Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents
et al., International Journal of Molecular Sciences, doi:10.3390/ijms26136175, Jun 2025
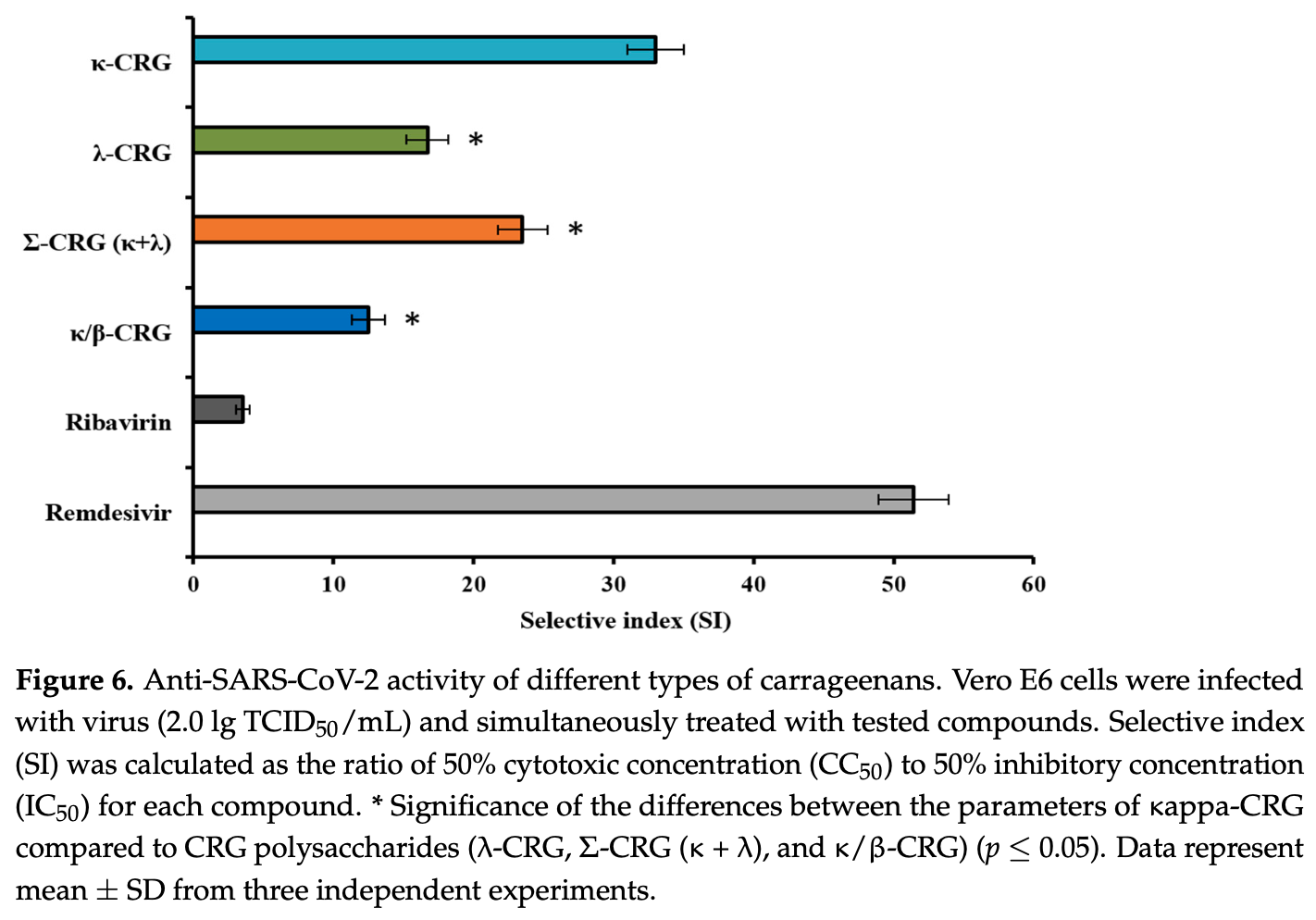
In vitro and in silico study showing that carrageenans and a carrageenan-echinochrome complex inhibit SARS-CoV-2 replication in Vero E6 cells. Authors isolated different types of carrageenans (kappa, lambda, kappa/beta) from red algae and used molecular docking to demonstrate their interactions with SARS-CoV-2's receptor-binding domain (RBD) and human ACE2 receptor through hydrogen bonding and ionic interactions. Kappa-carrageenan exhibited the strongest binding affinity to ACE2 and the most significant antiviral effect with a selective index (SI) of 33. The kappa-carrageenan/echinochrome complex showed even higher antiviral activity, with the strongest virucidal effect against SARS-CoV-2 particles (SI above 70) and 45% reduction in viral RNA levels. The complex was most effective when directly applied to virus particles before infection, suggesting a direct virucidal mechanism. All tested carrageenans demonstrated low cytotoxicity with CC50 values above 2000 μg/mL.
19 preclinical studies support the efficacy of iota-carrageenan for COVID-19:
1.
Herida et al., Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC155.071.
2.
Krylova et al., Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents, International Journal of Molecular Sciences, doi:10.3390/ijms26136175.
3.
Rohilla et al., Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV-2, Stresses, doi:10.3390/stresses3030039.
4.
Thet, H., The potential of carrageenan for the drug discovery of COVID-19 via molecular docking with angiotensin-converting enzyme 2 (ACE2) and the main protease (Mpro) of SARS-CoV-2, Journal of Bioinformatics and Genomics, doi:10.18454/jbg.2022.18.2.001.
