In Vitro Development of Local Antiviral Formulations with Potent Virucidal Activity Against SARS-CoV-2 and Influenza Viruses
et al., Pharmaceutics, doi:10.3390/pharmaceutics17030349, Mar 2025
58th treatment shown to reduce risk in
September 2025, now with p = 0.0035 from 4 studies.
Lower risk for recovery and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
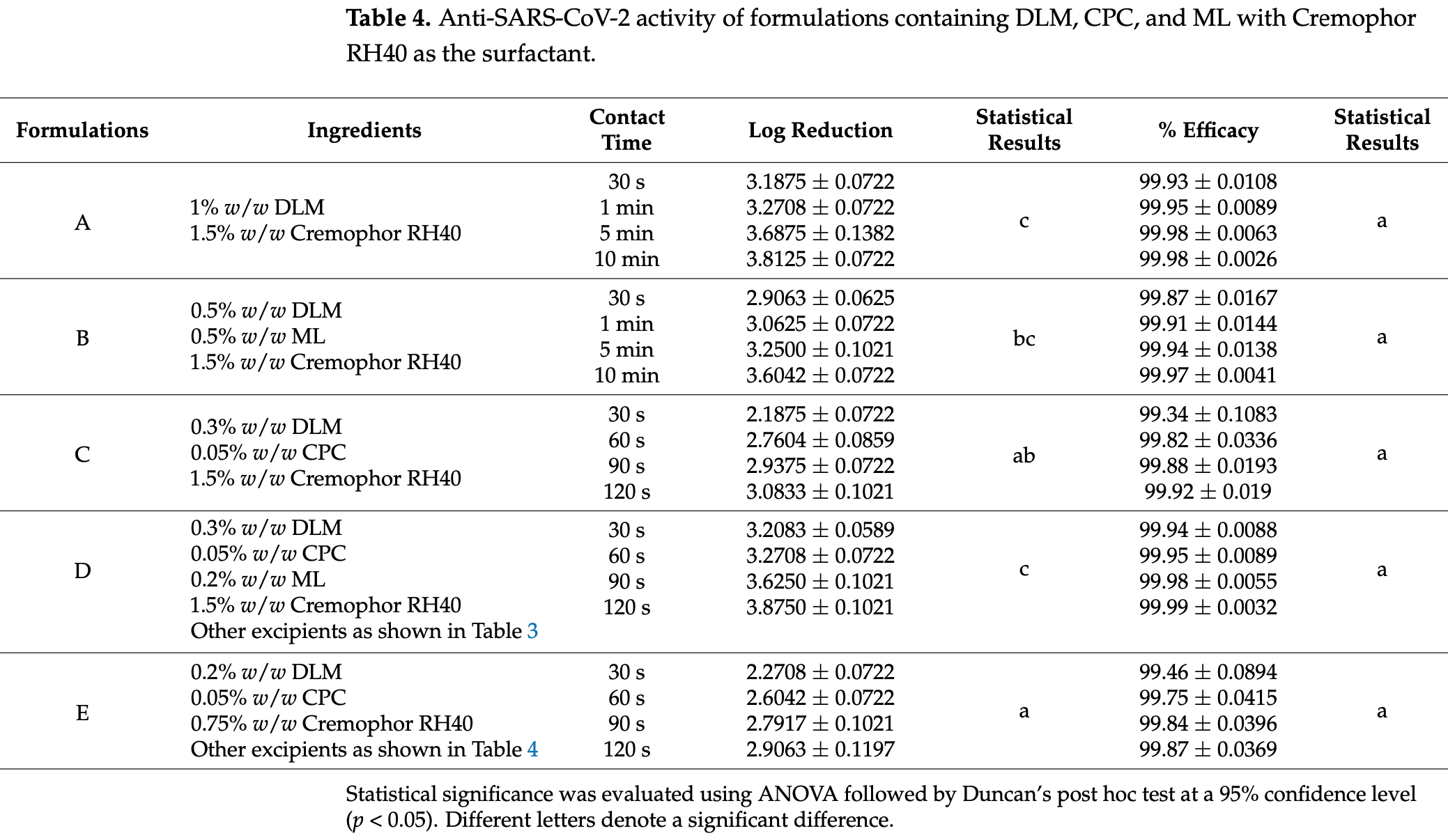
In vitro study showing potent virucidal activity of D-limonene (DLM), monolaurin (ML), and cetylpyridinium chloride (CPC) formulations against SARS-CoV-2 and influenza viruses. Authors developed two optimized formulations: an oral formulation D (0.3% DLM, 0.2% ML, 0.05% CPC, 1.5% Cremophor RH40) that achieved 99.99% efficacy with 3.9 log reduction against SARS-CoV-2 within 120 seconds, and a nasal formulation E (0.2% DLM, 0.05% CPC, 0.75% Cremophor RH40) that demonstrated 99.9% efficacy with 2.9 log reduction against SARS-CoV-2.
