Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study
et al., Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC155.071, Oct 2025
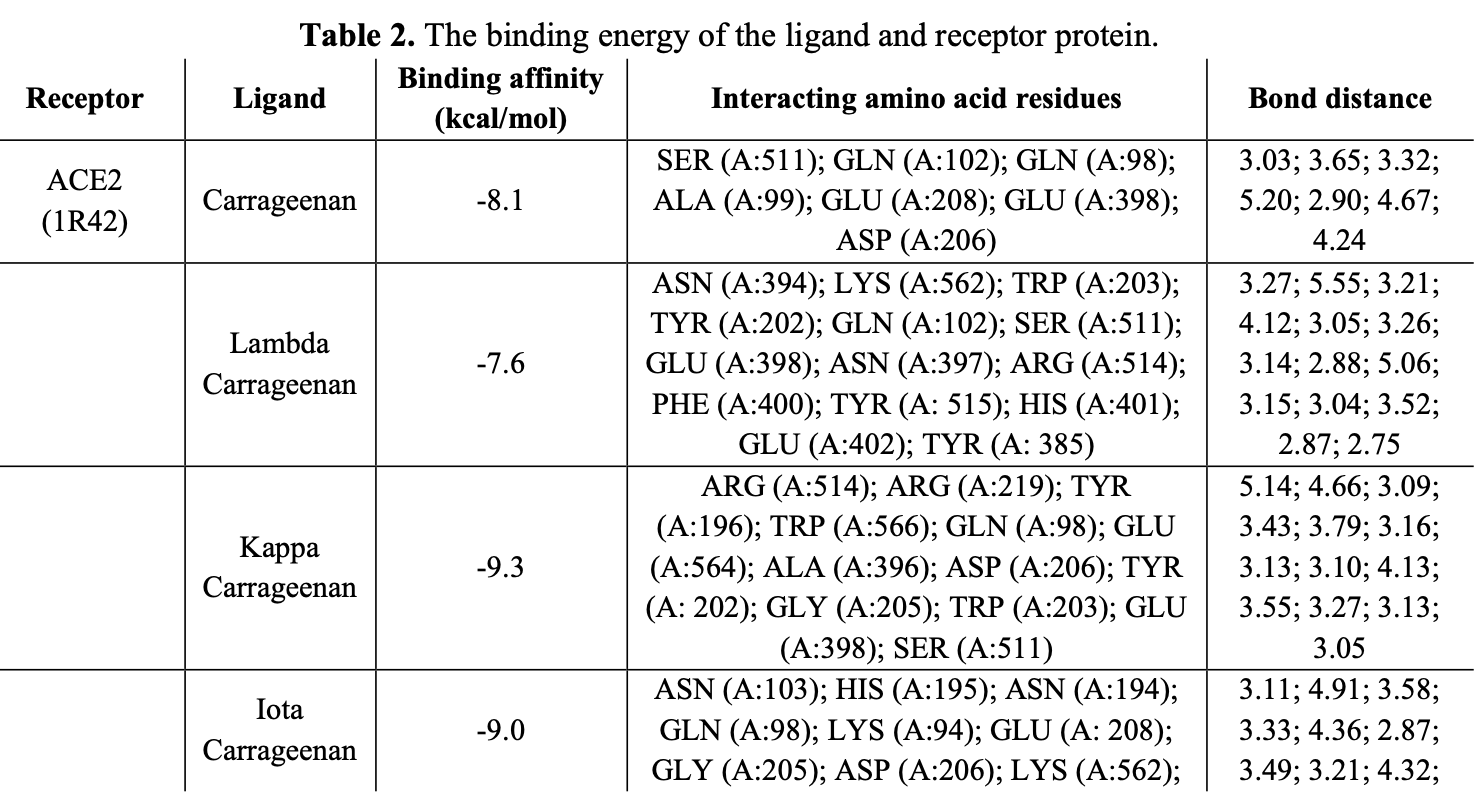
In silico study showing potential antiviral benefits with marine sulfated polysaccharides, specifically carrageenan, against SARS-CoV-2. Authors used molecular docking to screen compounds against the human ACE2 receptor, Spike protein RBD, and Mpro. Kappa-carrageenan showed the strongest binding affinity (-9.3 kcal/mol) with ACE2, suggesting inhibition of viral entry. Authors also noted strong Mpro binding, potentially hindering replication. All compounds complied with Lipinski’s Rule of Five, indicating favorable drug-likeness and oral bioavailability comparable to or exceeding the control drugs remdesivir and nafamostat.