5.
Alsaidi et al., Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model, Marine Drugs, doi:10.3390/md19080418.
6.
Sattari et al., Repositioning Therapeutics for COVID-19: Virtual Screening of the Potent Synthetic and Natural Compounds as SARS-CoV-2 3CLpro Inhibitors, Research Square, doi:10.21203/rs.3.rs-37994/v1.
7.
Hoffmann et al., Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity, Biomacromolecules, doi:10.1021/acs.biomac.5c00576.
8.
Yathindranath et al., Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection, International Journal of Nanomedicine, doi:10.2147/IJN.S448005.
9.
Setz et al., Iota-Carrageenan Inhibits Replication of the SARS-CoV-2 Variants of Concern Omicron BA.1, BA.2 and BA.5, Nutraceuticals, doi:10.3390/nutraceuticals3030025.
10.
Meister et al., Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2, Journal of Hospital Infection, doi:10.1016/j.jhin.2021.10.019.
11.
Bovard et al., Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochemistry and Biophysics Reports, doi:10.1016/j.bbrep.2021.101187.
12.
Fröba et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, International Journal of Molecular Sciences, doi:10.3390/ijms222413202.
13.
Varese et al., Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model, bioRxiv, doi:10.1101/2021.04.27.441512.
14.
Morokutti-Kurz et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE, doi:10.1371/journal.pone.0237480.
15.
Song et al., Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food & Function, doi:10.1039/D0FO02017F.
Krylova et al., 26 Jun 2025, peer-reviewed, 8 authors, study period 11 March, 2020 - 5 May, 2023.
Contact: krylovanatalya@gmail.com (corresponding author), olga_iun@inbox.ru, niiem_vl@mail.ru, adorob@mail.ru, kravchenko_89@mail.ru, galin56@mail.ru, glazunov@piboc.dvo.ru, imyer@mail.ru.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents
International Journal of Molecular Sciences, doi:10.3390/ijms26136175
The diversity of structural types of carrageenans (CRGs)-sulfated polysaccharides of red algae-determines their different biological activities. The different types of CRGs (kappa, lambda, kappa/beta-CRGs) were isolated from the red algae of the Pacific coast. Molecular docking was performed to determine potential interactions of CRGs with the receptorbinding domain (RBD) of SARS-CoV-2 and its cellular receptor-angiotensin-converting enzyme type 2 (ACE2). CRGs interacted with ACE2 and RBD via hydrogen bonding and ionic interactions. The strongest binding affinity of CRGs and ACE2 was observed for kappa-CRG. Molecular docking was confirmed by results studying the effects of CRGs against SARS-CoV-2 in vitro. The ability of CRGs, as well as the complex CRG with sea urchin echinochrome (Ech), to inhibit SARS-CoV-2 replication in Vero E6 cells was studied using cytopathic effect (CPE) inhibition and RT-PCR assays. The simultaneous treatment of cells with CRGs and the virus revealed that kappa-CRG exhibited the most significant antiviral effect among all the polysaccharides, with a selective index (SI) of 33. The kappa-CRG/Ech complex exhibited the highest virucidal effect on SARS-CoV-2 particles with an SI above 70 (more than two times higher than that of CRG and Ech) and reduced viral RNA levels by 45% (IC = 45%). Our results illustrate that CRGs and kappa-CRG/Ech complex can act as protective agents against SARS-CoV-2.
References
Ahmadi, Moghadamtousi, Abubakar, Zandi, Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review, BioMed Res. Int, doi:10.1155/2015/825203
Anastyuk, Barabanova, Correc, Nazarenko, Davydova et al., Analysis of structural heterogeneity of kappa/beta-carrageenan oligosaccharides from Tichocarpus crinitus by negative-ion ESI and tandem MALDI mass spectrometry, Carbohydr. Polym, doi:10.1016/j.carbpol.2011.04.081
Andrew, Jayaraman, Marine sulfated polysaccharides as potential antiviral drug candidates to treat Corona Virus disease (COVID-19), Carbohydr. Res, doi:10.1016/j.carres.2021.108326
Aung, Molecular Docking of Carrageenans for Main Protease (Mpro) and Angiotensin-Converting Enzyme 2 (ACE2) of SARS-CoV-2, Drug Des, doi:10.35248/2169-0138.22.11.221
Bansal, Jonsson, Taylor, Figueroa, Dugour et al., Iota-carrageenan inhibits SARS-CoV-2 in vitro at concentrations easily achievable by nasal and nebulization formulations, PLoS ONE, doi:10.1371/journal.pone.0259943
Beck, Antioxidants and viral infections: Host immune response and viral pathogenicity, J. Am. Coll. Nutr, doi:10.1080/07315724.2001.10719172
Bovard, Van Der Toorn, Schlage, Constant, Renggli et al., Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochem. Biophys. Rep, doi:10.1016/j.bbrep.2021.101187
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antivir. Res, doi:10.1016/j.antiviral.2020.104787