Ponphaiboon et al., 8 Mar 2025, Thailand, peer-reviewed, 10 authors.
Contact: limmatvapirat_c@su.ac.th (corresponding author), augusto_sc@hotmail.co.th, krongrawa_w@su.ac.th, limmatvapirat_s@su.ac.th, sukannika@go.buu.ac.th, akanitt.jit@mahidol.ac.th, pornsawan.lea@mahidol.ac.th, somsak@cri.or.th, prasat@cri.or.th.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
In Vitro Development of Local Antiviral Formulations with Potent Virucidal Activity Against SARS-CoV-2 and Influenza Viruses
Pharmaceutics, doi:10.3390/pharmaceutics17030349
Background/Object: This study investigates the in vitro antiviral potential of D-limonene (DLM), monolaurin (ML), and cetylpyridinium chloride (CPC) in formulations targeting SARS-CoV-2 and influenza viruses. The aim was to develop oral and nasal formulations with optimized concentrations of these active ingredients to evaluate their efficacy, safety, and stability. Methods: Oral (formulation D) and nasal (formulation E) products were developed using specific concentrations of DLM (0.2-0.3% w/w), ML (0.1-0.2% w/w), and CPC (0.05-0.075% w/w). In vitro virucidal activity assays were conducted to assess the antiviral efficacy of the formulations against SARS-CoV-2 and influenza viruses. Stability testing was also performed under various storage conditions. Results: Formulation D (0.3% w/w DLM, 0.2% w/w ML, 0.05% w/w CPC, and 1.5% w/w Cremophor RH40) demonstrated a 3.875 ± 0.1021 log reduction and 99.99 ± 0.0032% efficacy against SARS-CoV-2 within 120 s. Formulation E (0.2% w/w DLM, 0.05% w/w CPC, and 0.75% w/w Cremophor RH40) showed a 2.9063 ± 0.1197 log reduction and 99.87 ± 0.0369% efficacy against SARS-CoV-2. Both formulations achieved >99.99% efficacy and log reductions exceeding 4.000 against various influenza strains. Stability testing confirmed optimal performance at 4 • C with no microbial contamination. Conclusions: The findings suggest that both formulations exhibit broad-spectrum antiviral activity against SARS-CoV-2 and influenza viruses in vitro. These results support their potential for further clinical evaluations and therapeutic applications, particularly in oral and nasal spray formulations.
Supplementary Materials: The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics17030349/s1 , Figure S1 : Physical characteristics (turbid or clear) of formulations containing DLM and surfactant at different ratios, measured 1 day after preparation and after a temperature cycling test (6 cycles); Table S1 : Cytotoxicity and neutralization validation control data for oral formulation D in MDCK cells; Table S2 : Cytotoxicity and neutralization validation control data for nasal formulation E in MDCK cells; Table S3 : Test results for oral formulation D and nasal formulation E at a dilution of 1:32 against FluA(H1N1pdm) (A/Thailand/104/2009); Table S4 : Virus recovery data for FluA(H1N1pdm) (A/Thailand/104/2009); Table S5 : Virucidal activity of oral formulation D at a dilution of 1:32 against FluA(H1N1pdm) (A/Thailand/104/2009); Table S6 : Virucidal activity of nasal formulation E at a dilution of 1:32 against FluA(H1N1pdm) (A/Thailand/104/2009); Table S7 : Test results for oral formulation D and nasal formulation E at a dilution of 1:32 against FluA(H3N2) (ATCC VR-1881™); Table S8 : Virus recovery data for FluA(H3N2) (ATCC VR-1881™); Table S9 : Virucidal activity of oral formulation D at a dilution of 1:32 against FluA(H3N2) (ATCC VR-1881™); Table S10 : Virucidal activity of nasal formulation E at a dilution of 1:32 against FluA(H3N2) (ATCC VR-1881™); Table S11 : Test results for oral formulation D and nasal..
