Efficacy of Nasal Spray, Mouth Spray, and Mouthwash Containing Limonene, Cetylpyridinium Chloride, and Monolaurin in COVID-19 Management: A Double-Blind, Randomized, Placebo-Controlled Trial
et al., MDPI AG, doi:10.20944/preprints202509.1594.v1, TCTR20240803002, Sep 2025
58th treatment shown to reduce risk in
September 2025, now with p = 0.0035 from 4 studies.
Lower risk for recovery and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
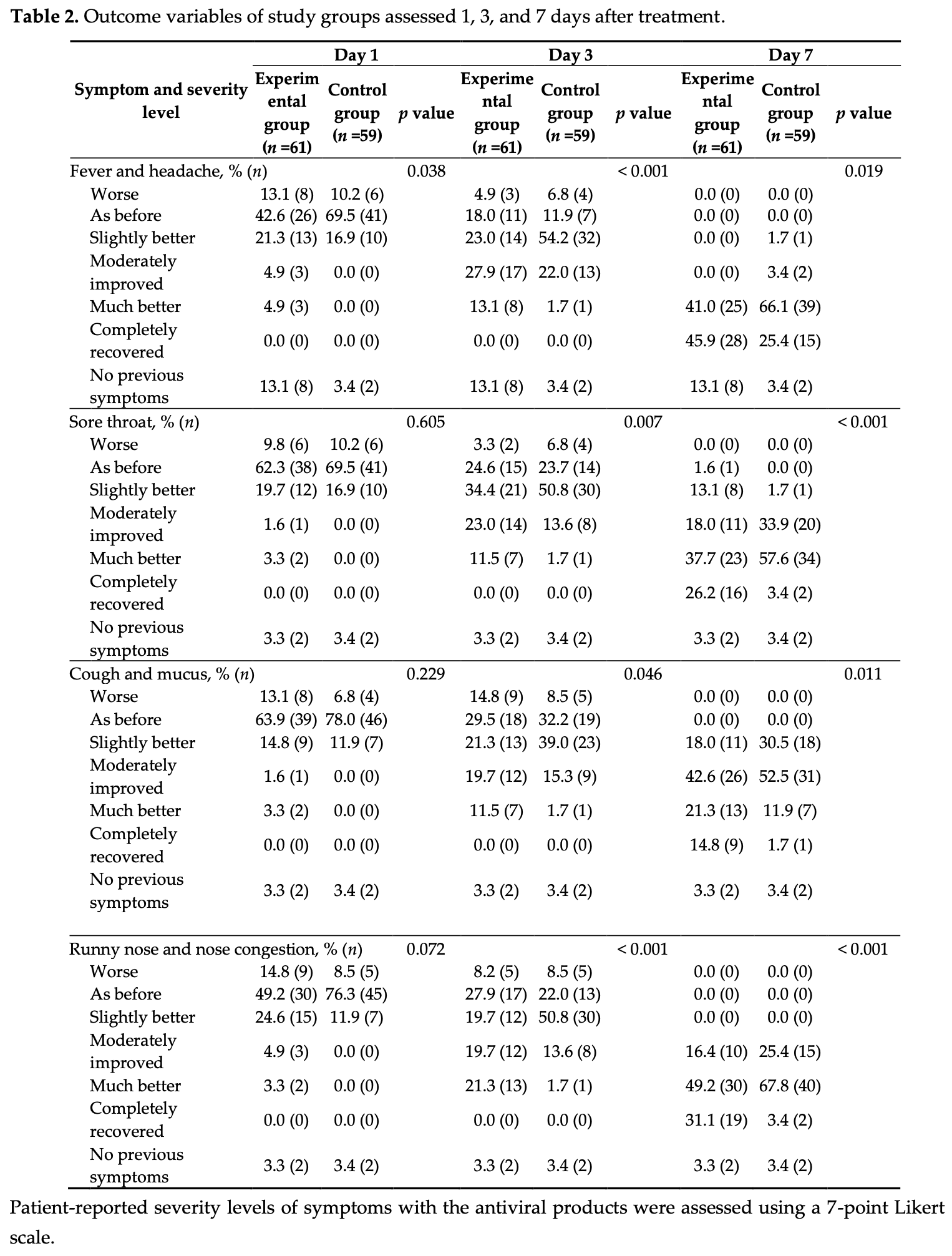
RCT 120 low-risk COVID-19 patients showing improved recovery with nasal and oral formulations containing cetylpyridinium chloride, D-limonene, and monolaurin (the nasal formulation contained D-limonene and cetylpyridinium chloride, while the oral formulation contained D-limonene, monolaurin, and cetylpyridinium chloride). No patients progressed to severe disease. No adverse events were reported in either group during the 7 day treatment period or 1 month followup. Placebo contents are not specified - authors note only "a homogenized liquid carrier", however any liquid rinse may have some efficacy via mechanical clearance.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
|
risk of no recovery, 36.0% lower, RR 0.64, p = 0.006, treatment 25 of 53 (47.2%), control 42 of 57 (73.7%), NNT 3.8, day 7, fever and headache.
