Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia
et al., Biochemistry and Biophysics Reports, doi:10.1016/j.bbrep.2021.101187, Dec 2021
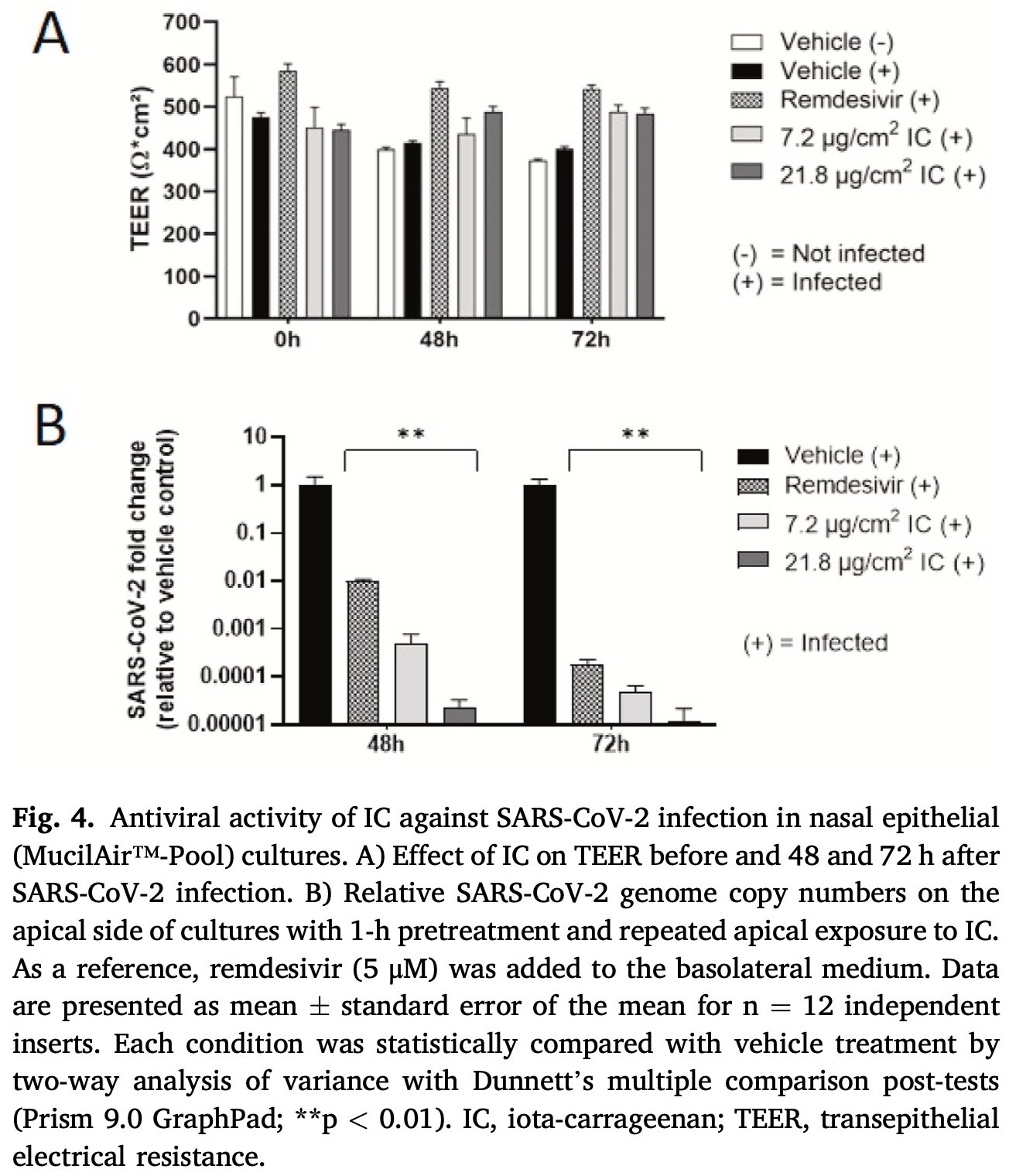
In vitro study showing iota-carrageenan inhibits SARS-CoV-2 in reconstituted human airway epithelia. Authors note that the absence of toxicity or any functional or structural impairment of the bronchial mucociliary epithelium indicates that topical treatment with nebulized iota-carrageenan is well tolerated at effective concentrations.