19 preclinical studies support the efficacy of iota-carrageenan for COVID-19:
1.
Herida et al., Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC155.071.
2.
Krylova et al., Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents, International Journal of Molecular Sciences, doi:10.3390/ijms26136175.
3.
Rohilla et al., Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV-2, Stresses, doi:10.3390/stresses3030039.
4.
Thet, H., The potential of carrageenan for the drug discovery of COVID-19 via molecular docking with angiotensin-converting enzyme 2 (ACE2) and the main protease (Mpro) of SARS-CoV-2, Journal of Bioinformatics and Genomics, doi:10.18454/jbg.2022.18.2.001.
5.
Alsaidi et al., Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model, Marine Drugs, doi:10.3390/md19080418.
6.
Sattari et al., Repositioning Therapeutics for COVID-19: Virtual Screening of the Potent Synthetic and Natural Compounds as SARS-CoV-2 3CLpro Inhibitors, Research Square, doi:10.21203/rs.3.rs-37994/v1.
7.
Hoffmann et al., Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity, Biomacromolecules, doi:10.1021/acs.biomac.5c00576.
8.
Yathindranath et al., Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection, International Journal of Nanomedicine, doi:10.2147/IJN.S448005.
9.
Setz et al., Iota-Carrageenan Inhibits Replication of the SARS-CoV-2 Variants of Concern Omicron BA.1, BA.2 and BA.5, Nutraceuticals, doi:10.3390/nutraceuticals3030025.
10.
Meister et al., Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2, Journal of Hospital Infection, doi:10.1016/j.jhin.2021.10.019.
11.
Bovard et al., Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochemistry and Biophysics Reports, doi:10.1016/j.bbrep.2021.101187.
12.
Fröba et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, International Journal of Molecular Sciences, doi:10.3390/ijms222413202.
13.
Varese et al., Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model, bioRxiv, doi:10.1101/2021.04.27.441512.
14.
Morokutti-Kurz et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE, doi:10.1371/journal.pone.0237480.
15.
Song et al., Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food & Function, doi:10.1039/D0FO02017F.
Herida et al., 8 Oct 2025, peer-reviewed.
In silico studies are an important part of preclinical research, however results may be very different in vivo.
Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study
Biointerface Research in Applied Chemistry, doi:10.33263/briac155.071
In recent years, several viral diseases have emerged suddenly, leading to widespread infection and fatalities. SARS-CoV-2, which appeared in late 2019, mutates frequently, and current vaccines have limited effectiveness in fully preventing SARS-CoV-2 infections. As a result, natural antiviral medicines have gained attention, particularly sulfated polysaccharides from seaweeds, which are promising sources of bioactive compounds for antiviral activity and immune support. This study screened the types of sulfated polysaccharides, such as carrageenan, fucoidan, and ulvan, using computational analysis to evaluate their antiviral potential against SARS-CoV-2. Molecular docking was conducted to examine potential interactions with human ACE2, SARS-CoV-2's RBD, and main protease. The results of molecular docking analysis showed that kappa carrageenan exhibited better docking scores of -9.3 kcal/mol with ACE2 and -8.1 kcal/mol with spike protein-RBD. Meanwhile, carrageenan showed a better docking score of -7.6 kcal/mol with the main protease. The prediction of drug compounds based on RO5 indicates that all bioactive test compounds have the potential to be used as therapeutic agents. It is concluded that the sulfated polysaccharides derived from red seaweed, namely carrageenan and its derivatives, exhibit greater potential in demonstrating antiviral activity against SARS-CoV-2 compared to fucoidan and ulvan.
Author Contributions
Institutional Review Board Statement Not applicable.
Informed Consent Statement Not applicable.
