi-asserted-by": "publisher",
"first-page": "1700",
"journal-title": "N Engl J Med",
"key": "ref9",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.0202",
"author": "Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "632",
"journal-title": "JAMA",
"key": "ref10",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1038/s41579-021-00630-8",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "685",
"journal-title": "Nat Rev Microbiol",
"key": "ref11",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1038/s41579-020-00468-6",
"author": "V’kovski",
"doi-asserted-by": "publisher",
"first-page": "155",
"journal-title": "Nat Rev Microbiol",
"key": "ref12",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2034577",
"author": "Polack",
"doi-asserted-by": "publisher",
"first-page": "2603",
"journal-title": "N Engl J Med",
"key": "ref13",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.712122",
"author": "Walsh",
"doi-asserted-by": "publisher",
"first-page": "712122",
"journal-title": "Front Immunol",
"key": "ref14",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1371/journal.pmed.1003495",
"author": "Politch",
"doi-asserted-by": "publisher",
"first-page": "e1003495",
"journal-title": "PLoS Med",
"key": "ref15",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04797-9",
"author": "Sneller",
"doi-asserted-by": "publisher",
"first-page": "375",
"journal-title": "Nature",
"key": "ref16",
"volume": "606",
"year": "2022"
},
{
"DOI": "10.1002/14651858.CD013825.pub2",
"author": "Kreuzberger",
"doi-asserted-by": "publisher",
"first-page": "Cd013825",
"journal-title": "Cochrane Database Syst Rev",
"key": "ref17",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1007/s40121-021-00515-6",
"author": "Nathan",
"doi-asserted-by": "publisher",
"first-page": "1933",
"journal-title": "Infect Dis Ther",
"key": "ref18",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03777-9",
"author": "Planas",
"doi-asserted-by": "publisher",
"first-page": "276",
"journal-title": "Nature",
"key": "ref19",
"volume": "596",
"year": "2021"
},
{
"DOI": "10.1126/sciadv.abf1738",
"author": "Chan",
"doi-asserted-by": "publisher",
"first-page": "eabf1738",
"journal-title": "Sci Adv",
"key": "ref20",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.732690",
"author": "Kuba",
"doi-asserted-by": "publisher",
"first-page": "732690",
"journal-title": "Front Immunol",
"key": "ref21",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.virol.2006.01.029",
"author": "Han",
"doi-asserted-by": "publisher",
"first-page": "15",
"journal-title": "Virology",
"key": "ref22",
"volume": "350",
"year": "2006"
},
{
"DOI": "10.1016/j.addr.2020.11.007",
"author": "Schütz",
"doi-asserted-by": "publisher",
"first-page": "47",
"journal-title": "Adv Drug Deliv Rev",
"key": "ref23",
"volume": "167",
"year": "2020"
},
{
"DOI": "10.1007/s40262-013-0072-7",
"author": "Haschke",
"doi-asserted-by": "publisher",
"first-page": "783",
"journal-title": "Clin Pharmacokinet",
"key": "ref24",
"volume": "52",
"year": "2013"
},
{
"DOI": "10.1038/s41467-021-24013-y",
"author": "Higuchi",
"doi-asserted-by": "publisher",
"first-page": "3802",
"journal-title": "Nat Commun",
"key": "ref25",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1002/adma.201802233",
"author": "Wei",
"doi-asserted-by": "publisher",
"first-page": "e1802233",
"journal-title": "Adv Mater",
"key": "ref26",
"volume": "30",
"year": "2018"
},
{
"DOI": "10.1073/pnas.2014352117",
"author": "Rao",
"doi-asserted-by": "publisher",
"first-page": "27141",
"journal-title": "Proc Natl Acad Sci",
"key": "ref27",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-18880-0",
"author": "Jia",
"doi-asserted-by": "publisher",
"first-page": "5165",
"journal-title": "Nat Commun",
"key": "ref28",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1172/JCI148517",
"author": "Ahn",
"doi-asserted-by": "publisher",
"first-page": "148517",
"journal-title": "J Clin Investig",
"key": "ref29",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1016/0003-2697(74)90034-7",
"author": "Scopes",
"doi-asserted-by": "publisher",
"first-page": "277",
"journal-title": "Analy Biochem",
"key": "ref30",
"volume": "59",
"year": "1974"
},
{
"DOI": "10.1016/j.ejphar.2010.12.004",
"author": "J-l",
"doi-asserted-by": "publisher",
"first-page": "17",
"journal-title": "Eur J Pharmacol",
"key": "ref31",
"volume": "654",
"year": "2011"
},
{
"DOI": "10.3390/cancers14215179",
"author": "Yathindranath",
"doi-asserted-by": "publisher",
"first-page": "5179",
"journal-title": "Cancers",
"key": "ref32",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1021/acsnano.1c05922",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "14022",
"journal-title": "ACS Nano",
"key": "ref33",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1007/s00018-005-5372-0",
"author": "Valenti",
"doi-asserted-by": "publisher",
"first-page": "2576",
"journal-title": "Cell Mol Life Sci",
"key": "ref34",
"volume": "62",
"year": "2005"
},
{
"DOI": "10.3168/jds.2021-21247",
"author": "Wotring",
"doi-asserted-by": "publisher",
"first-page": "2791",
"journal-title": "J Dairy Sci",
"key": "ref35",
"volume": "105",
"year": "2022"
},
{
"DOI": "10.3390/md19080437",
"author": "Álvarez-viñas",
"doi-asserted-by": "publisher",
"first-page": "437",
