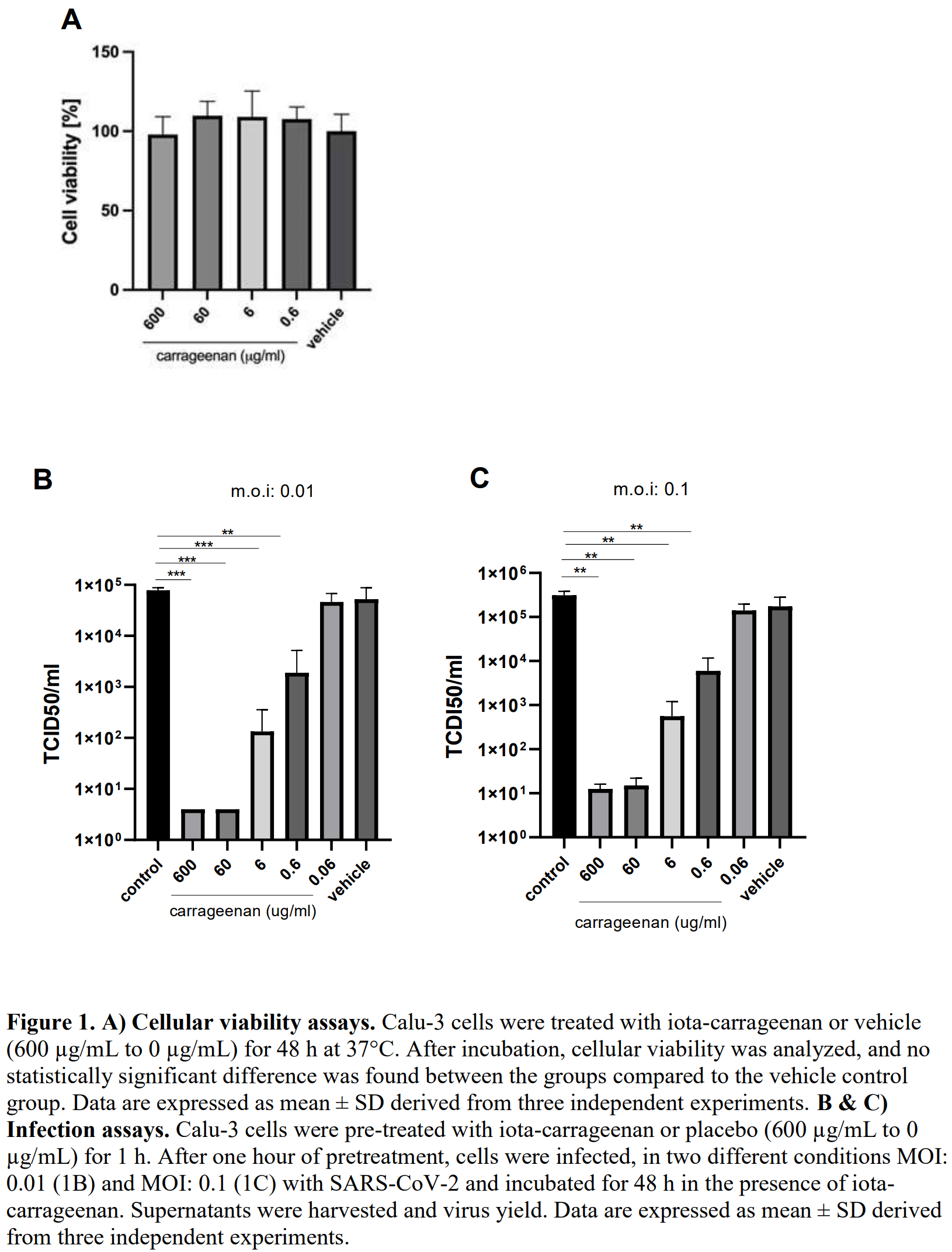
Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model
et al., bioRxiv, doi:10.1101/2021.04.27.441512, Apr 2021
In vitro Calu-3 (human respiratory epithelial cell line) study showing that iota-carrageenan inhibits SARS-CoV-2.
19 preclinical studies support the efficacy of iota-carrageenan for COVID-19:
1.
Herida et al., Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC155.071.
2.
Krylova et al., Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents, International Journal of Molecular Sciences, doi:10.3390/ijms26136175.
