The Saliva of Probands Sucking an Iota-Carrageenan Containing Lozenge Inhibits Viral Binding and Replication of the Most Predominant Common Cold Viruses and SARS-CoV-2
et al., International Journal of General Medicine, doi:10.2147/IJGM.S325861, NCT04533906, Sep 2021
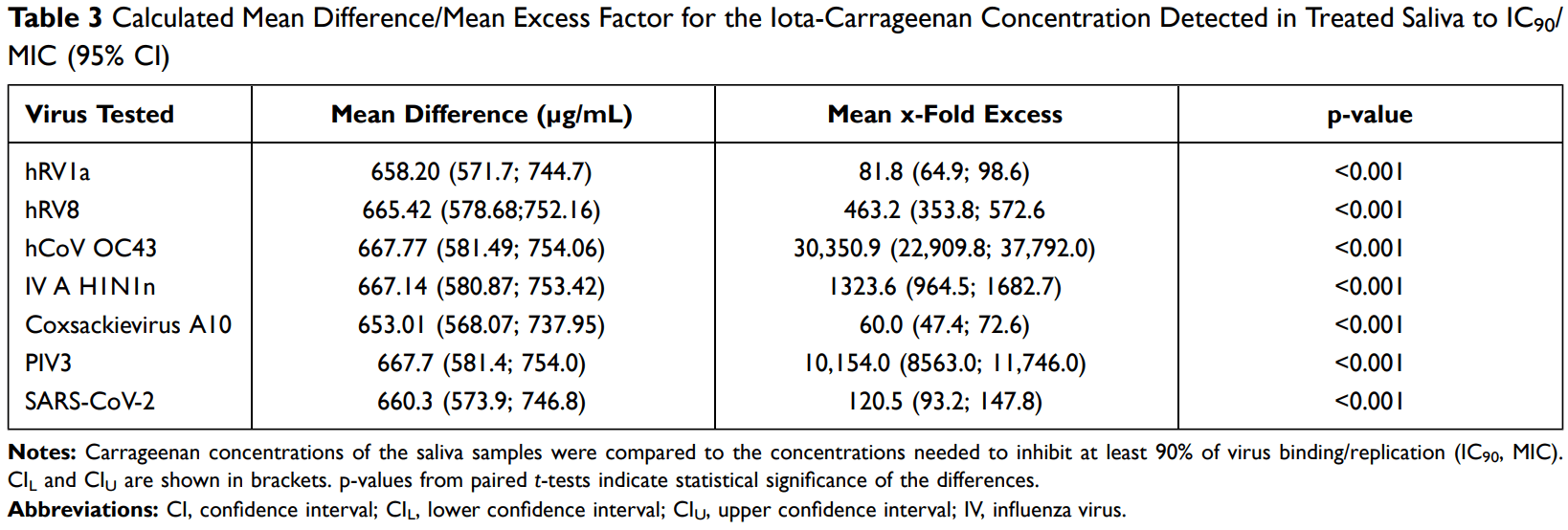
Prospective study of 31 subjects analyzing iota-carrageenan concentration in saliva after using an iota-carrageenan containing lozenge, showing sufficient concentration to neutralize common respiratory viruses including SARS-CoV-2. For SARS-CoV-2, the IC90 was exceeded by 121-fold (p < 0.001).
Morokutti-Kurz et al., 7 Sep 2021, prospective, peer-reviewed, 12 authors, trial NCT04533906 (history).
The Saliva of Probands Sucking an Iota-Carrageenan Containing Lozenge Inhibits Viral Binding and Replication of the Most Predominant Common Cold Viruses and SARS-CoV-2
International Journal of General Medicine, doi:10.2147/ijgm.s325861
The aim of this study was to investigate whether sucking of an iota-carrageenan containing lozenge releases sufficient iota-carrageenan into the saliva of healthy subjects to neutralize representatives of the most common respiratory virus families causing common cold and SARS-CoV-2. Patients and Methods: In this monocentric, open label, prospective clinical trial, 31 healthy subjects were included to suck a commercially available iota-carrageenan containing lozenge. Saliva samples from 27 subjects were used for ex vivo efficacy analysis. The study's primary objective was to assess if the mean iota-carrageenan concentration of the saliva samples exceeded 5 µg/mL, which is the concentration known to reduce replication of human rhinovirus (hRV) 1a and 8 by 90%. The iota-carrageenan concentration of the saliva samples was analyzed by UV-Vis spectroscopy. The antiviral effectiveness of the individual saliva samples was determined in vitro against a panel of respiratory viruses including hRV1a, hRV8, human coronavirus OC43, influenza virus A H1N1pdm09, coxsackievirus A10, parainfluenza virus 3 and SARS-CoV-2 using standard virological assays.