References
Astm E, Standard Practice to Assess Virucidal Activity of Chemicals Intended for Disinfection of Inanimate, Nonporous Environmental Surfaces. American Society for Testing and Materials
Bañó-Polo, Martínez-Gil, Sánchez Del Pino, Massoli, Mingarro et al., Cetylpyridinium chloride promotes disaggregation of SARS-CoV-2 virus-like particles, J. Oral Microbiol, doi:10.1080/20002297.2022.2030094
Belal, Shaalan, Haggag, Gradient HPLC-diode array detector stability-indicating determination of lidocaine hydrochloride and cetylpyridinium chloride in two combined oral gel dosage forms, J. AOAC Int, doi:10.1093/jaoac/94.2.503
Bernal, Uribe, Flores, Hernández, Gómez-Sandoval et al., Oral antiseptics against SARS-CoV-2: A literature review, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph19148768
Bidra, Pelletier, Westover, Frank, Brown et al., Comparison of in vitro inactivation of SARS-CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses, J. Prosthodont, doi:10.1111/jopr.13220
Camargo, Sarti, Alécio, Sabatini, Adorno et al., Limonene quantification by gas chromatography with mass spectrometry (GC-MS) and its effects on hydrogen and volatile fatty acids production in anaerobic reactors, Artigo Quím. Nova, doi:10.21577/0100-4042.20170557
Christiansen, Backensfeld, Weitschies, Effects of non-ionic surfactants on in vitro triglyceride digestion and their susceptibility to digestion by pancreatic enzymes, Eur. J. Pharm. Sci, doi:10.1016/j.ejps.2010.07.005
Coupland, Hayes, Physical approaches to masking bitter taste: Lessons from food and pharmaceuticals, Pharm. Res, doi:10.1007/s11095-014-1480-6
Fadilah, Jittmittraphap, Leaungwutiwong, Pripdeevech, Dhanushka et al., Virucidal activity of essential oils from Citrus × aurantium L. against influenza A virus H1N1: Limonene as a potential household disinfectant against virus, Nat. Prod. Commun, doi:10.1177/1934578X211072713
Gorjian, Mihankhah, Khaligh, Influence of tween nature and type on physicochemical properties and stability of spearmint essential oil (Mentha spicata L.) stabilized with basil seed mucilage nanoemulsion, J. Mol. Liq, doi:10.1016/j.molliq.2022.119379
Gupta, Jeyakumar, Lawrence, Journey of limonene as an antimicrobial agent, J. Pure Appl. Microbiol, doi:10.22207/JPAM.15.3.01
Horvát, Fehér, Wolburg, Sipos, Veszelka et al., Sodium hyaluronate as a mucoadhesive component in nasal formulation enhances delivery of molecules to brain tissue, Eur. J. Pharm. Biopharm, doi:10.1016/j.ejpb.2008.10.009
Kiss, Walter, Bocsik, Veszelka, Ózsvári et al., Kinetic analysis of the toxicity of pharmaceutical excipients Cremophor EL and RH40 on endothelial and epithelial cells, J. Pharm. Sci, doi:10.1002/jps.23458
Koch-Heier, Hoffmann, Schindler, Lussi, Planz, Inactivation of SARS-CoV-2 through treatment with the mouth rinsing solutions ViruProX ® and BacterX ® Pro, Microorganisms, doi:10.3390/microorganisms9030521
Lei, Yang, Hu, Sun, On the calculation of TCID 50 for quantitation of virus infectivity, Virol. Sin, doi:10.1007/s12250-020-00230-5
Lin, Li, Sun, Zhang, Wang et al., D-limonene: Promising and sustainable natural bioactive compound, Appl. Sci, doi:10.3390/app14114605
Maikhunthod, Chaipayang, Jittmittraphap, Thippornchai, Boonchuen et al., Exploring the therapeutic potential of Thai medicinal plants: In vitro screening and in silico docking of phytoconstituents for novel antiSARS-CoV-2 agents, BMC Complement. Altern. Med, doi:10.1186/s12906-024-04586-z
Moghadami, A Narrative review of influenza: A seasonal and pandemic disease, Iran J. Med. Sci
Ni, Yang, Yang, Bao, Li et al., Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19, Crit. Care, doi:10.1186/s13054-020-03120-0
Parikh-Das, Sharma, Du, Charles, Superiority of essential oils versus 0.075% CPC-containing mouthrinse: A two-week randomized clinical trial, J. Clin. Dent
Ponphaiboon, Limmatvapirat, Chaidedgumjorn, Limmatvapirat, Optimization and comparison of GC-FID and HPLC-ELSD methods for determination of lauric acid, mono-, di-, and trilaurins in modified coconut oil, J. Chromatogr. B, doi:10.1016/j.jchromb.2018.09.023