|
|
risk of no recovery, 24.5% lower, RR 0.76, p < 0.001, treatment 43 of 59 (72.9%), control 55 of 57 (96.5%), NNT 4.2, day 7, sore throat.
|
|
risk of no recovery, 13.7% lower, RR 0.86, p = 0.02, treatment 50 of 59 (84.7%), control 56 of 57 (98.2%), NNT 7.4, day 7, cough and mucus.
|
|
risk of no recovery, 29.7% lower, RR 0.70, p < 0.001, treatment 40 of 59 (67.8%), control 55 of 57 (96.5%), NNT 3.5, day 7, runny nose and nasal congestion.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ponphaiboon et al., 19 Sep 2025, Double Blind Randomized Controlled Trial, placebo-controlled, Thailand, preprint, 12 authors, study period 17 May, 2022 - 16 May, 2023, this trial uses multiple treatments in the treatment arm (combined with limonene and monolaurin) - results of individual treatments may vary, trial TCTR20240803002.
Contact: limmatvapirat_c@su.ac.th (corresponding author).
Efficacy of Nasal Spray, Mouth Spray, and Mouthwash Containing Limonene, Cetylpyridinium Chloride, and Monolaurin in COVID-19 Management: A Double-Blind, Randomized, Placebo-Controlled Trial
doi:10.20944/preprints202509.1594.v1
Background/Objectives: COVID-19 predominantly affects the respiratory tract, leading to symptoms such as fever, sore throat, cough, and nasal congestion. This study aimed to evaluate the efficacy and safety of nasal spray, mouth spray, and mouthwash containing limonene, cetylpyridinium chloride (CPC), and monolaurin in alleviating symptoms among patients with mild-to-moderate COVID-19. Methods: A double-blind, randomized, placebo-controlled trial was conducted at Dontum Hospital, Thailand, from May to November 2022. A total of 120 RT-PCR-confirmed COVID-19 patients were randomly assigned to receive either the active antiviral formulations or placebo products. Symptom severity was assessed on Days 1, 3, and 7 using a 7-point Likert scale. Patient satisfaction regarding symptom relief and product attributes (color, smell, taste) was evaluated on Day 7 using a 5-point Likert scale. The study was approved by the Ethics Committee of Silpakorn University (COE 65.0517-081) and registered with the Thai Clinical Trials Registry (TCTR20240803002). Results: Compared to the placebo group, the experimental group exhibited significantly faster symptom resolution, particularly for sore throat, cough with mucus, and nasal congestion, with notable improvements observed as early as Day 3 (p < 0.05). By Day 7, a higher proportion of patients in the experimental group reported complete recovery (p < 0.05). Additionally, patient satisfaction scores for symptom relief and product characteristics were significantly higher in the experimental group (p < 0.001).
Declaration of competing interest: All authors declare that they have no financial interests in the patents related to the formulations used in this study.