Results: The mean iota-carrageenan concentration detected in the saliva exceeds the concentration needed to inhibit 90% of hRV1a and hRV8 replication by 134-fold (95% CI 116.3-160.8-fold; p < 0.001). Thus, the study met the primary endpoint. Furthermore, the iota-carrageenan saliva concentration was 60 to 30,351-fold higher than needed to reduce viral replication/binding of all tested viruses by at least 90% (p < 0.001). The effect was most pronounced in hCoV OC43; in case of SARS-CoV-2, the IC 90 was exceeded by 121-fold (p < 0.001). Conclusion: Sucking an iota-carrageenan containing lozenge releases sufficient iota-carrageenan to neutralize and inactivate the most abundant respiratory viruses as well as pandemic SARS-CoV-2. The lozenges are therefore an appropriate measure to reduce the viral load at the site of infection, hereby presumably limiting transmission within a population as well as translocation to the lower respiratory tract. Trial Registration: NCT04533906.
Disclosure Martina Morokutti-Kurz, Nicole Unger-Manhart, Philipp Graf, Julia Kodnar are employed by Marinomed Biotech AG. Eva Prieschl-Grassauer and Andreas Grassauer are co-founder of Marinomed Biotech AG and inventor on patent #WO2008067982 held by Marinomed Biotech AG that relates to the content of the manuscript. Markus Savli reports personal fees from Marinomed Biotech AG. Andreas Grassauer, Eva Prieschl-Grassauer and Martina Morokutti-Kurz are inventors of a patent submission related to the content of the manuscript; the number of this patent application is EP20186334. The authors report no other conflicts of interest in this work.
International Journal of General Medicine
Dovepress
References
Bansal, Jonsson, Taylor, Iota-carrageenan and xylitol inhibit SARS-CoV-2 in cell culture, bioRxiv, doi:10.1101/2020.08.19.225854
Charlton, Babady, Ginocchio, Practical guidance for clinical microbiology laboratories: viruses causing acute respiratory tract infections, Clin Microbiol Rev, doi:10.1128/CMR.00042-18
Collins, Dawes, The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa, J Dent Res, doi:10.1177/00220345870660080201
Denny, The clinical impact of human respiratory virus infections, Am J Respir Crit Care Med, doi:10.1164/ajrccm/152.4_Pt_2.S4
Eccles, Iota-carrageenan as an antiviral treatment for the common cold, Open Virol J, doi:10.2174/1874357902014010009
Eccles, Meier, Jawad, Weinmüllner, Grassauer et al., Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold, Respir Res, doi:10.1186/1465-9921-11-108
Eccles, Winther, Johnston, Robinson, Trampisch et al., Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial, Respir Res, doi:10.1186/s12931-015-0281-8
Fazekas, Eickhoff, Pruckner, Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold, BMC Complement Altern Med, doi:10.1186/1472-6882-12-147
Figueroa, Lombardo, Dogliotti, Efficacy of a nasal spray containing iota-carrageenan in the prophylaxis of COVID, doi:10.1101/2021.04.13.21255409%A0
Graf, Bernkop-Schnürch, Egyed, Koller, Prieschl-Grassauer et al., Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis, Int J Gen Med, doi:10.2147/IJGM.S167123
Grassauer, Weinmuellner, Meier, Pretsch, Prieschl-Grassauer et al., Iota-carrageenan is a potent inhibitor of rhinovirus infection, Virol J, doi:10.1186/1743-422X-5-107
Große, Ruetalo, Businger, Evidence that quinine exhibits antiviral activity against SARS-CoV-2 infection in vitro, Preprints, doi:10.2147/IJGM.S325861
Hemilä, Chalker, Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: re-analysis of randomized trial data, Pharmacol Res Perspect
Huijghebaert, Vanham, Van Winckel, Allegaert, Does trypsin oral spray (Viruprotect ® / ColdZyme ® ) protect against COVID-19 and common colds or induce mutation? Caveats in medical device regulations in the European union, Int J Environ Res Public Health, doi:10.3390/ijerph18105066
Jang, Shin, Lee, Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2, Sci Rep, doi:10.1038/s41598-020-80896-9