3.
Rohilla et al., Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV-2, Stresses, doi:10.3390/stresses3030039.
4.
Thet, H., The potential of carrageenan for the drug discovery of COVID-19 via molecular docking with angiotensin-converting enzyme 2 (ACE2) and the main protease (Mpro) of SARS-CoV-2, Journal of Bioinformatics and Genomics, doi:10.18454/jbg.2022.18.2.001.
5.
Alsaidi et al., Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model, Marine Drugs, doi:10.3390/md19080418.
6.
Sattari et al., Repositioning Therapeutics for COVID-19: Virtual Screening of the Potent Synthetic and Natural Compounds as SARS-CoV-2 3CLpro Inhibitors, Research Square, doi:10.21203/rs.3.rs-37994/v1.
7.
Hoffmann et al., Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity, Biomacromolecules, doi:10.1021/acs.biomac.5c00576.
8.
Yathindranath et al., Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection, International Journal of Nanomedicine, doi:10.2147/IJN.S448005.
9.
Setz et al., Iota-Carrageenan Inhibits Replication of the SARS-CoV-2 Variants of Concern Omicron BA.1, BA.2 and BA.5, Nutraceuticals, doi:10.3390/nutraceuticals3030025.
10.
Meister et al., Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2, Journal of Hospital Infection, doi:10.1016/j.jhin.2021.10.019.
11.
Bovard et al., Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochemistry and Biophysics Reports, doi:10.1016/j.bbrep.2021.101187.
12.
Fröba et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, International Journal of Molecular Sciences, doi:10.3390/ijms222413202.
13.
Varese et al., Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model, bioRxiv, doi:10.1101/2021.04.27.441512.
14.
Morokutti-Kurz et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE, doi:10.1371/journal.pone.0237480.
15.
Song et al., Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food & Function, doi:10.1039/D0FO02017F.
Varese et al., 27 Apr 2021, preprint, 5 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model
doi:10.1101/2021.04.27.441512
There are, except for remdesivir, no approved antivirals for the treatment or prevention of SARS-CoV-2 infections. Iota-carrageenan formulated into a nasal spray has already been proven safe and effective in viral respiratory infections. We explored this antiviral activity in Calu-3, a human respiratory model cell line. A formula of iota-carrageenan and sodium chloride, as a nasal spray, already approved for human use, effectively inhibited SARS-CoV-2 infection in vitro, providing a more substantial reference for further clinical studies or developments.
Conflict of Interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Author Contributions AC, AVD, and JMF conceptualized the study. AC, AV, CP and AVD developed the methodology. JMF wrote and prepared the original draft. AC, AVD, and CP wrote, reviewed, and edited the manuscript. AC and AVD supervised the study. All authors contributed to the article and approved the submitted version.
References
Banerjee, Nasir, Budylowski, Yip, Aftanas et al., Isolation, Sequence, Infectivity, and Replication Kinetics of Severe Acute Respiratory Syndrome Coronavirus 2, Emerg Infect Dis, doi:10.3201/eid2609.201495
Bansal, Jonsson, Taylor, Figueroa, Dugour et al., Iota-carrageenan and Xylitol inhibit SARS-CoV-2 in cell culture, Biorxiv, doi:10.1101/2020.08.19.225854
Barrett, Terpening, Snow, Cobb, Kistner, Vero cell technology for rapid development of inactivated whole virus vaccines for emerging viral diseases, Expert Rev Vaccines, doi:10.1080/14760584.2017.1357471
Chen, Han, Wang, Zhao, Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines, Int J Biol Macromol, doi:10.1016/j.ijbiomac.2020.07.106
Dong, Du, Gardner, An interactive web-based dashboard to track COVID-19 in real time, Lancet Infect Dis, doi:10.1016/s1473-3099(20)30120-1
Duszyk, CFTR and lysozyme secretion in human airway epithelial cells, Pflügers Archiv, doi:10.1007/s004240100643
Felgenhauer, Schoen, Gad, Hartmann, Schaubmar et al., Inhibition of SARS-CoV-2 by type I and type III interferons, J Biol Chem, doi:10.1074/jbc.ac120.013788
Figueroa, Lombardo, Dogliotti, Flynn, Giugliano et al., Efficacy of a nasal spray containing Iota-Carrageenan in the prophylaxis of COVID-19 in hospital personnel dedicated to patients care with COVID-19 disease A pragmatic multicenter, randomized, double-blind, placebo-controlled trial, CARR-COV, doi:10.1101/2021.04.13.21255409