Correc, Barabanova, Tuvikene, Truus, Yermak et al., Comparison of the structures of hybrid κ/βcarrageenans extracted from Furcellaria lumbricalis and Tichocarpus crinitus, Carbohydr. Polym, doi:10.1016/j.carbpol.2011.11.052
Falshaw, Bixler, Johndro, Structure and performance of commercial kappa-2 carrageenan extracts. Pt III. Structure analysis and performance in two dairy applications of extracts from the New Zealand red seaweed, Food Hydrocoll, doi:10.1016/S0268-005X(02)00045-0
Fedoreyev, Krylova, Mishchenko, Vasileva, Pislyagin et al., Antiviral and antioxidant properties of echinochrome A, Mar. Drugs, doi:10.3390/md16120509
Figueroa, Lombardo, Dogliotti, Flynn, Giugliano et al., Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease, Int. J. Gen. Med, doi:10.2147/IJGM.S328486
Geahchan, Ehrlich, Rahman, The Anti-Viral Applications of Marine Resources for COVID-19 Treatment: An Overview, Mar. Drugs, doi:10.3390/md19080409
Hamming, Timens, Bulthuis, Lely, Navis et al., Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis, J. Pathol, doi:10.1002/path.1570
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 entry into cells, Nat. Rev. Mol. Cell Biol, doi:10.1038/s41580-021-00418-x
Jang, Shin, Lee, Kwon, Shin et al., Antiviral activity of lambdacarrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2, Sci. Rep, doi:10.1038/s41598-020-80896-9
Jawad, Adhikari, Podgornik, Ching, Binding Interactions between Receptor-Binding Domain of Spike Protein and Human Angiotensin Converting Enzyme-2 in Omicron Variant, J. Phys. Chem. Lett, doi:10.1021/acs.jpclett.2c00423
Jin, Zhang, Mitra, Mccandless, Sharma et al., The structure-activity relationship of the interactions of SARS-CoV-2 spike glycoproteins with glucuronomannan and sulfated galactofucan from Saccharina japonica, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2020.09.184
Jousselin, Pliego-Cortés, Damour, Garcia, Bodet et al., Anti-SARS-CoV-2 Activity of Polysaccharides Extracted from Halymenia floresii and Solieria chordalis (Rhodophyta), Mar. Drugs, doi:10.3390/md21060348
Kalitnik, Marcov, Anastyuk, Barabanova, Glazunov et al., Gelling polysaccharide from Chondrus armatus and its oligosaccharides: The structural peculiarities and anti-inflammatory activity, Carbohydr. Polym, doi:10.1016/j.carbpol.2014.04.070
Kim, Vasileva, Mishchenko, Fedoreyev, Han, Multifaceted Clinical Effects of Echinochrome, Mar. Drugs, doi:10.3390/md19080412
Knutsen, Myslabodski, Larsen, Usov, A modified system of nomenclature for red algal Galactans, Bot. Mar, doi:10.1515/botm.1994.37.2.163
Krylova, Gorbach, Iunikhina, Pott, Glazunov et al., Antiherpetic Activity of Carrageenan Complex with Echinochrome A and Its Liposomal Form, Int. J. Mol. Sci, doi:10.3390/ijms232415754
Krylova, Kravchenko, Iunikhina, Pott, Likhatskaya et al., Influence of the Structural Features of Carrageenans from Red Algae of the Far Eastern Seas on Their Antiviral Properties, Mar. Drugs, doi:10.3390/md20010060
Kuznetsova, Krylova, Kokoulin, Persiyanova, Maistrovskaya et al., Polysaccharides from Marine Bacteria and Their Anti-SARS-CoV-2Activity, Microbiol. Res
Latha, Hrishikesh, Shiban, Chandrashekar, Bharath, In silico, in vitro screening of plant extracts for anti-SARS-CoV-2 activity and evaluation of their acute and sub-acute toxicity, Phytomed Plus, doi:10.1016/j.phyplu.2022.100233
Lenharo, WHO declares end to COVID-19's emergency phase, Nature, doi:10.1038/d41586-023-01559-z
Lim, Moore, Vasilieva, Sui, Wong et al., Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus, Nature, doi:10.1038/nature02145
Mukherjee, Ghosh, Hahn, Wangen, Strojan et al., Chemically sulfated polysaccharides from natural sources: Assessment of extraction-sulfation efficiencies, structural features and antiviral activities, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2019.05.005
Padmi, Prasedya, Kharisma, Widyananda, Ansori et al., Macroalgae Bioactive Compounds for the potential Antiviral of SARS-CoV-2: An In Silico Study, J. Pure Appl. Microbiol, doi:10.22207/JPAM.16.2.26
Pauwels, Balzarini, Baba, Snoeck, Schols et al., Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds, J. Virol. Methods, doi:10.1016/0166-0934(88)90134-6
Pereira, Amado, Critchley, Van De Velde, Ribeiro-Claro, Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman), Food Hydrocoll, doi:10.1016/j.foodhyd.2008.11.014
Poorolajal, The global pandemics are getting more frequent and severe, J. Res. Health Sci, doi:10.34172/jrhs.2021.40
Salih, Thissera, Yaseen, Hassane, El-Seedi et al., Marine Sulfated Polysaccharides as Promising Antiviral Agents: A Comprehensive Report and Modeling Study Focusing on SARS-CoV-2, Mar. Drugs, doi:10.3390/md19080406
Schütz, Conzelmann, Fois, Groß, Weil et al., Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures, Am. J. Physiol. Lung Cell Mol. Physiol, doi:10.1152/ajplung.00552.2020