Koenighofer, Lion, Bodenteich, Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials, Multidiscip Respir Med, doi:10.1186/2049-6958-9-57
Leibbrandt, Meier, König-Schuster, Iota-carrageenan is a potent inhibitor of influenza A virus infection, PLoS One, doi:10.1371/journal.pone.0014320
Ludwig, Enzenhofer, Schneider, Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial, Respir Res, doi:10.1186/1465-9921-14-124
Morokutti-Kurz, Dovepress Powered by TCPDF
Morokutti-Kurz, Fröba, Graf, Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS One, doi:10.1371/journal.pone.0237480
Morokutti-Kurz, Graf, Prieschl-Grassauer, Amylmetacresol/ 2,4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat, Int J Gen Med, doi:10.2147/IJGM.S120665
Schütz, Conzelmann, Fois, Carrageenan containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00552.2020
Snell, New treatments for viral respiratory tract infectionsopportunities and problems, J Antimicrob Chemother, doi:10.1093/jac/47.3.251
Yasir, Goyal, Bansal, Sonthalia, StatPearls [Internet
DOI record:
{
"DOI": "10.2147/ijgm.s325861",
"ISSN": [
"1178-7074"
],
"URL": "http://dx.doi.org/10.2147/IJGM.S325861",
"author": [
{
"affiliation": [],
"family": "Morokutti-Kurz",
"given": "Martina",
"sequence": "first"
},
{
"affiliation": [],
"family": "Unger-Manhart",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Graf",
"given": "Philipp",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rauch",
"given": "Pia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kodnar",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Große",
"given": "Maximilian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Setz",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Savli",
"given": "Markus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ehrenreich",
"given": "Friedrich",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grassauer",
"given": "Andreas",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0118-4297",
"affiliation": [],
"authenticated-orcid": true,
"family": "Prieschl-Grassauer",
"given": "Eva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schubert",
"given": "Ulrich",
"sequence": "additional"
}
],
"container-title": "International Journal of General Medicine",
"container-title-short": "IJGM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
9,
6
]
],
"date-time": "2021-09-06T17:12:22Z",
"timestamp": 1630948342000
},
"deposited": {
"date-parts": [
[
2023,
11,
8
]
],
"date-time": "2023-11-08T11:34:46Z",
"timestamp": 1699443286000
},
"indexed": {
"date-parts": [
[
2024,
2,
17
]
],
"date-time": "2024-02-17T17:41:04Z",
"timestamp": 1708191664861
},
"is-referenced-by-count": 6,
"issued": {
"date-parts": [
[
2021,
9
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
1
]
],
"date-time": "2021-09-01T00:00:00Z",
"timestamp": 1630454400000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/getfile.php?fileID=73355",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/getfile.php?fileID=73355",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "5241-5249",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2021,
9
]
]
},
"published-online": {
"date-parts": [
[
2021,
9
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1164/ajrccm/152.4_Pt_2.S4",
"author": "Denny",
"doi-asserted-by": "publisher",
"first-page": "S4",
"journal-title": "Am J Respir Crit Care Med",
"key": "ref1",
"volume": "152",
"year": "1995"
},
{
"DOI": "10.1093/jac/47.3.251",
"author": "Snell",
"doi-asserted-by": "publisher",
"first-page": "251",
"journal-title": "J Antimicrob Chemother",
"key": "ref2",
"volume": "47",
"year": "2001"
},
{
"DOI": "10.1186/1465-9921-11-108",
"author": "Eccles",
"doi-asserted-by": "publisher",
"first-page": "108",
"journal-title": "Respir Res",
"key": "ref3",
"volume": "11",
"year": "2010"
},
{
"DOI": "10.1186/1472-6882-12-147",
"author": "Fazekas",
"doi-asserted-by": "publisher",
"first-page": "147",
"journal-title": "BMC Complement Altern Med",
"key": "ref4",
"volume": "12",
"year": "2012"
},
{
"DOI": "10.1186/2049-6958-9-57",
"author": "Koenighofer",
"doi-asserted-by": "publisher",