References
Alhassan, Asiamah, Opuni, Alhassan, The Likert scale: exploring the unknowns and their potential to mislead the world, UDS Int. J. Dev, doi:10.47740/586.UDSIJD6i
Alsaleh, Alhussien, Alyamani, Efficacy of povidone-iodine nasal rinse and mouth wash in COVID-19 management: a prospective, randomized pilot clinical trial (povidone-iodine in COVID-19 management), BMC Infect. Dis, doi:10.1186/s12879-024-09137-y
Anderson, Patterson, Richards, CPC-containing oral rinses inactivate SARS-CoV-2 variants and are active in the presence of human saliva, J. Med. Microbiol, doi:10.1099/jmm.0.001508
Bacanlı, Başaran, Başaran, The antioxidant and antigenotoxic properties of citrus phenolics limonene and naringin, Food Chem. Toxicol, doi:10.1016/j.fct.2015.04.015
Barker, Bakkum, Chapman, The clinical use of monolaurin as a dietary supplement: a review of the literature, J. Chiropr. Med, doi:10.1016/j.jcm.2019.02.004
Bañó-Polo, Martínez-Gil, Sánchez Del Pino, Massoli, Mingarro et al., Cetylpyridinium chloride promotes disaggregation of SARS-CoV-2 virus-like particles, J. Oral Microbiol, doi:10.1080/20002297.2022.2030094
Beck, Handy, Levander, Host nutritional status: the neglected virulence factor, Trends Microbiol, doi:10.1016/j.tim.2004.07.007
Cecchini, Cecchini, SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression, Med. Hypotheses, doi:10.1016/j.mehy.2020.110102.Disclaimer/Publisher'sNote
Chaudhary, Siddiqui, Athar, Alam, D-limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis, Hum. Exp. Toxicol, doi:10.1177/0960327111434948
Chen, Zhou, Dong, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet, doi:10.1016/S0140-6736(20)30211-7
Chong, Dividing the emergency department into red, yellow, and green zones to control COVID-19 infection; a letter to editor, Arch. Acad. Emerg. Med
Chowdhury, Sikdar, Turin, Sample size calculation in clinical studies: Some common scenarios, Int. J. Stat. Med. Res, doi:10.6000/1929-6029.2017.06.04.3
Corrêa, Weimer, Rossi, Hoffmann, Koester et al., Lime and orange essential oils and D-limonene as a potential COVID-19 inhibitor: computational, in chemico, and cytotoxicity analysis, Food Biosci, doi:10.1016/j.fbio.2022.102348
Corrêa, Weimer, Rossi, Hoffmann, Koester et al., Lime and orange essential oils and d-limonene as a potential COVID-19 inhibitor: Computational, in chemico, and cytotoxicity analysis, Food Biosci, doi:10.1016/j.fbio.2022.102348
Drusano, Infection site concentrations: their therapeutic importance and the macrolide and macrolidelike class of antibiotics, Pharmacotherapy, doi:10.1592/phco.2005
Fadilah, Jittmittraphap, Leaungwutiwong, Pripdeevech, Dhanushka et al., Virucidal activity of essential oils from Citrus × aurantium L. against influenza A virus H1N1: limonene as a potential household disinfectant against virus, Nat. Prod. Commun, doi:10.1177/1934578X211072713
Fais, Juskeviciene, Francardo, Drug-free nasal spray as a barrier against SARS-CoV-2 and its delta variant: in vitro study of safety and efficacy in human nasal airway epithelia, Int. J. Mol. Sci, doi:10.3390/ijms23074062
Falk-Filipsson, Löf, Hagberg, Hjelm, Wang, d-limonene exposure to humans by inhalation: uptake, distribution, elimination, and effects on the pulmonary function, J. Toxicol. Environ. Health, doi:10.1080/15287399309531702
Fazekas, Eickhoff, Pruckner, Lessons learned from a double-blind randomised placebocontrolled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold, BMC Complement. Altern. Med, doi:10.1186/1472-6882-12-147
Felsenstein, Herbert, Mcnamara, Hedrich, COVID-19: immunology and treatment options, Clin. Immunol, doi:10.1016/j.clim.2020.108448
Gupta, Jeyakumar, Lawrence, Journey of limonene as an antimicrobial agent, J. Pure Appl. Microbiol, doi:10.22207/JPAM.15.3.01
Herrera, Serrano, Roldán, Sanz, Is the oral cavity relevant in SARS-CoV-2 pandemic?, Clin. Oral Investig, doi:10.1007/s00784-020-03413-2
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Idrees, Mcgowan, Fawzy, Abuderman, Balasubramaniam et al., Efficacy of mouth rinses and nasal spray in the inactivation of SARS-CoV-2: a systematic review and meta-analysis of in vitro and in vivo studies, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph191912148