Shah, Woo, Omicron: A Heavily Mutated SARS-CoV-2 Variant Exhibits Stronger Binding to ACE2 and Potently Escapes Approved COVID-19 Therapeutic Antibodies, Front. Immunol, doi:10.3389/fimmu.2021.830527
Shao, Guo, Xu, Li, Zhao, Specific Inhibitory Effect of κ-Carrageenan Polysaccharide on Swine Pandemic 2009 H1N1 Influenza Virus, PLoS ONE, doi:10.1371/journal.pone.0126577
Shchelkanov, Sakhuriya, Burunova, Pavlova, Kornilaeva et al., Dehydrogenase activity of infected cells and biological properties of HIV-1 variants, Biochemistry
Shikov, Pozharitskaya, Krishtopina, Makarov, Naphthoquinone pigments from sea urchins: Chemistry and pharmacology, Phytochem. Rev, doi:10.1007/s11101-018-9547-3
Shulgin, Spirin, Lebedev, Kravchenko, Glasunov et al., Comparative study of HIV-1 inhibition efficiency by carrageenans from red seaweeds family Gigartinaceae, Tichocarpaceae and Phyllophoraceae, Heliyon, doi:10.1016/j.heliyon.2024.e33407
Sliwoski, Kothiwale, Meiler, Lowe, Jr, Computational methods in drug discovery, Pharmacol. Rev, doi:10.1124/pr.112.007336
Torres, Sodero, Jofily, Silva, Jr, Key Topics in Molecular Docking for Drug Design, Int. J. Mol. Sci, doi:10.3390/ijms20184574
Van De Velde, Knutsen, Usov, Rollema, Cerezo, 1 H and 13 C high resolution NMR spectroscopy of carrageenans: Application in research and industry, Trends Food Sci. Technol, doi:10.1016/S0924-2244(02)00066-3
Van De Velde, Lourenço, Pinheiro, Bakker, Carrageenan: A food-grade and biocompatible support for immobilisation techniques, Adv. Synth. Catal, doi:10.1002/1615-4169(200209)344:8%3C815::AID-ADSC815%3E3.0.CO;2-H
Wang, Liu, Zhang, Wang, Hong et al., Structural basis for SARS-CoV-2 Delta variant recognition of ACE2 receptor and broadly neutralizing antibodies, Nat. Commun, doi:10.1038/s41467-022-28528-w
Wang, Wang, Guan, The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview, Mar. Drugs, doi:10.3390/md10122795
Wise, COVID-19: WHO declares end of global health emergency, BMJ, doi:10.1136/bmj.p1041
Yermak, Anastyuk, Kravchenko, Helbert, Glazunov et al., New Insights into the Structure of Kappa/Beta-Carrageenan: A Novel Potential Inhibitor of HIV-1, Int. J. Mol. Sci, doi:10.3390/ijms222312905
Yermak, Khotimchenko, Chemical properties, biological activities and applications of carrageenan from red algae
Yermak, Kim, Titlynov, Isakov, Solov'eva, Chemical structure and gel properties of carrageenans from algae belonging to the Gigartinaceae and Tichocarpaceae, collected from the Russian Pacific Coast, J. Appl. Phycol, doi:10.1023/A:1008071925884
Yermak, Kravchenko, Khasina, Menchinskaya, Pislyagin et al., The Anti-Inflammatory Effect of Carrageenan/Echinochrom Complex at Experimental Endotoxemia, Int. J. Mol. Sci, doi:10.3390/ijms231911702
Yermak, Mischchenko, Davydova, Glazunov, Tarbeeva et al., Carrageenans-Sulfated Polysaccharides from Red Seaweeds as Matrices for the Inclusion of Echinochrome, Mar. Drugs, doi:10.3390/md15110337
Yim, Kim, Kim, Chun, Oh et al., Inhibition of SARS-CoV-2 Virus Entry by the Crude Polysaccharides of Seaweeds and Abalone Viscera In Vitro, Mar. Drugs, doi:10.3390/md19040219
DOI record:
{
"DOI": "10.3390/ijms26136175",
"ISSN": [
"1422-0067"
],
"URL": "http://dx.doi.org/10.3390/ijms26136175",
"abstract": "<jats:p>The diversity of structural types of carrageenans (CRGs)—sulfated polysaccharides of red algae—determines their different biological activities. The different types of CRGs (kappa, lambda, kappa/beta-CRGs) were isolated from the red algae of the Pacific coast. Molecular docking was performed to determine potential interactions of CRGs with the receptor-binding domain (RBD) of SARS-CoV-2 and its cellular receptor—angiotensin—converting enzyme type 2 (ACE2). CRGs interacted with ACE2 and RBD via hydrogen bonding and ionic interactions. The strongest binding affinity of CRGs and ACE2 was observed for kappa-CRG. Molecular docking was confirmed by results studying the effects of CRGs against SARS-CoV-2 in vitro. The ability of CRGs, as well as the complex CRG with sea urchin echinochrome (Ech), to inhibit SARS-CoV-2 replication in Vero E6 cells was studied using cytopathic effect (CPE) inhibition and RT-PCR assays. The simultaneous treatment of cells with CRGs and the virus revealed that kappa-CRG exhibited the most significant antiviral effect among all the polysaccharides, with a selective index (SI) of 33. The kappa-CRG/Ech complex exhibited the highest virucidal effect on SARS-CoV-2 particles with an SI above 70 (more than two times higher than that of CRG and Ech) and reduced viral RNA levels by 45% (IC = 45%). Our results illustrate that CRGs and kappa-CRG/Ech complex can act as protective agents against SARS-CoV-2.</jats:p>",
"alternative-id": [