Conflicts of Interest The authors declare that this research is not impacted by any competing interests.
Publisher's Note & Disclaimer The statements, opinions, and data presented in this publication are solely those of the individual author(s) and contributor(s) and do not necessarily reflect the views of the publisher and/or the editor(s). The publisher and/or the editor(s) disclaim any responsibility for the accuracy, completeness, or reliability of the content. Neither the publisher nor the editor(s) assume any legal liability for any errors, omissions, or consequences arising from the use of the information presented in this publication. Furthermore, the publisher and/or the editor(s) disclaim any liability for any injury, damage, or loss to persons or property that may result from the use of any ideas, methods, instructions, or products mentioned in the content. Readers are encouraged to independently verify any information before relying on it, and the publisher assumes no responsibility for any consequences arising from the use of materials contained in this publication.
References
Afriza, Suriyah, Ichwan, In silico analysis of molecular interactions between the antiapoptotic protein survivin and dentatin, nordentatin, and quercetin, J. Phys. Conf. Ser, doi:10.1088/1742-6596/1073/3/032001
Agu, Afiukwa, Orji, Ezeh, Ofoke et al., Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management, Sci. Rep, doi:10.1038/s41598-023-40160-2
Andrew, Jayaraman, Marine sulfated polysaccharides as potential antiviral drug candidates to treat Corona Virus disease (COVID-19), Carbohydr. Res, doi:10.1016/j.carres.2021.108326
Arasu, Balakrishnan, Velusamy, Ramesh, Can Mandated BCG Vaccine Promote herd Immunity against Novel Coronavirus? A Potential Solution at Hand to Tackle Covid-19 Pandemic, Curr. Immunol. Rev, doi:10.2174/1573395516999201112092557
Arunkumar, Sathaiah, Manikka, Verma, Patra et al., Marine algal antagonists targeting 3CL protease and spike glycoprotein of SARS-CoV-2: a computational approach for anti-COVID-19 drug discovery, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2021.1921032
Astuti, Ysrafil, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response, Diabetes Metab. Syndr.: Clin. Res. Rev, doi:10.1016/j.dsx.2020.04.020
Baell, Congreve, Leeson, Abad-Zapatero, Ask the Experts: Past, Present and Future of the Rule of Five, Future Med. Chem, doi:10.4155/fmc.13.61
Cascella, Rajnik, Aleem, Dulebohn, Di Napoli et al., Evaluation, and Treatment of Coronavirus (COVID-19)
Dallakyan, Olson, Small-Molecule Library Screening by Docking with PyRx, doi:10.1007/978-1-4939-2269-7_19
Deshpande, Tiwari, Nyayanit, Modak, In silico molecular docking analysis for repurposing therapeutics against multiple proteins from SARS-CoV-2, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2020.173430
Diksha, Pai, A Compendium of Global Traditional and Alternative Medicine: An Only Alternative to Thwart the Disease in the Current Scenario of the COVID Pandemic, Altern. Integ. Med
Dorp, Van; Tan, Lam, Richard, Owen et al., Recurrent mutations in SARS-CoV-2 genomes isolated from mink point to rapid host-adaptation, Nature Communications
Fauziyyah, Rahmasari, Sauriasari, The Efficacy of Remdesivir in Reducing SARS-CoV-2 Viral Load and Its Safety on COVID-19 Patients: A Systematic Review, Jurnal Respirologi Indonesia, doi:10.36497/jri.v42i1.211
Ferreira De Freitas, Schapira, A systematic analysis of atomic protein-ligand interactions in the PDB, Med. Chem. Commun, doi:10.1039/C7MD00381A
Frediansyah, Nainu, Dhama, Mudatsir, Harapan, Remdesivir and its antiviral activity against COVID-19: A systematic review, Clin. Epidemiol. Glob. Health, doi:10.1016/j.cegh.2020.07.011
Fröba, Große, Setz, Rauch, Auth et al., Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta, Int. J. Mol. Sci, doi:10.3390/ijms222413202