Ponphaiboon, Limmatvapirat, Limmatvapirat, Development and evaluation of a dry emulsion of ostrich oil as a dietary supplement, Foods, doi:10.3390/foods13162570
Popkin, Zilka, Dimaano, Fujioka, Rackley et al., Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo, Pathog. Immun, doi:10.20411/pai.v2i2.200
Reed, Muench, A simple method of estimating fifty percent endpoints, Am. J. Epidemiol, doi:10.1093/oxfordjournals.aje.a118408
Rius-Salvador, García-Múrria, Rusu, Bañó-Polo, León et al., Cetylpyridinium chloride and chlorhexidine show antiviral activity against influenza A virus and respiratory syncytial virus in vitro, PLoS ONE, doi:10.1371/journal.pone.0297291
Rodríguez-Casanovas, Rosa, Bello-Lemus, Rasperini, Acosta-Hoyos, Virucidal activity of different mouthwashes using a novel biochemical assay, Healthcare, doi:10.3390/healthcare10010063
Salade, Wauthoz, Goole, Amighi, How to characterize a nasal product. The state of the art of in vitro and ex vivo specific methods, Int. J. Pharm, doi:10.1016/j.ijpharm.2019.02.026
Subroto, Indiarto, Bioactive monolaurin as an antimicrobial and its potential to improve the immune system and against COVID-19: A review, Food Res, doi:10.26656/fr.2017.4(6).324
Subroto, Monoacylglycerols and diacylglycerols for fat-based food products: A review, Food Res, doi:10.26656/fr.2017.4(4).398
Tarragó-Gil, Gil-Mosteo, Aza-Pascual-Salcedo, Alvarez, Ainaga et al., Randomized clinical trial to assess the impact of oral intervention with cetylpyridinium chloride to reduce salivary SARS-CoV-2 viral load, J. Clin. Periodontol, doi:10.1111/jcpe.13746
Toschi, Mercado, Henz, Antiviral effect of oral antiseptic solutions commonly used in dentistry practice: A scoping review, Dent. Rev, doi:10.1016/j.dentre.2023.100064
Vázquez-Blanco, González-Freire, Dávila-Pousa, Crespo-Diz, pH determination as a quality standard for the elaboration of oral liquid compounding formula, Farm. Hosp, doi:10.7399/fh.10932
Weerapol, Manmuan, Limmatvapirat, Limmatvapirat, Sirirak et al., Enhancing the efficacy of monolaurin against SARS-CoV-2 and influenza A (H1N1) with a nanoemulsion formulation, OpenNano, doi:10.1016/j.onano.2024.100207
Zou, Ruan, Huang, Liang, Huang et al., SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N. Engl. J. Med, doi:10.1056/NEJMc2001737
DOI record:
{
"DOI": "10.3390/pharmaceutics17030349",
"ISSN": [
"1999-4923"
],
"URL": "http://dx.doi.org/10.3390/pharmaceutics17030349",
"abstract": "<jats:p>Background/Object: This study investigates the in vitro antiviral potential of D-limonene (DLM), monolaurin (ML), and cetylpyridinium chloride (CPC) in formulations targeting SARS-CoV-2 and influenza viruses. The aim was to develop oral and nasal formulations with optimized concentrations of these active ingredients to evaluate their efficacy, safety, and stability. Methods: Oral (formulation D) and nasal (formulation E) products were developed using specific concentrations of DLM (0.2–0.3% w/w), ML (0.1–0.2% w/w), and CPC (0.05–0.075% w/w). In vitro virucidal activity assays were conducted to assess the antiviral efficacy of the formulations against SARS-CoV-2 and influenza viruses. Stability testing was also performed under various storage conditions. Results: Formulation D (0.3% w/w DLM, 0.2% w/w ML, 0.05% w/w CPC, and 1.5% w/w Cremophor RH40) demonstrated a 3.875 ± 0.1021 log reduction and 99.99 ± 0.0032% efficacy against SARS-CoV-2 within 120 s. Formulation E (0.2% w/w DLM, 0.05% w/w CPC, and 0.75% w/w Cremophor RH40) showed a 2.9063 ± 0.1197 log reduction and 99.87 ± 0.0369% efficacy against SARS-CoV-2. Both formulations achieved >99.99% efficacy and log reductions exceeding 4.000 against various influenza strains. Stability testing confirmed optimal performance at 4 °C with no microbial contamination. Conclusions: The findings suggest that both formulations exhibit broad-spectrum antiviral activity against SARS-CoV-2 and influenza viruses in vitro. These results support their potential for further clinical evaluations and therapeutic applications, particularly in oral and nasal spray formulations.</jats:p>",
"alternative-id": [
"pharmaceutics17030349"
],
"author": [
{
"affiliation": [
{