"journal-title": "Mar Drugs",
"key": "ref36",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2021.103255",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "103255",
"journal-title": "EBioMedicine",
"key": "ref37",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1038/mtna.2012.28",
"author": "Belliveau",
"doi-asserted-by": "publisher",
"first-page": "e37",
"journal-title": "Mol Ther Nucleic Acids",
"key": "ref38",
"volume": "1",
"year": "2012"
},
{
"DOI": "10.1038/s41578-021-00358-0",
"author": "Hou",
"doi-asserted-by": "publisher",
"first-page": "1078",
"journal-title": "Nat Rev Mater",
"key": "ref39",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3390/pharmaceutics5030498",
"author": "Tam",
"doi-asserted-by": "publisher",
"first-page": "498",
"journal-title": "Pharmaceutics",
"key": "ref40",
"volume": "5",
"year": "2013"
},
{
"DOI": "10.3390/nano9030474",
"author": "Bayón-Cordero",
"doi-asserted-by": "publisher",
"first-page": "474",
"journal-title": "Nanomaterials",
"key": "ref41",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1016/j.bbamem.2007.07.008",
"author": "Zhao",
"doi-asserted-by": "publisher",
"first-page": "2764",
"journal-title": "Biochim Biophys Acta",
"key": "ref42",
"volume": "1768",
"year": "2007"
},
{
"DOI": "10.1016/S0005-2736(00)00343-6",
"author": "Semple",
"doi-asserted-by": "publisher",
"first-page": "152",
"journal-title": "Biochim Biophys Acta",
"key": "ref43",
"volume": "1510",
"year": "2001"
},
{
"DOI": "10.1002/smll.202103025",
"author": "van der Meel",
"doi-asserted-by": "publisher",
"first-page": "2103025",
"journal-title": "Small",
"key": "ref44",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1016/j.ejps.2022.106234",
"author": "Tam",
"doi-asserted-by": "publisher",
"first-page": "106234",
"journal-title": "Eur J Pharm Sci",
"key": "ref45",
"volume": "176",
"year": "2022"
},
{
"DOI": "10.1021/nn301630n",
"author": "Hak",
"doi-asserted-by": "publisher",
"first-page": "5648",
"journal-title": "ACS Nano",
"key": "ref46",
"volume": "6",
"year": "2012"
},
{
"DOI": "10.1002/smll.201001349",
"author": "Papp",
"doi-asserted-by": "publisher",
"first-page": "2900",
"journal-title": "Small",
"key": "ref47",
"volume": "6",
"year": "2010"
},
{
"DOI": "10.1002/chem.200900923",
"author": "Martinez-Avila",
"doi-asserted-by": "publisher",
"first-page": "9874",
"journal-title": "Chem Eur J",
"key": "ref48",
"volume": "15",
"year": "2009"
},
{
"DOI": "10.1002/smll.200902384",
"author": "Baram-Pinto",
"doi-asserted-by": "publisher",
"first-page": "1044",
"journal-title": "Small",
"key": "ref49",
"volume": "6",
"year": "2010"
},
{
"DOI": "10.1021/ja710321g",
"author": "Bowman",
"doi-asserted-by": "publisher",
"first-page": "6896",
"journal-title": "J Am Chem Soc",
"key": "ref50",
"volume": "130",
"year": "2008"
},
{
"DOI": "10.1038/s41467-021-27893-2",
"author": "El-Shennawy",
"doi-asserted-by": "publisher",
"first-page": "405",
"journal-title": "Nat Commun",
"key": "ref51",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1021/acsami.7b09931",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "39953",
"journal-title": "ACS Appl Mater Interfaces",
"key": "ref52",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.1039/D1CC02267A",
"author": "Mesias",
"doi-asserted-by": "publisher",
"first-page": "6979",
"journal-title": "Chem Comm",
"key": "ref53",
"volume": "57",
"year": "2021"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"author": "Sungnak",
"doi-asserted-by": "publisher",
"first-page": "681",
"journal-title": "Nat Med",
"key": "ref54",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2021.103316",
"author": "Nikiforuk",
"doi-asserted-by": "publisher",
"first-page": "103316",
"journal-title": "eBioMedicine",
"key": "ref55",
"volume": "66",
"year": "2021"
},
{
"DOI": "10.1016/0169-409X(93)90015-V",
"author": "Gizurarson",
"doi-asserted-by": "publisher",
"first-page": "329",
"journal-title": "Adv Drug Deliv Rev",
"key": "ref56",
"volume": "11",
"year": "1993"
},
{
"DOI": "10.1038/s44222-022-00012-6",
"author": "Cahn",
"doi-asserted-by": "publisher",
"first-page": "83",
"journal-title": "Nat Rev Bioeng",
"key": "ref57",
"volume": "1",
"year": "2023"
},
{
"DOI": "10.1016/S0168-3659(99)00233-3",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "63",
"journal-title": "J Control Release",
"key": "ref58",
"volume": "65",
"year": "2000"
},
{
"DOI": "10.1021/acsnano.5b03876",
"author": "Xu",
"doi-asserted-by": "publisher",
"first-page": "9217",
"journal-title": "ACS Nano",
"key": "ref59",
"volume": "9",
"year": "2015"
},
{
"DOI": "10.1016/j.ejpb.2005.10.011",
"author": "Serra",
"doi-asserted-by": "publisher",
"first-page": "11",
"journal-title": "Eur J Pharm Biopharm",
"key": "ref60",
"volume": "63",
"year": "2006"
}
],
"reference-count": 60,
"references-count": 60,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/lipid-nanoparticle-based-inhibitors-for-sars-cov-2-host-cell-infection-peer-reviewed-fulltext-article-IJN"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Organic Chemistry",
"Drug Discovery",
"General Medicine",
"Biomaterials",
"Bioengineering",
"Biophysics",
"Pharmaceutical Science"
],
"subtitle": [],
"title": "Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection",
"type": "journal-article",
"volume": "Volume 19"
}