Gallina, Bork, Bordo, Structural analysis of protein-ligand interactions: the binding of endogenous compounds and of synthetic drugs, J. Mol. Recognit, doi:10.1002/jmr.2332
Gil, Ginex, Maestro, Nozal, Barrado-Gil et al., COVID-19: Drug Targets and Potential Treatments, J. Med. Chem, doi:10.1021/acs.jmedchem.0c00606
Han, Quan, Guo, Zhang, Lu et al., The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019, J. Med. Virol, doi:10.1002/jmv.25711
Hoffmann, Schroeder, Kleine-Weber, Müller, Drosten et al., Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19, Antimicrob. Agents Chemother, doi:10.1128/aac.00754-20
Jayaram, Singh, Mukherjee, Mathur, Shekhar et al., Sanjeevini: a freely accessible web-server for target directed lead molecule discovery, BMC Bioinformatics, doi:10.1186/1471-2105-13-S17-S7
Khailany, Safdar, Ozaslan, Genomic characterization of a novel SARS-CoV-2, Gene Rep, doi:10.1016/j.genrep.2020.100682
Kurian, Molecular Docking-Based Screening of Five Heterocyclic Quinone Compounds for Antifungal Activity on Yeast Sec14p and Validation by Redocking, J. Pharm. Res, doi:10.18579/jopcr/v23.2.31
Lipinski, Lead-and drug-like compounds: the rule-of-five revolution, Drug Discov. Today Technol, doi:10.1016/j.ddtec.2004.11.007
Liu, Blet, Smyth, Li, The Science Underlying COVID-19: Implications for the Cardiovascular System, Circulation, doi:10.1161/CIRCULATIONAHA.120.047549
Lohit, Singh, Kumar, Singh, Yadav et al., Description and in silico ADME studies of US-FDA approved drugs or drugs under clinical trial which violate the Lipinski's rule of 5, Lett. Drug Des. Discov, doi:10.2174/1570180820666230224112505
Mei, Tan, Current Strategies of Antiviral Drug Discovery for COVID-19, Front. Mol. Biosci, doi:10.3389/fmolb.2021.671263
Mittal, Manjunath, Ranjan, Kaushik, Kumar et al., COVID-19 pandemic: Insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2, PLOS Pathog, doi:10.1371/journal.ppat.1008762
Muthukumar, Chidambaram, Sukumaran, Sulfated polysaccharides and its commercial applications in food industries-A review, J. Food Sci. Technol, doi:10.1007/s13197-020-04837-0
Möbitz, Design Principles for Balancing Lipophilicity and Permeability in beyond Rule of 5 Space, ChemMedChem, doi:10.1002/cmdc.202300395
Pollastri, Overview on the Rule of Five, Curr. Protoc. Pharmacol, doi:10.1002/0471141755.ph0912s49
Pratama, Poerwono, Siswodihardjo, Introducing a two-dimensional graph of docking score difference vs. similarity of ligand-receptor interactions, Indones. J. Biotechnol, doi:10.22146/ijbiotech.62194
Rai, Barik, Singh, Suresh, Singh et al., Molecular docking, binding mode analysis, molecular dynamics, and prediction of ADMET/toxicity properties of selective potential antiviral agents against SARS-CoV-2 main protease: an effort toward drug repurposing to combat COVID-19, Mol. Divers, doi:10.1007/s11030-021-10188-5
Reshi, Su, Hong, RNA Viruses: ROS-Mediated Cell Death, Int. J. Cell Biol, doi:10.1155/2014/467452
Rohilla, Srivastava, Singh, Yadav, Singh et al., Algae Polysaccharides (Carrageenan and Alginate)-A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV2, Stresses, doi:10.3390/stresses3030039
Salehi, Abedi, Balakrishnan, Gholamrezanezhad, Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients, Am. J. Roentgenol, doi:10.2214/AJR.20.23034
Salih, Thissera, Yaseen, Hassane, El-Seedi et al., Marine Sulfated Polysaccharides as Promising Antiviral Agents: A Comprehensive Report and Modeling Study Focusing on SARS CoV-2, Mar. Drugs, doi:10.3390/md19080406
Shawon, Akter, Hossen, Akter, Sayeed et al., Current landscape of natural products against coronaviruses: perspectives in COVID-19 treatment and antiviral mechanism, Curr. Pharm. Des, doi:10.2174/1381612826666201106093912
Shi, Wang, Lu, Qin, Hu et al., Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds, Carbohydr. Res, doi:10.1016/j.carres.2017.10.020