"name": "Department of Industrial Pharmacy, Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand"
},
{
"name": "Natural Products Research Center (NPRC), Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand"
}
],
"family": "Ponphaiboon",
"given": "Juthaporn",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0009-0000-2254-4883",
"affiliation": [
{
"name": "Department of Industrial Pharmacy, Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand"
},
{
"name": "Natural Products Research Center (NPRC), Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand"
},
{
"name": "Pharmaceutical Intellectual Center “Prachote Plengwittaya”, Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand"
}
],
"authenticated-orcid": false,
"family": "Krongrawa",
"given": "Wantanwa",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3829-4062",
"affiliation": [
{
"name": "Department of Industrial Pharmacy, Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand"
},
{
"name": "Natural Products Research Center (NPRC), Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand"
}
],
"authenticated-orcid": false,
"family": "Limmatvapirat",
"given": "Sontaya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Burapha University, Chonburi 20131, Thailand"
}
],
"family": "Tubtimsri",
"given": "Sukannika",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5155-2727",
"affiliation": [
{
"name": "Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand"
}
],
"authenticated-orcid": false,
"family": "Jittmittraphap",
"given": "Akanitt",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6215-8290",
"affiliation": [
{
"name": "Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand"
}
],
"authenticated-orcid": false,
"family": "Leaungwutiwong",
"given": "Pornsawan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9776-5330",
"affiliation": [
{
"name": "Laboratory of Natural Products and Medicinal Chemistry, Chulabhorn Research Institute, Bangkok 10210, Thailand"
},
{
"name": "Program in Chemical Sciences, Chulabhorn Graduate Institute, Bangkok 10210, Thailand"
}
],
"authenticated-orcid": false,
"family": "Mahidol",
"given": "Chulabhorn",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5842-4330",
"affiliation": [
{
"name": "Laboratory of Natural Products and Medicinal Chemistry, Chulabhorn Research Institute, Bangkok 10210, Thailand"
},
{
"name": "Program in Chemical Sciences, Chulabhorn Graduate Institute, Bangkok 10210, Thailand"
},
{
"name": "Center of Excellence on Environmental Health and Toxicology (EHT), OPS, Ministry of Higher Education, Science, Research and Innovation, Bangkok 10400, Thailand"
}
],
"authenticated-orcid": false,
"family": "Ruchirawat",
"given": "Somsak",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5210-3162",
"affiliation": [
{
"name": "Laboratory of Natural Products and Medicinal Chemistry, Chulabhorn Research Institute, Bangkok 10210, Thailand"
},
{
"name": "Program in Chemical Sciences, Chulabhorn Graduate Institute, Bangkok 10210, Thailand"
},
{
"name": "Center of Excellence on Environmental Health and Toxicology (EHT), OPS, Ministry of Higher Education, Science, Research and Innovation, Bangkok 10400, Thailand"
}
],
"authenticated-orcid": false,
"family": "Kittakoop",
"given": "Prasat",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7378-7065",
"affiliation": [
{
"name": "Department of Industrial Pharmacy, Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand"
},
{
"name": "Natural Products Research Center (NPRC), Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand"
}
],
"authenticated-orcid": false,
"family": "Limmatvapirat",
"given": "Chutima",
"sequence": "additional"
}
],
"container-title": "Pharmaceutics",
"container-title-short": "Pharmaceutics",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
3,
10
]
],
"date-time": "2025-03-10T12:46:41Z",
"timestamp": 1741610801000
},
"deposited": {
"date-parts": [
[
2025,
3,
10
]
],
"date-time": "2025-03-10T12:56:05Z",
"timestamp": 1741611365000
},
"funder": [
{
"award": [
"SURDI Postdoctoral/67"
],
"name": "Silpakorn University under the Postdoctoral fellowship program"
}
],
"indexed": {
"date-parts": [
[
2025,
3,
11
]