"ijms26136175"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-9048-6803",
"affiliation": [
{
"name": "G.P. Somov Institute of Epidemiology and Microbiology, Rospotrebnadzor, 690087 Vladivostok, Russia"
}
],
"authenticated-orcid": false,
"family": "Krylova",
"given": "Natalya V.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far-Eastern Branch of the Russian Academy of Science, 690022 Vladivostok, Russia"
}
],
"family": "Kravchenko",
"given": "Anna O.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1937-1680",
"affiliation": [
{
"name": "G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far-Eastern Branch of the Russian Academy of Science, 690022 Vladivostok, Russia"
}
],
"authenticated-orcid": false,
"family": "Likhatskaya",
"given": "Galina N.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6723-582X",
"affiliation": [
{
"name": "G.P. Somov Institute of Epidemiology and Microbiology, Rospotrebnadzor, 690087 Vladivostok, Russia"
}
],
"authenticated-orcid": false,
"family": "Iunikhina",
"given": "Olga V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far-Eastern Branch of the Russian Academy of Science, 690022 Vladivostok, Russia"
}
],
"family": "Glazunov",
"given": "Valery P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "G.P. Somov Institute of Epidemiology and Microbiology, Rospotrebnadzor, 690087 Vladivostok, Russia"
}
],
"family": "Zaporozhets",
"given": "Tatyana S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "G.P. Somov Institute of Epidemiology and Microbiology, Rospotrebnadzor, 690087 Vladivostok, Russia"
}
],
"family": "Shchelkanov",
"given": "Mikhail Y.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far-Eastern Branch of the Russian Academy of Science, 690022 Vladivostok, Russia"
}
],
"family": "Yermak",
"given": "Irina M.",
"sequence": "additional"
}
],
"container-title": "International Journal of Molecular Sciences",
"container-title-short": "IJMS",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
6,
27
]
],
"date-time": "2025-06-27T07:33:25Z",
"timestamp": 1751009605000
},
"deposited": {
"date-parts": [
[
2025,
6,
28
]
],
"date-time": "2025-06-28T04:31:11Z",
"timestamp": 1751085071000
},
"funder": [
{
"award": [
"122041800135-3"
],
"name": "Rospotrebnadzor of the Russian Federation in the framework of the implementation of the state task"
},
{
"DOI": "10.13039/501100006769",
"award": [
"21-74-20019-P"
],
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/501100006769",
"id-type": "DOI"
}
],
"name": "Russian Science Foundation"
}
],
"indexed": {
"date-parts": [
[
2025,
6,
28
]
],
"date-time": "2025-06-28T05:10:01Z",
"timestamp": 1751087401944,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issue": "13",
"issued": {
"date-parts": [
[
2025,
6,
26
]
]
},
"journal-issue": {
"issue": "13",
"published-online": {
"date-parts": [
[
2025,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
26
]
],
"date-time": "2025-06-26T00:00:00Z",
"timestamp": 1750896000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1422-0067/26/13/6175/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "6175",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2025,
6,
26
]
]
},
"published-online": {
"date-parts": [
[
2025,
6,
26
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.3390/md19080409",
"doi-asserted-by": "crossref",
"key": "ref_1",
"unstructured": "Geahchan, S., Ehrlich, H., and Rahman, M.A. (2021). The Anti-Viral Applications of Marine Resources for COVID-19 Treatment: An Overview. Mar. Drugs, 19."
},
{
"DOI": "10.1136/bmj.p1041",
"article-title": "COVID-19: WHO declares end of global health emergency",
"author": "Wise",
"doi-asserted-by": "crossref",
"first-page": "1041",
"journal-title": "BMJ",
"key": "ref_2",
"volume": "381",
"year": "2023"
},
{
"DOI": "10.34172/jrhs.2021.40",
"article-title": "The global pandemics are getting more frequent and severe",
"author": "Poorolajal",
"doi-asserted-by": "crossref",
"first-page": "e00502",
"journal-title": "J. Res. Health Sci.",
"key": "ref_3",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1038/d41586-023-01559-z",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "Lenharo, M. (2023). WHO declares end to COVID-19’s emergency phase. Nature, 882."
},
{
"DOI": "10.1155/2015/825203",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Ahmadi, A., Moghadamtousi, S.Z., Abubakar, S., and Zandi, K. (2015). Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. BioMed Res. Int., 2015."
},
{
"DOI": "10.3390/md19040219",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Yim, S.K., Kim, K., Kim, I.H., Chun, S.H., Oh, T.H., Kim, J.U., Kim, J.U., Jung, W.H., Moon, H.S., and Ku, B.S. (2021). Inhibition of SARS-CoV-2 Virus Entry by the Crude Polysaccharides of Seaweeds and Abalone Viscera In Vitro. Mar. Drugs, 19."