Gomaa, Elshoubaky, Antiviral Activity of Sulfated Polysaccharides Carrageenan from Some Marine Seaweeds, International Journal of Current Pharmaceutical Review and Research
Grassauer, Weinmuellner, Meier, Pretsch, Prieschl-Grassauer et al., Iota-Carrageenan is a potent inhibitor of rhinovirus infection, Virol J, doi:10.1186/1743-422x-5-107
Gubernatorova, Gorshkova, Polinova, Drutskaya, IL-6: relevance for immunopathology of SARS-CoV-2, Cytokine Growth F R, doi:10.1016/j.cytogfr.2020.05.009
Hans, Malik, Naik, Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review, Bioresour Technology Reports, doi:10.1016/j.biteb.2020.100623
Hoffmann, Mösbauer, Hofmann-Winkler, Kaul, Kleine-Weber et al., Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2, Nature, doi:10.1038/s41586-020-2575-3
Holwerda, V'kovski, Wider, Thiel, Dijkman, Identification of an Antiviral Compound from the Pandemic Response Box that Efficiently Inhibits SARS-CoV-2
Koenighofer, Lion, Bodenteich, Prieschl-Grassauer, Grassauer et al., Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials, Multidiscip Resp Med, doi:10.1186/2049-6958-9-57
Leibbrandt, Meier, König-Schuster, Weinmüllner, Kalthoff et al., Iota-Carrageenan Is a Potent Inhibitor of Influenza A Virus Infection, Plos One, doi:10.1371/journal.pone.0014320
Morokutti-Kurz, Graf, Grassauer, Prieschl-Grassauer, SARS-CoV-2 invitro neutralization assay reveals inhibition of virus entry by iota-carrageenan, Biorxiv, doi:10.1101/2020.07.28.224733
Morokutti-Kurz, König-Schuster, Koller, Graf, Graf et al., The Intranasal Application of Zanamivir and Carrageenan Is Synergistically Active against Influenza A Virus in the Murine Model, Plos One, doi:10.1371/journal.pone.0128794
Murgolo, Therien, Howell, Klein, Koeplinger et al., SARS-CoV-2 tropism, entry, replication, and propagation: Considerations for drug discovery and development, Plos Pathog, doi:10.1371/journal.ppat.1009225
Osada, Kohara, Yamaji, Hirayama, Kasai et al., The Genome Landscape of the African Green Monkey Kidney-Derived Vero Cell Line, Dna Res, doi:10.1093/dnares/dsu029
Park, Kim, Park, Maharjan, Kim et al., Differential Signaling and Virus Production in Calu-3 Cells and Vero Cells upon SARS-CoV-2 Infection, Biomol Ther, doi:10.4062/biomolther.2020.226
Saccon, Chen, Mikaeloff, Rodriguez, Szekely et al., Tropism of SARS-CoV-2 in commonly used laboratory cell lines and their proteomic landscape during infection, Biorxiv, doi:10.1101/2020.08.28.271684
Salgado-Benvindo, Thaler, Tas, Ogando, Bredenbeek et al., Suramin Inhibits SARS-CoV-2 Infection in Cell Culture by Interfering with Early Steps of the Replication Cycle, Antimicrob Agents Ch, doi:10.1128/aac.00900-20
Song, Peng, Wang, Liu, Dong et al., Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food Funct, doi:10.1039/d0fo02017f
Yamamoto, Kiso, Sakai-Tagawa, Iwatsuki-Horimoto, Imai et al., The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner, Viruses, doi:10.3390/v12060629
Zhu, Chidekel, Shaffer, Cultured Human Airway Epithelial Cells (Calu-3): A Model of Human Respiratory Function, Structure, and Inflammatory Responses, Critical Care Res Pract, doi:10.1155/2010/394578
Zhu, Miller, Singhaus, Shaffer, Chidekel, Effects of oxygen concentration and exposure time on cultured human airway epithelial cells, Pediatr Crit Care Me, doi:10.1097/pcc.0b013e318166fbb5
DOI record:
{
"DOI": "10.1101/2021.04.27.441512",
"URL": "http://dx.doi.org/10.1101/2021.04.27.441512",
"abstract": "<jats:title>Abstract</jats:title><jats:p>There are, except for remdesivir, no approved antivirals for the treatment or prevention of SARS-CoV-2 infections. Iota-carrageenan formulated into a nasal spray has already been proven safe and effective in viral respiratory infections. We explored this antiviral activity in Calu-3, a human respiratory model cell line. A formula of iota-carrageenan and sodium chloride, as a nasal spray, already approved for human use, effectively inhibited SARS-CoV-2 infection in vitro, providing a more substantial reference for further clinical studies or developments.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
4,
27
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-3129-2048",
"affiliation": [],
"authenticated-orcid": false,
"family": "Varese",
"given": "Augusto",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-4564-7361",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ceballos",