],
"date-time": "2025-03-11T04:12:06Z",
"timestamp": 1741666326885,
"version": "3.38.0"
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2025,
3,
8
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2025,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
8
]
],
"date-time": "2025-03-08T00:00:00Z",
"timestamp": 1741392000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4923/17/3/349/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "349",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2025,
3,
8
]
]
},
"published-online": {
"date-parts": [
[
2025,
3,
8
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1186/s13054-020-03120-0",
"article-title": "Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19",
"author": "Ni",
"doi-asserted-by": "crossref",
"first-page": "422",
"journal-title": "Crit. Care",
"key": "ref_1",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2001737",
"article-title": "SARS-CoV-2 viral load in upper respiratory specimens of infected patients",
"author": "Zou",
"doi-asserted-by": "crossref",
"first-page": "1177",
"journal-title": "N. Engl. J. Med.",
"key": "ref_2",
"volume": "382",
"year": "2020"
},
{
"article-title": "A Narrative review of influenza: A seasonal and pandemic disease",
"author": "Moghadami",
"first-page": "2",
"journal-title": "Iran J. Med. Sci.",
"key": "ref_3",
"volume": "42",
"year": "2017"
},
{
"article-title": "Virucidal activity of essential oils from Citrus × aurantium L. against influenza A virus H1N1: Limonene as a potential household disinfectant against virus",
"author": "Fadilah",
"first-page": "1934578X211072713",
"journal-title": "Nat. Prod. Commun.",
"key": "ref_4",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.3390/app14114605",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Lin, H., Li, Z., Sun, Y., Zhang, Y., Wang, S., Zhang, Q., Cai, T., Xiang, W., Zeng, C., and Tang, J. (2024). D-limonene: Promising and sustainable natural bioactive compound. Appl. Sci., 14."
},
{
"DOI": "10.22207/JPAM.15.3.01",
"article-title": "Journey of limonene as an antimicrobial agent",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1094",
"journal-title": "J. Pure Appl. Microbiol.",
"key": "ref_6",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1016/j.onano.2024.100207",
"article-title": "Enhancing the efficacy of monolaurin against SARS-CoV-2 and influenza A (H1N1) with a nanoemulsion formulation",
"author": "Weerapol",
"doi-asserted-by": "crossref",
"first-page": "100207",
"journal-title": "OpenNano",
"key": "ref_7",
"volume": "17",
"year": "2024"
},
{
"DOI": "10.26656/fr.2017.4(6).324",
"article-title": "Bioactive monolaurin as an antimicrobial and its potential to improve the immune system and against COVID-19: A review",
"author": "Subroto",
"doi-asserted-by": "crossref",
"first-page": "2355",
"journal-title": "Food Res.",
"key": "ref_8",
"volume": "4",
"year": "2020"
},
{
"DOI": "10.26656/fr.2017.4(4).398",
"article-title": "Monoacylglycerols and diacylglycerols for fat-based food products: A review",
"author": "Subroto",
"doi-asserted-by": "crossref",
"first-page": "932",
"journal-title": "Food Res.",
"key": "ref_9",
"volume": "4",
"year": "2020"
},
{
"DOI": "10.1111/jcpe.13746",
"article-title": "Randomized clinical trial to assess the impact of oral intervention with cetylpyridinium chloride to reduce salivary SARS-CoV-2 viral load",
"author": "Alvarez",
"doi-asserted-by": "crossref",
"first-page": "288",
"journal-title": "J. Clin. Periodontol.",
"key": "ref_10",
"volume": "50",
"year": "2023"
},
{
"DOI": "10.20411/pai.v2i2.200",
"article-title": "Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo",
"author": "Popkin",
"doi-asserted-by": "crossref",
"first-page": "253",
"journal-title": "Pathog. Immun.",
"key": "ref_11",
"volume": "2",
"year": "2017"
},
{
"DOI": "10.1080/20002297.2022.2030094",
"doi-asserted-by": "crossref",
"key": "ref_12",
"unstructured": "Bañó-Polo, M., Martínez-Gil, L., Sánchez del Pino, M.M., Massoli, A., Mingarro, I., Léon, R., and Garcia-Murria, M.J. (2022). Cetylpyridinium chloride promotes disaggregation of SARS-CoV-2 virus-like particles. J. Oral Microbiol., 14."