Kabara, Vrable, Lie, Jie, Antimicrobial lipids: natural and synthetic acids and monoglycerides, Lipids, doi:10.1007/BF02570908
Kawana, Kitamura, Nakagomi, Inactivation of human viruses by povidone-iodine in comparison with other antiseptics, Dermatology, doi:10.1159/000246027
Keles, Mild SARS-CoV-2 infections in children might be based on evolutionary biology and linked with host reactive oxidative stress and antioxidant capabilities, New Microbes New Infect, doi:10.1016/j.nmni.2020.100723
Khomich, Kochetkov, Bartosch, Ivanov, Redox biology of respiratory viral infections, Viruses, doi:10.3390/v10080392
Kumar, Mishra, Dunn, Townsend, Oguadinma et al., Biocides and novel antimicrobial agents for the mitigation of coronaviruses, Front. Microbiol, doi:10.3389/fmicb.2020.01351
Lebel, Vaillancourt, Morin, Grenier, Antimicrobial activity, biocompatibility and antiinflammatory properties of cetylpyridinium chloride-based mouthwash containing sodium fluoride and xylitol: An in vitro study, Oral Health Prev. Dent, doi:10.3290/j.ohpd.b871071
Li, Guan, Wu, Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia, N. Engl. J. Med, doi:10.1056/NEJMoa2001316
Lin, Li, Sun, D-Limonene: promising and sustainable natural bioactive compound, Appl. Sci, doi:10.3390/app14114605
Low, Lani, Tiong, Poh, Abubakar et al., COVID-19 therapeutic potential of natural products, Int. J. Mol. Sci, doi:10.3390/ijms24119589
Luo, Wang, Luo, Limonene anti-TMV activity and its mode of action, Pestic. Biochem. Physiol, doi:10.1016/j.pestbp.2023.105512
Mahidol, Ruchirawat, Sutananta, Kittakoop, Wanichacheva et al., Antiviral product for oral and throat and production method, Thai Petty Patent
Mahidol, Ruchirawat, Sutananta, Kittakoop, Wanichacheva et al., Nasal spray product for killing viruses and production method, Thai Petty Patent
Meeran, Seenipandi, Javed, Sharma, Hashiesh et al., Can limonene be a possible candidate for evaluation as an agent or adjuvant against infection, immunity, and inflammation in COVID-19?, Heliyon, doi:10.1016/j.heliyon.2020.e05703
Miller, Hakim, Chew, Thompson, Thomson et al., Adipose tissue accumulation of d-limonene with the consumption of a lemonade preparation rich in d-limonene content, Nutr. Cancer, doi:10.1080/01635581003693066
Miranda, Damaceno, Faveri, Figueiredo, Soares et al., In vitro antimicrobial effect of cetylpyridinium chloride on complex multispecies subgingival biofilm, Braz. Dent. J, doi:10.1590/0103-6440202002630
Nagatake, Ahmed, Oishi, Prevention of respiratory infections by povidone-iodine gargle, Dermatology, doi:10.1159/000057722
Ni, Yang, Yang, Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19, Crit. Care, doi:10.1186/s13054-020-03120-0
Nicola, Alsafi, Sohrabi, Kerwan, Al-Jabir et al., The socio-economic implications of the coronavirus pandemic (COVID-19): a review, Int. J. Surg
Okamoto, Saito, Okabayashi, Komine, Virucidal activity and mechanism of action of cetylpyridinium chloride against SARS-CoV-2, J. Oral Maxillofac. Surg. Med. Pathol, doi:10.1016/j.ajoms.2022.04.001
Panatto, Orsi, Bruzzone, Efficacy of the Sentinox spray in reducing viral load in mild COVID-19 and its virucidal activity against other respiratory viruses: results of a randomized controlled trial and an in vitro study, Viruses, doi:10.3390/v14051033
Parikh-Das, Sharma, Du, Charles, Superiority of essential oils versus 0.075% CPCcontaining mouthrinse: a two-week randomized clinical trial, J. Clin. Dent
Peiris, Chu, Cheng, Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study, Lancet, doi:10.1016/S0140-6736(03)13412-5
Pelletier, Tessema, Frank, Westover, Brown et al., Efficacy of povidone-iodine nasal and oral antiseptic preparations against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), Ear Nose Throat J, doi:10.1177/0145561320957237
Perlman, Another decade, another coronavirus, N. Engl. J. Med, doi:10.1056/NEJMe2001126