19 preclinical studies support the efficacy of iota-carrageenan for COVID-19:
1.
Herida et al., Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC155.071.
2.
Krylova et al., Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents, International Journal of Molecular Sciences, doi:10.3390/ijms26136175.
3.
Rohilla et al., Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV-2, Stresses, doi:10.3390/stresses3030039.
4.
Thet, H., The potential of carrageenan for the drug discovery of COVID-19 via molecular docking with angiotensin-converting enzyme 2 (ACE2) and the main protease (Mpro) of SARS-CoV-2, Journal of Bioinformatics and Genomics, doi:10.18454/jbg.2022.18.2.001.
5.
Alsaidi et al., Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model, Marine Drugs, doi:10.3390/md19080418.
6.
Sattari et al., Repositioning Therapeutics for COVID-19: Virtual Screening of the Potent Synthetic and Natural Compounds as SARS-CoV-2 3CLpro Inhibitors, Research Square, doi:10.21203/rs.3.rs-37994/v1.
7.
Hoffmann et al., Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity, Biomacromolecules, doi:10.1021/acs.biomac.5c00576.
8.
Yathindranath et al., Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection, International Journal of Nanomedicine, doi:10.2147/IJN.S448005.
9.
Setz et al., Iota-Carrageenan Inhibits Replication of the SARS-CoV-2 Variants of Concern Omicron BA.1, BA.2 and BA.5, Nutraceuticals, doi:10.3390/nutraceuticals3030025.
10.
Meister et al., Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2, Journal of Hospital Infection, doi:10.1016/j.jhin.2021.10.019.
11.
Bovard et al., Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochemistry and Biophysics Reports, doi:10.1016/j.bbrep.2021.101187.
12.
Fröba et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, International Journal of Molecular Sciences, doi:10.3390/ijms222413202.
13.
Varese et al., Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model, bioRxiv, doi:10.1101/2021.04.27.441512.
14.
Morokutti-Kurz et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE, doi:10.1371/journal.pone.0237480.
15.
Song et al., Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food & Function, doi:10.1039/D0FO02017F.
Bovard et al., 15 Dec 2021, Switzerland, peer-reviewed, 7 authors.
Contact: julia.hoeng@pmi.com.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Iota-carrageenan extracted from red algae is a potent inhibitor of SARS‐CoV-2 infection in reconstituted human airway epithelia
Biochemistry and Biophysics Reports, doi:10.1016/j.bbrep.2021.101187
Iota-carrageenan (IC) nasal spray, a medical device approved for treating respiratory viral infections, has previously been shown to inhibit the ability of a variety of respiratory viruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), to enter and replicate in the cell by interfering with the virus binding to the cell surface. The aim of this study was to further investigate the efficacy and safety of IC in SARS-CoV-2 infection in advanced in vitro models of the human respiratory epithelium, the primary target and entry port for SARS-CoV-2. We extended the in vitro safety assessment of nebulized IC in a 3-dimensional model of reconstituted human bronchial epithelium, and we demonstrated the efficacy of IC in protecting reconstituted nasal epithelium against viral infection and replication of a patient-derived SARS-CoV-2 strain. The results obtained from these two advanced models of human respiratory tract epithelia confirm previous findings from in vitro SARS-CoV-2 infection assays and demonstrate that topically applied IC can effectively prevent SARS-CoV-2 infection and replication. Moreover, the absence of toxicity and functional and structural impairment of the mucociliary epithelium demonstrates that the nebulized IC is well tolerated.