},
{
"DOI": "10.1111/jopr.13220",
"article-title": "Comparison of in vitro inactivation of SARS-CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses",
"author": "Bidra",
"doi-asserted-by": "crossref",
"first-page": "599",
"journal-title": "J. Prosthodont.",
"key": "ref_13",
"volume": "29",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0297291",
"doi-asserted-by": "crossref",
"key": "ref_14",
"unstructured": "Rius-Salvador, M., García-Múrria, M.J., Rusu, L., Bañó-Polo, M., León, R., Geller, R., Mingarro, I., and Martinez-Gil, L. (2024). Cetylpyridinium chloride and chlorhexidine show antiviral activity against influenza A virus and respiratory syncytial virus in vitro. PLoS ONE, 19."
},
{
"DOI": "10.3390/microorganisms9030521",
"doi-asserted-by": "crossref",
"key": "ref_15",
"unstructured": "Koch-Heier, J., Hoffmann, H., Schindler, M., Lussi, A., and Planz, O. (2021). Inactivation of SARS-CoV-2 through treatment with the mouth rinsing solutions ViruProX® and BacterX® Pro. Microorganisms, 9."
},
{
"DOI": "10.3390/healthcare10010063",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Rodríguez-Casanovas, H.J., la Rosa, M.D., Bello-Lemus, Y., Rasperini, G., and Acosta-Hoyos, A.J. (2021). Virucidal activity of different mouthwashes using a novel biochemical assay. Healthcare, 10."
},
{
"DOI": "10.3390/ijerph19148768",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Bernal, C.G.G., Uribe, E.R., Flores, J.S., Hernández, J.J.V., Gómez-Sandoval, J.R., Salazar, S.Y.M., Maldonado, A.F.G., Aguilar Martínez, J.A., and Martínez, S.M.L. (2022). Oral antiseptics against SARS-CoV-2: A literature review. Int. J. Environ. Res. Public Health, 19."
},
{
"DOI": "10.1016/j.dentre.2023.100064",
"article-title": "Antiviral effect of oral antiseptic solutions commonly used in dentistry practice: A scoping review",
"author": "Toschi",
"doi-asserted-by": "crossref",
"first-page": "100064",
"journal-title": "Dent. Rev.",
"key": "ref_18",
"volume": "3",
"year": "2023"
},
{
"article-title": "Superiority of essential oils versus 0.075% CPC-containing mouthrinse: A two-week randomized clinical trial",
"author": "Sharma",
"first-page": "94",
"journal-title": "J. Clin. Dent.",
"key": "ref_19",
"volume": "24",
"year": "2013"
},
{
"article-title": "Limonene quantification by gas chromatography with mass spectrometry (GC-MS) and its effects on hydrogen and volatile fatty acids production in anaerobic reactors",
"author": "Camargo",
"first-page": "844",
"journal-title": "Artigo Quím. Nova",
"key": "ref_20",
"volume": "43",
"year": "2020"
},
{
"DOI": "10.1016/j.jchromb.2018.09.023",
"article-title": "Optimization and comparison of GC-FID and HPLC-ELSD methods for determination of lauric acid, mono-, di-, and trilaurins in modified coconut oil",
"author": "Ponphaiboon",
"doi-asserted-by": "crossref",
"first-page": "110",
"journal-title": "J. Chromatogr. B",
"key": "ref_21",
"volume": "1099",
"year": "2018"
},
{
"DOI": "10.1093/jaoac/94.2.503",
"article-title": "Gradient HPLC-diode array detector stability-indicating determination of lidocaine hydrochloride and cetylpyridinium chloride in two combined oral gel dosage forms",
"author": "Belal",
"doi-asserted-by": "crossref",
"first-page": "503",
"journal-title": "J. AOAC Int.",
"key": "ref_22",
"volume": "94",
"year": "2011"
},
{
"DOI": "10.2139/ssrn.4781672",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Ponphaiboon, J., Limmatvapirat, S., and Limmatvapirat, C. (2024). Development and evaluation of a dry emulsion of ostrich oil as a dietary supplement. Foods, 13."
},
{
"key": "ref_24",
"unstructured": "The United States Pharmacopeial Convention (2020). <61> Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests, in the United States Pharmacopeia 43 and the National Formulary 38, The United States Pharmacopeial Convention."
},
{
"DOI": "10.1186/s12906-024-04586-z",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Maikhunthod, B., Chaipayang, S., Jittmittraphap, A., Thippornchai, N., Boonchuen, P., Tittabutr, P., Eumkeb, G., Sabuakham, S., Rungrotmongkol, T., and Mahalapbutr, P. (2024). Exploring the therapeutic potential of Thai medicinal plants: In vitro screening and in silico docking of phytoconstituents for novel antiSARS-CoV-2 agents. BMC Complement. Altern. Med., 24."