Ponphaiboon, Krongrawa, Limmatvapirat, Tubtimsri, Jittmittraphap et al., In vitro development of local antiviral formulations with potent virucidal activity against SARS-CoV-2 and influenza viruses, Pharmaceutics, doi:10.3390/pharmaceutics17030349
Popkin, Zilka, Dimaano, Fujioka, Rackley et al., Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo, Pathog. Immun, doi:10.20411/pai.v2i2.200
Projan, Brown-Skrobot, Schlievert, Vandenesch, Novick, Glycerol monolaurate inhibits the production of β-lactamase, toxic shock syndrome toxin-1 and other staphylococcal exoproteins by interfering with signal transduction, J. Bacteriol, doi:10.1128/jb.176.14.4204-4209.1994
Razazi, Kakanezhadi, Raisi, Pedram, Dezfoulian et al., D-limonene inhibits peritoneal adhesion formation in rats via anti-inflammatory, anti-angiogenic, and antioxidative effects, doi:10.1007/s10787-023-01417-4
Rodríguez-Casanovas, Rosa, Bello-Lemus, Rasperini, Acosta-Hoyos, Virucidal activity of different mouthwashes using a novel biochemical assay, Healthcare, doi:10.3390/healthcare10010063
Rothan, Byrareddy, The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak, J. Autoimmun, doi:10.1016/j.jaut.2020.102433
Schlievert, Kilgore, Seo, Leung, Glycerol monolaurate contributes to the antimicrobial and anti-inflammatory activity of human milk, Sci. Rep, doi:10.1038/s41598-019-51130-y
Schulz, Altman, Moher, CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials, BMJ, doi:10.1136/bmj.c332
Steyer, Marušić, Kolenc, Triglav, A throat lozenge with fixed combination of cetylpyridinium chloride and benzydamine hydrochloride has direct virucidal effect on SARS-CoV-2, COVID, doi:10.3390/covid1020037
Subroto, Indiarto, Bioactive monolaurin as an antimicrobial and its potential to improve the immune system and against COVID-19: a review, Food Res, doi:10.26656/fr.2017
Subroto, Indiarto, Bioactive monolaurin as an antimicrobial and its potential to improve the immune system and against COVID-19: a review, Food Res, doi:10.26656/fr.2017.4(6).491
Subroto, Monoacylglycerols and diacylglycerols for fat-based food products: a review, Food Res, doi:10.26656/fr.2017.4(4).398
Sun, D-limonene: Safety and clinical applications, Altern. Med. Rev
Tarragó-Gil, Gil-Mosteo, Aza-Pascual-Salcedo, Randomized clinical trial to assess impact of oral intervention with cetylpyridinium chloride to reduce salivary SARS-CoV-2 viral load, J. Clin. Periodontol, doi:10.1111/jcpe.13746
Weerapol, Manmuan, Limmatvapirat, Limmatvapirat, Sirirak et al., Enhancing the efficacy of monolaurin against SARS-CoV-2 and influenza A (H1N1) with a nanoemulsion formulation, OpenNano, doi:10.1016/j.onano.2024.100207
Weerapol, Manmuan, Limmatvapirat, Limmatvapirat, Sirirak et al., Enhancing the efficacy of monolaurin against SARS-CoV-2 and influenza A (H1N1) with a nanoemulsion formulation, OpenNano, doi:10.1016/j.onano.2024.100207
Wölfel, Corman, Guggemos, Virological assessment of hospitalized patients with COVID-2019, Nature, doi:10.1038/s41586-020-2196-x
Yang, Chen, Li, D-limonene is a potential monoterpene to inhibit PI3K/Akt/IKK-α/NF-κB p65 signaling pathway in coronavirus disease 2019 pulmonary fibrosis, Front. Med, doi:10.3389/fmed.2021.591830
Zou, Ruan, Huang, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N. Engl. J. Med, doi:10.1056/NEJMc2001737
DOI record:
{
"DOI": "10.20944/preprints202509.1594.v1",
"URL": "http://dx.doi.org/10.20944/preprints202509.1594.v1",
"abstract": "<jats:p>Introduction: COVID-19 predominantly affects the respiratory tract, leading to symptoms such as fever, sore throat, cough, and nasal congestion. This study aimed to evaluate the efficacy and safety of nasal spray, mouth spray, and mouthwash containing limonene, cetylpyridinium chloride (CPC), and monolaurin in alleviating symptoms among patients with mild-to-moderate COVID-19.\nMethods: A double-blind, randomized, placebo-controlled trial was conducted at Dontum Hospital, Thailand, from May to November 2022. A total of 120 RT-PCR–confirmed COVID-19 patients were randomly assigned to receive either the active antiviral formulations or placebo products. Symptom severity was assessed on Days 1, 3, and 7 using a 7-point Likert scale. Patient satisfaction regarding symptom relief and product attributes (color, smell, taste) was evaluated on Day 7 using a 5-point Likert scale. The study was approved by the Ethics Committee of Silpakorn University (COE 65.0517-081) and registered with the Thai Clinical Trials Registry (TCTR20240803002).