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Baxter, Targeted omics analyses, and metabolic enzyme activity assays demonstrate maintenance of key mucociliary characteristics in long term cultures of reconstituted human airway epithelia, Toxicol. Vitro
Bichiri, Rente, Jesus, Safety and efficacy of iota-carrageenan nasal spray in treatment and prevention of the common cold, Med Pharm Rep
Bovard, A lung/liver-on-a-chip platform for acute and chronic toxicity studies, Lab Chip
Bovard, Comparison of the basic morphology and function of 3D lung epithelial cultures derived from several donors, Current Research in Toxicology
Burton, Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them, Cochrane Database Syst. Rev
Burton, Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection, Cochrane Database Syst. Rev
Colafrancesco, COVID-19 gone bad: a new character in the spectrum of the hyperferritinemic syndrome?, Autoimmun. Rev
Eccles, Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold, Respir. Res
Ecdc, COVID-19 situation update worldwide, as of week
Fazekas, Lessons learned from a double-blind randomised placebocontrolled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold, BMC Compl. Alternative Med
Giovane, Current pharmacological modalities for management of novel coronavirus disease 2019 (COVID-19) and the rationale for their utilization: a review, Rev. Med. Virol
Graf, Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis, Int. J. Gen. Med
Grassauer, Iota-Carrageenan is a potent inhibitor of rhinovirus infection, Virol. J
Hamming, Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis, J. Pathol
Hemilä, Chalker, Influenza Virus Infections: Re-analysis of Randomized Trial Data
Hu, Characteristics of SARS-CoV-2 and COVID-19, Nat. Rev. Microbiol
Huang, Wiszniewski, Constant, The use of in vitro 3D cell models in drug development for respiratory diseases
Iskandar, 3-D nasal cultures: systems toxicological assessment of a candidate modified-risk tobacco product, ALTEX
Iverson, Leveraging 3D model systems to understand viral interactions with the respiratory mucosa, Viruses
Kavanagh, Inhaled hydroxychloroquine to improve efficacy and reduce harm in the treatment of COVID-19, Med. Hypotheses
Kim, Read, Fauci, Therapy for early COVID-19: a critical need, Jama
Koenighofer, Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials, Multidiscip Respir Med
Leibbrandt, Iota-carrageenan is a potent inhibitor of influenza A virus infection, PLoS One
Lenz, A dose-controlled system for air-liquid interface cell exposure and application to zinc oxide nanoparticles, Part. Fibre Toxicol
Ludwig, Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial, Respir. Res
Morokutti-Kurz, Graf, Prieschl-Grassauer, Amylmetacresol/2,4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat, Int. J. Gen. Med
Morokutti-Kurz, Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS One
Pizzorno, Characterization and treatment of SARS-CoV-2 in nasal and bronchial human airway epithelia, Cell Rep Med
Sanders, Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review, Jama
Schütz, Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures, Am. J. Physiol. Lung Cell Mol. Physiol
Srinivasan, TEER measurement techniques for in vitro barrier model systems, J. Lab. Autom
Stathis, Review of the use of nasal and oral antiseptics during a global pandemic, Future Microbiol
Zou, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N. Engl. J. Med
DOI record:
{
"DOI": "10.1016/j.bbrep.2021.101187",
"ISSN": [
"2405-5808"
],
"URL": "http://dx.doi.org/10.1016/j.bbrep.2021.101187",
"alternative-id": [
"S2405580821002818"
],
"article-number": "101187",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Iota-carrageenan extracted from red algae is a potent inhibitor of SARS‐CoV-2 infection in reconstituted human airway epithelia"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Biochemistry and Biophysics Reports"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.bbrep.2021.101187"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Authors. Published by Elsevier B.V."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3467-1251",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bovard",