},
{
"key": "ref_26",
"unstructured": "(2020). Standard Practice to Assess Virucidal Activity of Chemicals Intended for Disinfection of Inanimate, Nonporous Environmental Surfaces (Standard No. ASTM E1053–20). Available online: https://www.astm.org/Standards/E1053.htm."
},
{
"DOI": "10.1093/oxfordjournals.aje.a118408",
"article-title": "A simple method of estimating fifty percent endpoints",
"author": "Reed",
"doi-asserted-by": "crossref",
"first-page": "493",
"journal-title": "Am. J. Epidemiol.",
"key": "ref_27",
"volume": "27",
"year": "1938"
},
{
"DOI": "10.1007/s12250-020-00230-5",
"article-title": "On the calculation of TCID50 for quantitation of virus infectivity",
"author": "Lei",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Virol. Sin.",
"key": "ref_28",
"volume": "36",
"year": "2021"
},
{
"article-title": "pH determination as a quality standard for the elaboration of oral liquid compounding formula",
"first-page": "221",
"journal-title": "Farm. Hosp.",
"key": "ref_29",
"volume": "42",
"year": "2018"
},
{
"DOI": "10.1016/j.ijpharm.2019.02.026",
"article-title": "How to characterize a nasal product. The state of the art of in vitro and ex vivo specific methods",
"author": "Salade",
"doi-asserted-by": "crossref",
"first-page": "47",
"journal-title": "Int. J. Pharm.",
"key": "ref_30",
"volume": "561",
"year": "2019"
},
{
"DOI": "10.1016/j.molliq.2022.119379",
"article-title": "Influence of tween nature and type on physicochemical properties and stability of spearmint essential oil (Mentha spicata L.) stabilized with basil seed mucilage nanoemulsion",
"author": "Gorjian",
"doi-asserted-by": "crossref",
"first-page": "119379",
"journal-title": "J. Mol. Liq.",
"key": "ref_31",
"volume": "359",
"year": "2022"
},
{
"DOI": "10.1007/s11095-014-1480-6",
"article-title": "Physical approaches to masking bitter taste: Lessons from food and pharmaceuticals",
"author": "Coupland",
"doi-asserted-by": "crossref",
"first-page": "2921",
"journal-title": "Pharm. Res.",
"key": "ref_32",
"volume": "31",
"year": "2014"
},
{
"DOI": "10.1016/j.ejps.2010.07.005",
"article-title": "Effects of non-ionic surfactants on in vitro triglyceride digestion and their susceptibility to digestion by pancreatic enzymes",
"author": "Christiansen",
"doi-asserted-by": "crossref",
"first-page": "376",
"journal-title": "Eur. J. Pharm. Sci.",
"key": "ref_33",
"volume": "41",
"year": "2010"
},
{
"DOI": "10.1002/jps.23458",
"article-title": "Kinetic analysis of the toxicity of pharmaceutical excipients Cremophor EL and RH40 on endothelial and epithelial cells",
"author": "Kiss",
"doi-asserted-by": "crossref",
"first-page": "1173",
"journal-title": "J. Pharm. Sci.",
"key": "ref_34",
"volume": "102",
"year": "2013"
},
{
"DOI": "10.1016/j.ejpb.2008.10.009",
"article-title": "Sodium hyaluronate as a mucoadhesive component in nasal formulation enhances delivery of molecules to brain tissue",
"author": "Wolburg",
"doi-asserted-by": "crossref",
"first-page": "252",
"journal-title": "Eur. J. Pharm. Biopharm.",
"key": "ref_35",
"volume": "72",
"year": "2009"
},
{
"key": "ref_36",
"unstructured": "The United States Pharmacopeial Convention (2020). <232> Elemental Impurities—Limits, in the United States Pharmacopeia 43 and the National Formulary 38, The United States Pharmacopeial Convention."
},
{
"key": "ref_37",
"unstructured": "The United States Pharmacopeial Convention (2020). <1111> Microbiological Examination of Nonsterile Products: Acceptance Criteria for Pharmaceutical Preparations and Substances for Pharmaceutical Use, in the United States Pharmacopeia 43 and the National Formulary 38, The United States Pharmacopeial Convention."
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4923/17/3/349"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "In Vitro Development of Local Antiviral Formulations with Potent Virucidal Activity Against SARS-CoV-2 and Influenza Viruses",
"type": "journal-article",
"volume": "17"
}