},
{
"DOI": "10.1016/j.carres.2021.108326",
"article-title": "Marine sulfated polysaccharides as potential antiviral drug candidates to treat Corona Virus disease (COVID-19)",
"author": "Andrew",
"doi-asserted-by": "crossref",
"first-page": "108326",
"journal-title": "Carbohydr. Res.",
"key": "ref_7",
"volume": "505",
"year": "2021"
},
{
"DOI": "10.1016/j.ijbiomac.2019.05.005",
"article-title": "Chemically sulfated polysaccharides from natural sources: Assessment of extraction-sulfation efficiencies, structural features and antiviral activities",
"author": "Mukherjee",
"doi-asserted-by": "crossref",
"first-page": "521",
"journal-title": "Int. J. Biol. Macromol.",
"key": "ref_8",
"volume": "136",
"year": "2019"
},
{
"DOI": "10.3390/md10122795",
"article-title": "The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "2795",
"journal-title": "Mar. Drugs",
"key": "ref_9",
"volume": "10",
"year": "2012"
},
{
"DOI": "10.1515/botm.1994.37.2.163",
"article-title": "A modified system of nomenclature for red algal Galactans",
"author": "Knutsen",
"doi-asserted-by": "crossref",
"first-page": "163",
"journal-title": "Bot. Mar.",
"key": "ref_10",
"volume": "37",
"year": "1994"
},
{
"DOI": "10.1016/S0268-005X(02)00045-0",
"article-title": "Structure and performance of commercial kappa-2 carrageenan extracts. Pt III. Structure analysis and performance in two dairy applications of extracts from the New Zealand red seaweed",
"author": "Falshaw",
"doi-asserted-by": "crossref",
"first-page": "129",
"journal-title": "Food Hydrocoll.",
"key": "ref_11",
"volume": "17",
"year": "2003"
},
{
"article-title": "Chemical properties, biological activities and applications of carrageenan from red algae",
"author": "Fingerman",
"first-page": "207",
"journal-title": "Recent Advances in Marine Biotechnology",
"key": "ref_12",
"volume": "Volume 9",
"year": "2003"
},
{
"DOI": "10.3390/md20010060",
"doi-asserted-by": "crossref",
"key": "ref_13",
"unstructured": "Krylova, N.V., Kravchenko, A.O., Iunikhina, O.V., Pott, A.B., Likhatskaya, G.N., Volod’ko, A.V., Zaporozhets, T.S., Shchelkanov, M.Y., and Yermak, I.M. (2022). Influence of the Structural Features of Carrageenans from Red Algae of the Far Eastern Seas on Their Antiviral Properties. Mar. Drugs, 20."
},
{
"DOI": "10.3390/md21060348",
"doi-asserted-by": "crossref",
"key": "ref_14",
"unstructured": "Jousselin, C., Pliego-Cortés, H., Damour, A., Garcia, M., Bodet, C., Robledo, D., Bourgougnon, N., and Lévêque, N. (2023). Anti-SARS-CoV-2 Activity of Polysaccharides Extracted from Halymenia floresii and Solieria chordalis (Rhodophyta). Mar. Drugs, 21."
},
{
"DOI": "10.1038/s41598-020-80896-9",
"doi-asserted-by": "crossref",
"key": "ref_15",
"unstructured": "Jang, Y., Shin, H., Lee, M.K., Kwon, O.S., Shin, J.S., Kim, Y.I., Kim, C.W., Lee, H.R., and Kim, M. (2021). Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci. Rep., 11."
},
{
"DOI": "10.1152/ajplung.00552.2020",
"article-title": "Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures",
"author": "Conzelmann",
"doi-asserted-by": "crossref",
"first-page": "L750",
"journal-title": "Am. J. Physiol. Lung Cell Mol. Physiol.",
"key": "ref_16",
"volume": "320",
"year": "2021"
},
{
"DOI": "10.1016/j.bbrep.2021.101187",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Bovard, D., van der Toorn, M., Schlage, W.K., Constant, S., Renggli, K., Peitsch, M.C., and Hoeng, J. (2022). Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia. Biochem. Biophys. Rep., 29."
},
{
"DOI": "10.1371/journal.pone.0259943",
"doi-asserted-by": "crossref",
"key": "ref_18",
"unstructured": "Bansal, S., Jonsson, C.B., Taylor, S.L., Figueroa, J.M., Dugour, A.V., Palacios, C., and Vega, J.C. (2021). Iota-carrageenan inhibits SARS-CoV-2 in vitro at concentrations easily achievable by nasal and nebulization formulations. PLoS ONE, 16."
},
{
"DOI": "10.2147/IJGM.S328486",
"article-title": "Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease",
"author": "Figueroa",
"doi-asserted-by": "crossref",
"first-page": "6277",
"journal-title": "Int. J. Gen. Med.",
"key": "ref_19",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1038/nature02145",
"article-title": "Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus",
"author": "Lim",
"doi-asserted-by": "crossref",
"first-page": "450",
"journal-title": "Nature",
"key": "ref_20",
"volume": "426",
"year": "2003"
},
{
"DOI": "10.1038/s41580-021-00418-x",
"article-title": "Mechanisms of SARS-CoV-2 entry into cells",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "ref_21",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/j.ijbiomac.2020.09.184",
"article-title": "The structure-activity relationship of the interactions of SARS-CoV-2 spike glycoproteins with glucuronomannan and sulfated galactofucan from Saccharina japonica",
"author": "Jin",
"doi-asserted-by": "crossref",
"first-page": "1649",
"journal-title": "Int. J. Biol. Macromol.",
"key": "ref_22",
"volume": "163",
"year": "2020"
},
{
"DOI": "10.1080/07315724.2001.10719172",
"article-title": "Antioxidants and viral infections: Host immune response and viral pathogenicity",
"author": "Beck",
"doi-asserted-by": "crossref",
"first-page": "384",
"journal-title": "J. Am. Coll. Nutr.",
"key": "ref_23",
"volume": "20",
"year": "2001"
},
{
"DOI": "10.1007/s11101-018-9547-3",
"article-title": "Naphthoquinone pigments from sea urchins: Chemistry and pharmacology",
"author": "Shikov",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "Phytochem. Rev.",
"key": "ref_24",
"volume": "17",
"year": "2018"
},
{
"DOI": "10.3390/md19080412",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Kim, H.K., Vasileva, E.A., Mishchenko, N.P., Fedoreyev, S.A., and Han, J. (2021). Multifaceted Clinical Effects of Echinochrome. Mar. Drugs, 19."