"given": "David",
"sequence": "first"
},
{
"affiliation": [],
"family": "van der Toorn",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schlage",
"given": "Walter K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0931-2663",
"affiliation": [],
"authenticated-orcid": false,
"family": "Constant",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Renggli",
"given": "Kasper",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5324-359X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Peitsch",
"given": "Manuel C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoeng",
"given": "Julia",
"sequence": "additional"
}
],
"container-title": "Biochemistry and Biophysics Reports",
"container-title-short": "Biochemistry and Biophysics Reports",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T23:07:38Z",
"timestamp": 1639609658000
},
"deposited": {
"date-parts": [
[
2022,
5,
31
]
],
"date-time": "2022-05-31T22:51:55Z",
"timestamp": 1654037515000
},
"indexed": {
"date-parts": [
[
2022,
5,
31
]
],
"date-time": "2022-05-31T23:10:34Z",
"timestamp": 1654038634868
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
1
]
],
"date-time": "2022-03-01T00:00:00Z",
"timestamp": 1646092800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
9
]
],
"date-time": "2021-12-09T00:00:00Z",
"timestamp": 1639008000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2405580821002818?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2405580821002818?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "101187",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
3
]
]
},
"published-print": {
"date-parts": [
[
2022,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/s41579-020-00459-7",
"article-title": "Characteristics of SARS-CoV-2 and COVID-19",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "141",
"issue": "3",
"journal-title": "Nat. Rev. Microbiol.",
"key": "10.1016/j.bbrep.2021.101187_bib2",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1016/j.autrev.2020.102573",
"article-title": "COVID-19 gone bad: a new character in the spectrum of the hyperferritinemic syndrome?",
"author": "Colafrancesco",
"doi-asserted-by": "crossref",
"first-page": "102573",
"issue": "7",
"journal-title": "Autoimmun. Rev.",
"key": "10.1016/j.bbrep.2021.101187_bib3",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1002/rmv.2136",
"article-title": "Current pharmacological modalities for management of novel coronavirus disease 2019 (COVID-19) and the rationale for their utilization: a review",
"author": "Giovane",
"doi-asserted-by": "crossref",
"first-page": "e2136",
"issue": "5",
"journal-title": "Rev. Med. Virol.",
"key": "10.1016/j.bbrep.2021.101187_bib4",
"volume": "30",
"year": "2020"
},
{
"article-title": "Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review",
"author": "Sanders",
"first-page": "1824",
"issue": "18",
"journal-title": "Jama",
"key": "10.1016/j.bbrep.2021.101187_bib5",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.22813",
"article-title": "Therapy for early COVID-19: a critical need",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "2149",
"issue": "21",
"journal-title": "Jama",
"key": "10.1016/j.bbrep.2021.101187_bib6",
"volume": "324",
"year": "2020"
},
{
"article-title": "Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them",
"author": "Burton",
"first-page": "Cd013627",
"issue": "9",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "10.1016/j.bbrep.2021.101187_bib7",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.2217/fmb-2020-0286",
"article-title": "Review of the use of nasal and oral antiseptics during a global pandemic",
"author": "Stathis",
"doi-asserted-by": "crossref",
"first-page": "119",
"issue": "2",
"journal-title": "Future Microbiol.",
"key": "10.1016/j.bbrep.2021.101187_bib8",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1002/path.1570",
"article-title": "Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis",
"author": "Hamming",