"first-page": "57",
"journal-title": "Multidiscip Respir Med",
"key": "ref5",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.1186/1465-9921-14-124",
"author": "Ludwig",
"doi-asserted-by": "publisher",
"first-page": "124",
"journal-title": "Respir Res",
"key": "ref6",
"volume": "14",
"year": "2013"
},
{
"DOI": "10.1186/s12931-015-0281-8",
"author": "Eccles",
"doi-asserted-by": "publisher",
"first-page": "121",
"journal-title": "Respir Res",
"key": "ref7",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1101/2021.04.13.21255409 ",
"author": "Figueroa",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref8",
"year": "2021"
},
{
"DOI": "10.1186/1743-422X-5-107",
"author": "Grassauer",
"doi-asserted-by": "publisher",
"first-page": "107",
"journal-title": "Virol J",
"key": "ref9",
"volume": "5",
"year": "2008"
},
{
"DOI": "10.1371/journal.pone.0014320",
"author": "Leibbrandt",
"doi-asserted-by": "publisher",
"first-page": "e14320",
"journal-title": "PLoS One",
"key": "ref10",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.2147/IJGM.S120665",
"author": "Morokutti-Kurz",
"doi-asserted-by": "publisher",
"first-page": "53",
"journal-title": "Int J Gen Med",
"key": "ref11",
"volume": "10",
"year": "2017"
},
{
"DOI": "10.1371/journal.pone.0237480",
"author": "Morokutti-Kurz",
"doi-asserted-by": "publisher",
"first-page": "e0237480",
"journal-title": "PLoS One",
"key": "ref12",
"volume": "16",
"year": "2021"
},
{
"key": "ref13",
"volume-title": "StatPearls [Internet]",
"year": "2020"
},
{
"DOI": "10.1101/2020.08.19.225854",
"author": "Bansal",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "ref14",
"year": "2020"
},
{
"DOI": "10.1128/CMR.00042-18",
"author": "Charlton",
"doi-asserted-by": "publisher",
"first-page": "e00042",
"journal-title": "Clin Microbiol Rev",
"key": "ref15",
"volume": "32",
"year": "2019"
},
{
"DOI": "10.1152/ajplung.00552.2020",
"author": "Schütz",
"doi-asserted-by": "publisher",
"first-page": "L750",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "ref16",
"volume": "320",
"year": "2021"
},
{
"DOI": "10.2147/IJGM.S167123",
"author": "Graf",
"doi-asserted-by": "publisher",
"first-page": "275",
"journal-title": "Int J Gen Med",
"key": "ref17",
"volume": "11",
"year": "2018"
},
{
"author": "Große",
"first-page": "2020070102",
"journal-title": "Preprints",
"key": "ref18",
"year": "2020"
},
{
"DOI": "10.2174/1874357902014010009",
"author": "Eccles",
"doi-asserted-by": "publisher",
"first-page": "9",
"journal-title": "Open Virol J",
"key": "ref19",
"volume": "14",
"year": "2020"
},
{
"key": "ref20",
"unstructured": "World Health Organization.WHO Manual on Animal Influenza Diagnosis and Surveillance. Available from: https://www.who.int/csr/resources/publications/influenza/whocdscsrncs20025rev.pdf. Accessed August 31, 2021."
},
{
"DOI": "10.1002/prp2.810",
"author": "Hemilä",
"doi-asserted-by": "crossref",
"first-page": "e00810",
"journal-title": "Pharmacol Res Perspect",
"key": "ref21",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1038/s41598-020-80896-9",
"author": "Jang",
"doi-asserted-by": "publisher",
"first-page": "821",
"journal-title": "Sci Rep",
"key": "ref22",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3390/ijerph18105066",
"author": "Huijghebaert",
"doi-asserted-by": "publisher",
"first-page": "5066",
"journal-title": "Int J Environ Res Public Health",
"key": "ref23",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1177/00220345870660080201",
"author": "Collins",
"doi-asserted-by": "publisher",
"first-page": "1300",
"journal-title": "J Dent Res",
"key": "ref24",
"volume": "66",
"year": "1987"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/the-saliva-of-probands-sucking-an-iota-carrageenan-containing-lozenge--peer-reviewed-fulltext-article-IJGM"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The Saliva of Probands Sucking an Iota-Carrageenan Containing Lozenge Inhibits Viral Binding and Replication of the Most Predominant Common Cold Viruses and SARS-CoV-2",
"type": "journal-article",
"volume": "Volume 14"
}