},
{
"DOI": "10.3390/md16120509",
"doi-asserted-by": "crossref",
"key": "ref_26",
"unstructured": "Fedoreyev, S.A., Krylova, N.V., Mishchenko, N.P., Vasileva, E.A., Pislyagin, E.A., Iunikhina, O.V., Lavrov, V.F., Svitich, O.A., Ebralidze, L.K., and Leonova, G.N. (2018). Antiviral and antioxidant properties of echinochrome A. Mar. Drugs, 16."
},
{
"DOI": "10.22207/JPAM.16.2.26",
"article-title": "Macroalgae Bioactive Compounds for the potential Antiviral of SARS-CoV-2: An In Silico Study",
"author": "Padmi",
"doi-asserted-by": "crossref",
"first-page": "1018",
"journal-title": "J. Pure Appl. Microbiol.",
"key": "ref_27",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.3390/md15110337",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Yermak, I.M., Mischchenko, N.P., Davydova, V.N., Glazunov, V.P., Tarbeeva, D.V., Kravchenko, A.O., Pimenova, E.A., and Sorokina, I.V. (2017). Carrageenans-Sulfated Polysaccharides from Red Seaweeds as Matrices for the Inclusion of Echinochrome. Mar. Drugs, 15."
},
{
"DOI": "10.3390/ijms232415754",
"doi-asserted-by": "crossref",
"key": "ref_29",
"unstructured": "Krylova, N.V., Gorbach, V.I., Iunikhina, O.V., Pott, A.B., Glazunov, V.P., Kravchenko, A.O., Shchelkanov, M.Y., and Yermak, I.M. (2022). Antiherpetic Activity of Carrageenan Complex with Echinochrome A and Its Liposomal Form. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1023/A:1008071925884",
"article-title": "Chemical structure and gel properties of carrageenans from algae belonging to the Gigartinaceae and Tichocarpaceae, collected from the Russian Pacific Coast",
"author": "Yermak",
"doi-asserted-by": "crossref",
"first-page": "41",
"journal-title": "J. Appl. Phycol.",
"key": "ref_30",
"volume": "11",
"year": "1999"
},
{
"DOI": "10.1016/j.foodhyd.2008.11.014",
"article-title": "Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman)",
"author": "Pereira",
"doi-asserted-by": "crossref",
"first-page": "1903",
"journal-title": "Food Hydrocoll.",
"key": "ref_31",
"volume": "23",
"year": "2009"
},
{
"DOI": "10.1016/S0924-2244(02)00066-3",
"article-title": "1H and 13C high resolution NMR spectroscopy of carrageenans: Application in research and industry",
"author": "Knutsen",
"doi-asserted-by": "crossref",
"first-page": "73",
"journal-title": "Trends Food Sci. Technol.",
"key": "ref_32",
"volume": "13",
"year": "2002"
},
{
"DOI": "10.1016/j.carbpol.2011.04.081",
"article-title": "Analysis of structural heterogeneity of kappa/beta-carrageenan oligosaccharides from Tichocarpus crinitus by negative-ion ESI and tandem MALDI mass spectrometry",
"author": "Anastyuk",
"doi-asserted-by": "crossref",
"first-page": "546",
"journal-title": "Carbohydr. Polym.",
"key": "ref_33",
"volume": "86",
"year": "2011"
},
{
"DOI": "10.1016/j.carbpol.2014.04.070",
"article-title": "Gelling polysaccharide from Chondrus armatus and its oligosaccharides: The structural peculiarities and anti-inflammatory activity",
"author": "Kalitnik",
"doi-asserted-by": "crossref",
"first-page": "768",
"journal-title": "Carbohydr. Polym.",
"key": "ref_34",
"volume": "115",
"year": "2015"
},
{
"DOI": "10.1016/j.carbpol.2011.11.052",
"article-title": "Comparison of the structures of hybrid κ/β-carrageenans extracted from Furcellaria lumbricalis and Tichocarpus crinitus",
"author": "Correc",
"doi-asserted-by": "crossref",
"first-page": "31",
"journal-title": "Carbohydr. Polym.",
"key": "ref_35",
"volume": "88",
"year": "2012"
},
{
"DOI": "10.3390/md19080406",
"doi-asserted-by": "crossref",
"key": "ref_36",
"unstructured": "Salih, A.E.M., Thissera, B., Yaseen, M., Hassane, A.S.I., El-Seedi, H.R., Sayed, A.M., and Rateb, M.E. (2021). Marine Sulfated Polysaccharides as Promising Antiviral Agents: A Comprehensive Report and Modeling Study Focusing on SARS-CoV-2. Mar. Drugs, 19."