Wan, Shang, Graham, Baric, Li, Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus, J. Virol, doi:10.1128/JVI.00127-20
Who, Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases
Yi, Taylor, Ziebarth, Wang, Predictive Models and Impact of Interfacial Contacts and Amino Acids on Protein-Protein Binding Affinity, ACS Omega, doi:10.1021/acsomega.3c06996
Zehra, Luthra, Siddiqui, Shamsi, Gaur et al., Corona virus versus existence of human on the earth: A computational and biophysical approach, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2020.06.007
Zhang, Lin, Sun, Curth, Drosten et al., Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors, Science, doi:10.1126/science.abb3405
Zheng, SARS-CoV-2: an Emerging Coronavirus that Causes a Global Threat, Int. J. Biol. Sci, doi:10.7150/ijbs.45053
Álvarez-Viñas, Souto, Flórez-Fernández, Torres, Bandín et al., Antiviral Activity of Carrageenans and Processing Implications, Mar. Drugs, doi:10.3390/md19080437
DOI record:
{
"DOI": "10.33263/briac155.071",
"ISSN": [
"2069-5837"
],
"URL": "http://dx.doi.org/10.33263/BRIAC155.071",
"abstract": "<jats:p>In recent years, several viral diseases have emerged suddenly, leading to widespread infection and fatalities. SARS-CoV-2, which appeared in late 2019, mutates frequently, and current vaccines have limited effectiveness in fully preventing SARS-CoV-2 infections. As a result, natural antiviral medicines have gained attention, particularly sulfated polysaccharides from seaweeds, which are promising sources of bioactive compounds for antiviral activity and immune support. This study screened the types of sulfated polysaccharides, such as carrageenan, fucoidan, and ulvan, using computational analysis to evaluate their antiviral potential against SARS-CoV-2. Molecular docking was conducted to examine potential interactions with human ACE2, SARS-CoV-2's RBD, and main protease. The results of molecular docking analysis showed that kappa carrageenan exhibited better docking scores of -9.3 kcal/mol with ACE2 and -8.1 kcal/mol with spike protein-RBD. Meanwhile, carrageenan showed a better docking score of -7.6 kcal/mol with the main protease. The prediction of drug compounds based on RO5 indicates that all bioactive test compounds have the potential to be used as therapeutic agents. It is concluded that the sulfated polysaccharides derived from red seaweed, namely carrageenan and its derivatives, exhibit greater potential in demonstrating antiviral activity against SARS-CoV-2 compared to fucoidan and ulvan.</jats:p>",
"container-title": "Biointerface Research in Applied Chemistry",
"container-title-short": "Biointerface Res Appl Chem",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
10,
14
]
],
"date-time": "2025-10-14T06:02:42Z",
"timestamp": 1760421762000
},
"deposited": {
"date-parts": [
[
2025,
10,
14
]
],
"date-time": "2025-10-14T06:06:12Z",
"timestamp": 1760421972000
},
"indexed": {
"date-parts": [
[
2025,
10,
14
]
],
"date-time": "2025-10-14T06:42:47Z",
"timestamp": 1760424167334,
"version": "build-2065373602"
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2025,
10,
15
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2025,
10,
15
]
]
}
},
"language": "en",
"member": "18784",
"original-title": [],
"page": "71",
"prefix": "10.33263",
"published": {
"date-parts": [
[
2025,
10,
15
]
]
},
"published-online": {
"date-parts": [
[
2025,
10,
15
]
]
},
"publisher": "AMG Transcend Association",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://biointerfaceresearch.com/wp-content/uploads/2025/08/BRIAC155.071.pdf"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Chemical Insights into the Antiviral Mechanisms of Marine Sulfated Polysaccharides: An In-Silico Screening and Molecular Docking Study",
"type": "journal-article",
"volume": "15"
}