"doi-asserted-by": "crossref",
"first-page": "631",
"issue": "2",
"journal-title": "J. Pathol.",
"key": "10.1016/j.bbrep.2021.101187_bib9",
"volume": "203",
"year": "2004"
},
{
"DOI": "10.1056/NEJMc2001737",
"article-title": "SARS-CoV-2 viral load in upper respiratory specimens of infected patients",
"author": "Zou",
"doi-asserted-by": "crossref",
"first-page": "1177",
"issue": "12",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.bbrep.2021.101187_bib10",
"volume": "382",
"year": "2020"
},
{
"article-title": "Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection",
"author": "Burton",
"first-page": "Cd013626",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "10.1016/j.bbrep.2021.101187_bib11",
"volume": "9",
"year": "2020"
},
{
"article-title": "Safety and efficacy of iota-carrageenan nasal spray in treatment and prevention of the common cold",
"author": "Bichiri",
"first-page": "28",
"issue": "1",
"journal-title": "Med Pharm Rep",
"key": "10.1016/j.bbrep.2021.101187_bib12",
"volume": "94",
"year": "2021"
},
{
"author": "Hemilä",
"key": "10.1016/j.bbrep.2021.101187_bib13",
"series-title": "Carrageenan Nasal Spray May Double the Rate of Recovery from Coronavirus and Influenza Virus Infections: Re-analysis of Randomized Trial Data",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0237480",
"article-title": "Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro",
"author": "Morokutti-Kurz",
"doi-asserted-by": "crossref",
"first-page": "e0237480",
"issue": "2",
"journal-title": "PLoS One",
"key": "10.1016/j.bbrep.2021.101187_bib14",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0014320",
"article-title": "Iota-carrageenan is a potent inhibitor of influenza A virus infection",
"author": "Leibbrandt",
"doi-asserted-by": "crossref",
"first-page": "e14320",
"issue": "12",
"journal-title": "PLoS One",
"key": "10.1016/j.bbrep.2021.101187_bib15",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.1186/1743-422X-5-107",
"article-title": "Iota-Carrageenan is a potent inhibitor of rhinovirus infection",
"author": "Grassauer",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "Virol. J.",
"key": "10.1016/j.bbrep.2021.101187_bib16",
"volume": "5",
"year": "2008"
},
{
"DOI": "10.1186/1465-9921-11-108",
"article-title": "Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold",
"author": "Eccles",
"doi-asserted-by": "crossref",
"first-page": "108",
"issue": "1",
"journal-title": "Respir. Res.",
"key": "10.1016/j.bbrep.2021.101187_bib17",
"volume": "11",
"year": "2010"
},
{
"DOI": "10.1186/1472-6882-12-147",
"article-title": "Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold",
"author": "Fazekas",
"doi-asserted-by": "crossref",
"first-page": "147",
"journal-title": "BMC Compl. Alternative Med.",
"key": "10.1016/j.bbrep.2021.101187_bib18",
"volume": "12",
"year": "2012"
},
{
"DOI": "10.1186/1465-9921-14-124",
"article-title": "Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial",
"author": "Ludwig",
"doi-asserted-by": "crossref",
"first-page": "124",
"issue": "1",
"journal-title": "Respir. Res.",
"key": "10.1016/j.bbrep.2021.101187_bib19",
"volume": "14",
"year": "2013"
},
{
"DOI": "10.1186/2049-6958-9-57",
"article-title": "Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials",
"author": "Koenighofer",
"doi-asserted-by": "crossref",
"first-page": "57",
"issue": "1",
"journal-title": "Multidiscip Respir Med",
"key": "10.1016/j.bbrep.2021.101187_bib20",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.2147/IJGM.S120665",
"article-title": "Amylmetacresol/2,4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat",
"author": "Morokutti-Kurz",
"doi-asserted-by": "crossref",
"first-page": "53",
"journal-title": "Int. J. Gen. Med.",
"key": "10.1016/j.bbrep.2021.101187_bib21",
"volume": "10",
"year": "2017"
},
{
"DOI": "10.1016/j.mehy.2020.110110",
"article-title": "Inhaled hydroxychloroquine to improve efficacy and reduce harm in the treatment of COVID-19",
"author": "Kavanagh",
"doi-asserted-by": "crossref",
"first-page": "110110",
"journal-title": "Med. Hypotheses",