},
{
"DOI": "10.1124/pr.112.007336",
"article-title": "Computational methods in drug discovery",
"author": "Sliwoski",
"doi-asserted-by": "crossref",
"first-page": "334",
"journal-title": "Pharmacol. Rev.",
"key": "ref_37",
"volume": "66",
"year": "2013"
},
{
"DOI": "10.1038/s41467-022-28528-w",
"article-title": "Structural basis for SARS-CoV-2 Delta variant recognition of ACE2 receptor and broadly neutralizing antibodies",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "871",
"journal-title": "Nat. Commun.",
"key": "ref_38",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"first-page": "104787",
"journal-title": "Antivir. Res.",
"key": "ref_39",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1002/path.1570",
"article-title": "Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis",
"author": "Hamming",
"doi-asserted-by": "crossref",
"first-page": "631",
"journal-title": "J. Pathol.",
"key": "ref_40",
"volume": "203",
"year": "2004"
},
{
"DOI": "10.3390/ijms20184574",
"doi-asserted-by": "crossref",
"key": "ref_41",
"unstructured": "Torres, P.H.M., Sodero, A.C.R., Jofily, P., and Silva, F.P. (2019). Key Topics in Molecular Docking for Drug Design. Int. J. Mol. Sci., 20."
},
{
"article-title": "Molecular Docking of Carrageenans for Main Protease (Mpro) and Angiotensin-Converting Enzyme 2 (ACE2) of SARS-CoV-2",
"author": "Aung",
"first-page": "221",
"journal-title": "Drug Des.",
"key": "ref_42",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0126577",
"doi-asserted-by": "crossref",
"key": "ref_43",
"unstructured": "Shao, Q., Guo, Q., Xu, W., Li, Z., and Zhao, T. (2015). Specific Inhibitory Effect of κ-Carrageenan Polysaccharide on Swine Pandemic 2009 H1N1 Influenza Virus. PLoS ONE, 10."
},
{
"DOI": "10.3390/ijms222312905",
"doi-asserted-by": "crossref",
"key": "ref_44",
"unstructured": "Yermak, I., Anastyuk, S., Kravchenko, A., Helbert, W., Glazunov, V., Shulgin, A., Spirin, P., and Prassolov, V. (2021). New Insights into the Structure of Kappa/Beta-Carrageenan: A Novel Potential Inhibitor of HIV-1. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.1016/j.heliyon.2024.e33407",
"article-title": "Comparative study of HIV-1 inhibition efficiency by carrageenans from red seaweeds family Gigartinaceae, Tichocarpaceae and Phyllophoraceae",
"author": "Shulgin",
"doi-asserted-by": "crossref",
"first-page": "e33407",
"journal-title": "Heliyon",
"key": "ref_45",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1002/1615-4169(200209)344:8<815::AID-ADSC815>3.0.CO;2-H",
"article-title": "Carrageenan: A food-grade and biocompatible support for immobilisation techniques",
"author": "Pinheiro",
"doi-asserted-by": "crossref",
"first-page": "815",
"journal-title": "Adv. Synth. Catal.",
"key": "ref_46",
"volume": "344",
"year": "2002"
},
{
"DOI": "10.3390/ijms231911702",
"doi-asserted-by": "crossref",
"key": "ref_47",
"unstructured": "Yermak, I.M., Kravchenko, A.O., Khasina, E.I., Menchinskaya, E.S., Pislyagin, E.A., Sokolova, E.V., Likhatskaya, G.N., and Aminin, D.L. (2022). The Anti-Inflammatory Effect of Carrageenan/Echinochrom Complex at Experimental Endotoxemia. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1021/acs.jpclett.2c00423",
"article-title": "Binding Interactions between Receptor-Binding Domain of Spike Protein and Human Angiotensin Converting Enzyme-2 in Omicron Variant",
"author": "Jawad",
"doi-asserted-by": "crossref",
"first-page": "3915",
"journal-title": "J. Phys. Chem. Lett.",
"key": "ref_48",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2021.830527",
"doi-asserted-by": "crossref",
"key": "ref_49",
"unstructured": "Shah, M., and Woo, H.G. (2022). Omicron: A Heavily Mutated SARS-CoV-2 Variant Exhibits Stronger Binding to ACE2 and Potently Escapes Approved COVID-19 Therapeutic Antibodies. Front. Immunol., 12."
},
{
"article-title": "Dehydrogenase activity of infected cells and biological properties of HIV-1 variants",
"author": "Shchelkanov",
"first-page": "431",
"journal-title": "Biochemistry",
"key": "ref_50",
"volume": "64",
"year": "1999"
},
{
"DOI": "10.1016/0166-0934(88)90134-6",
"article-title": "Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds",
"author": "Pauwels",
"doi-asserted-by": "crossref",
"first-page": "309",
"journal-title": "J. Virol. Methods",
"key": "ref_51",
"volume": "20",
"year": "1988"
},
{
"DOI": "10.1016/j.phyplu.2022.100233",
"article-title": "In silico, in vitro screening of plant extracts for anti-SARS-CoV-2 activity and evaluation of their acute and sub-acute toxicity",
"author": "Latha",
"doi-asserted-by": "crossref",
"first-page": "100233",
"journal-title": "Phytomed Plus",
"key": "ref_52",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.3390/microbiolres16050102",
"doi-asserted-by": "crossref",
"key": "ref_53",
"unstructured": "Kuznetsova, T.A., Krylova, N.V., Kokoulin, M.S., Persiyanova, E.V., Maistrovskaya, O.S., Milovankin, P.G., Belov, Y.A., and Shchelkanov, M.Y. (2025). Polysaccharides from Marine Bacteria and Their Anti-SARS-CoV-2Activity. Microbiol. Res., 16."
}
],
"reference-count": 53,
"references-count": 53,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1422-0067/26/13/6175"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents",
"type": "journal-article",
"volume": "26"
}