\nResults: Compared to the placebo group, the experimental group exhibited significantly faster symptom resolution, particularly for sore throat, cough with mucus, and nasal congestion, with notable improvements observed as early as Day 3 (p &lt; 0.05). By Day 7, a higher proportion of patients in the experimental group reported complete recovery (p &lt; 0.05). Additionally, patient satisfaction scores for symptom relief and product characteristics were significantly higher in the experimental group (p &lt; 0.001). No adverse events were reported in either group.\nConclusion: The nasal spray, mouth spray, and mouthwash formulations containing limonene, CPC, and monolaurin were effective and well-tolerated in managing mild-to-moderate COVID-19 symptoms. These findings suggest their potential as adjunctive therapies in outpatient settings. Further large-scale, multicenter studies are warranted to confirm these results and assess long-term clinical benefits.\nTrial Registration: Thai Clinical Trials Registry (TCTR20240803002).</jats:p>",
"accepted": {
"date-parts": [
[
2025,
9,
17
]
]
},
"author": [
{
"affiliation": [],
"family": "Ponphaiboon",
"given": "Juthaporn",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-3829-4062",
"affiliation": [],
"authenticated-orcid": false,
"family": "Limmatvapirat",
"given": "Sontaya",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0000-2254-4883",
"affiliation": [],
"authenticated-orcid": false,
"family": "Krongrawa",
"given": "Wantanwa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Auparigtatipong",
"given": "Witoon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ingsurarak",
"given": "Manachai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tubtimsri",
"given": "Sukannika",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5155-2727",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jittmittraphap",
"given": "Akanitt",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6215-8290",
"affiliation": [],
"authenticated-orcid": false,
"family": "Leaungwutiwong",
"given": "Pornsawan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahidol",
"given": "Chulabhorn",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5842-4330",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ruchirawat",
"given": "Somsak",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5210-3162",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kittakoop",
"given": "Prasat",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7378-7065",
"affiliation": [],
"authenticated-orcid": false,
"family": "Limmatvapirat",
"given": "Chutima",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
9,
22
]
],
"date-time": "2025-09-22T02:55:48Z",
"timestamp": 1758509748000
},
"deposited": {
"date-parts": [
[
2025,
9,
22
]
],
"date-time": "2025-09-22T02:55:59Z",
"timestamp": 1758509759000
},
"group-title": "Medicine and Pharmacology",
"indexed": {
"date-parts": [
[
2025,
9,
23
]
],
"date-time": "2025-09-23T00:13:36Z",
"timestamp": 1758586416470,
"version": "3.44.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
9,
19
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
9,
19
]
],
"date-time": "2025-09-19T00:00:00Z",
"timestamp": 1758240000000
}
}
],
"member": "1968",
"original-title": [],
"posted": {
"date-parts": [
[
2025,
9,
19
]
]
},
"prefix": "10.20944",
"published": {
"date-parts": [
[
2025,
9,
19
]
]
},
"publisher": "MDPI AG",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.preprints.org/manuscript/202509.1594/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Efficacy of Nasal Spray, Mouth Spray, and Mouthwash Containing Limonene, Cetylpyridinium Chloride, and Monolaurin in COVID-19 Management: A Double-Blind, Randomized, Placebo-Controlled Trial",
"type": "posted-content"
}