"key": "10.1016/j.bbrep.2021.101187_bib22",
"volume": "143",
"year": "2020"
},
{
"DOI": "10.1039/C8LC01029C",
"article-title": "A lung/liver-on-a-chip platform for acute and chronic toxicity studies",
"author": "Bovard",
"doi-asserted-by": "crossref",
"first-page": "3814",
"issue": "24",
"journal-title": "Lab Chip",
"key": "10.1016/j.bbrep.2021.101187_bib23",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.1016/j.crtox.2020.08.002",
"article-title": "Comparison of the basic morphology and function of 3D lung epithelial cultures derived from several donors",
"author": "Bovard",
"doi-asserted-by": "crossref",
"first-page": "56",
"journal-title": "Current Research in Toxicology",
"key": "10.1016/j.bbrep.2021.101187_bib24",
"volume": "1",
"year": "2020"
},
{
"article-title": "The use of in vitro 3D cell models in drug development for respiratory diseases",
"author": "Huang",
"first-page": "169",
"key": "10.1016/j.bbrep.2021.101187_bib25",
"series-title": "Drug Discovery and Development - Present and Future",
"year": "2011"
},
{
"DOI": "10.14573/altex.1605041s",
"article-title": "3-D nasal cultures: systems toxicological assessment of a candidate modified-risk tobacco product",
"author": "Iskandar",
"doi-asserted-by": "crossref",
"journal-title": "ALTEX",
"key": "10.1016/j.bbrep.2021.101187_bib26",
"year": "2016"
},
{
"DOI": "10.1016/j.xcrm.2020.100059",
"article-title": "Characterization and treatment of SARS-CoV-2 in nasal and bronchial human airway epithelia",
"author": "Pizzorno",
"doi-asserted-by": "crossref",
"first-page": "100059",
"issue": "4",
"journal-title": "Cell Rep Med",
"key": "10.1016/j.bbrep.2021.101187_bib27",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1016/j.tiv.2015.03.004",
"article-title": "Targeted omics analyses, and metabolic enzyme activity assays demonstrate maintenance of key mucociliary characteristics in long term cultures of reconstituted human airway epithelia",
"author": "Baxter",
"doi-asserted-by": "crossref",
"first-page": "864",
"issue": "5",
"journal-title": "Toxicol. Vitro",
"key": "10.1016/j.bbrep.2021.101187_bib28",
"volume": "29",
"year": "2015"
},
{
"DOI": "10.3390/v12121425",
"article-title": "Leveraging 3D model systems to understand viral interactions with the respiratory mucosa",
"author": "Iverson",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "Viruses",
"key": "10.1016/j.bbrep.2021.101187_bib29",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1186/1743-8977-6-32",
"article-title": "A dose-controlled system for air-liquid interface cell exposure and application to zinc oxide nanoparticles",
"author": "Lenz",
"doi-asserted-by": "crossref",
"first-page": "32",
"journal-title": "Part. Fibre Toxicol.",
"key": "10.1016/j.bbrep.2021.101187_bib30",
"volume": "6",
"year": "2009"
},
{
"DOI": "10.1177/2211068214561025",
"article-title": "TEER measurement techniques for in vitro barrier model systems",
"author": "Srinivasan",
"doi-asserted-by": "crossref",
"first-page": "107",
"issue": "2",
"journal-title": "J. Lab. Autom.",
"key": "10.1016/j.bbrep.2021.101187_bib31",
"volume": "20",
"year": "2015"
},
{
"DOI": "10.2147/IJGM.S167123",
"article-title": "Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis",
"author": "Graf",
"doi-asserted-by": "crossref",
"first-page": "275",
"journal-title": "Int. J. Gen. Med.",
"key": "10.1016/j.bbrep.2021.101187_bib32",
"volume": "11",
"year": "2018"
},
{
"DOI": "10.1152/ajplung.00552.2020",
"article-title": "Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures",
"author": "Schütz",
"doi-asserted-by": "crossref",
"first-page": "L750",
"issue": "5",
"journal-title": "Am. J. Physiol. Lung Cell Mol. Physiol.",
"key": "10.1016/j.bbrep.2021.101187_bib33",
"volume": "320",
"year": "2021"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2405580821002818"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Biochemistry",
"Biophysics"
],
"subtitle": [],
"title": "Iota-carrageenan extracted from red algae is a potent inhibitor of SARS‐CoV-2 infection in reconstituted human airway epithelia",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "29"
}