"given": "Ana",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3368-0538",
"affiliation": [],
"authenticated-orcid": false,
"family": "Palacios",
"given": "Carlos",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5680-1486",
"affiliation": [],
"authenticated-orcid": false,
"family": "Figueroa",
"given": "Juan Manuel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0187-0548",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dugour",
"given": "Andrea Vanesa",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
4,
27
]
],
"date-time": "2021-04-27T22:45:33Z",
"timestamp": 1619563533000
},
"deposited": {
"date-parts": [
[
2022,
5,
25
]
],
"date-time": "2022-05-25T19:49:05Z",
"timestamp": 1653508145000
},
"group-title": "Microbiology",
"indexed": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T23:48:20Z",
"timestamp": 1709336900933
},
"institution": [
{
"name": "bioRxiv"
}
],
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2021,
4,
27
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.04.27.441512",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
4,
27
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
4,
27
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.3201/eid2609.201495",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.1"
},
{
"DOI": "10.1101/2020.08.19.225854",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.2"
},
{
"DOI": "10.1080/14760584.2017.1357471",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.3"
},
{
"DOI": "10.1016/j.ijbiomac.2020.07.106",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.4"
},
{
"DOI": "10.1016/s1473-3099(20)30120-1",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.5"
},
{
"DOI": "10.1007/s004240100643",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.6"
},
{
"DOI": "10.1074/jbc.ac120.013788",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.7"
},
{
"DOI": "10.1101/2021.04.13.21255409",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.8"
},
{
"article-title": "Antiviral Activity of Sulfated Polysaccharides Carrageenan from Some Marine Seaweeds",
"first-page": "32",
"journal-title": "International Journal of Current Pharmaceutical Review and Research",
"key": "2021042904350956000_2021.04.27.441512v1.9",
"volume": "1",
"year": "2016"
},
{
"DOI": "10.1186/1743-422X-5-107",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.10"
},
{
"DOI": "10.1016/j.cytogfr.2020.05.009",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.11"
},
{
"DOI": "10.1016/j.biteb.2020.100623",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.12"
},
{
"DOI": "10.1038/s41586-020-2575-3",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.13"
},
{
"DOI": "10.3390/microorganisms8121872",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.14"
},
{
"DOI": "10.1186/2049-6958-9-57",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.15"
},
{
"DOI": "10.1371/journal.pone.0014320",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.16"
},
{
"DOI": "10.1101/2020.07.28.224733",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.17"
},
{
"DOI": "10.1371/journal.pone.0128794",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.18"
},
{
"DOI": "10.1371/journal.ppat.1009225",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.19"
},
{
"DOI": "10.1093/dnares/dsu029",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.20"
},
{
"DOI": "10.4062/biomolther.2020.226",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.21"
},
{
"DOI": "10.1101/2020.08.28.271684",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.22"
},
{
"DOI": "10.1128/aac.00900-20",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.23"
},
{
"DOI": "10.1039/d0fo02017f",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.24"
},
{
"DOI": "10.3390/v12060629",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.25"
},
{
"DOI": "10.1155/2010/394578",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.26"
},
{
"DOI": "10.1097/pcc.0b013e318166fbb5",
"doi-asserted-by": "publisher",
"key": "2021042904350956000_2021.04.27.441512v1.27"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {
"is-preprint-of": [
{
"asserted-by": "subject",
"id": "10.3389/fviro.2021.746824",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://biorxiv.org/lookup/doi/10.1101/2021.04.27.441512"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model",
"type": "posted-content"
}