Randomized clinical trial to assess the impact of oral intervention with cetylpyridinium chloride to reduce salivary SARS‐CoV‐2 viral load
et al., Journal of Clinical Periodontology, doi:10.1111/jcpe.13746, NCT04820803, Nov 2022
58th treatment shown to reduce risk in
September 2025, now with p = 0.0035 from 4 studies.
Lower risk for recovery and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
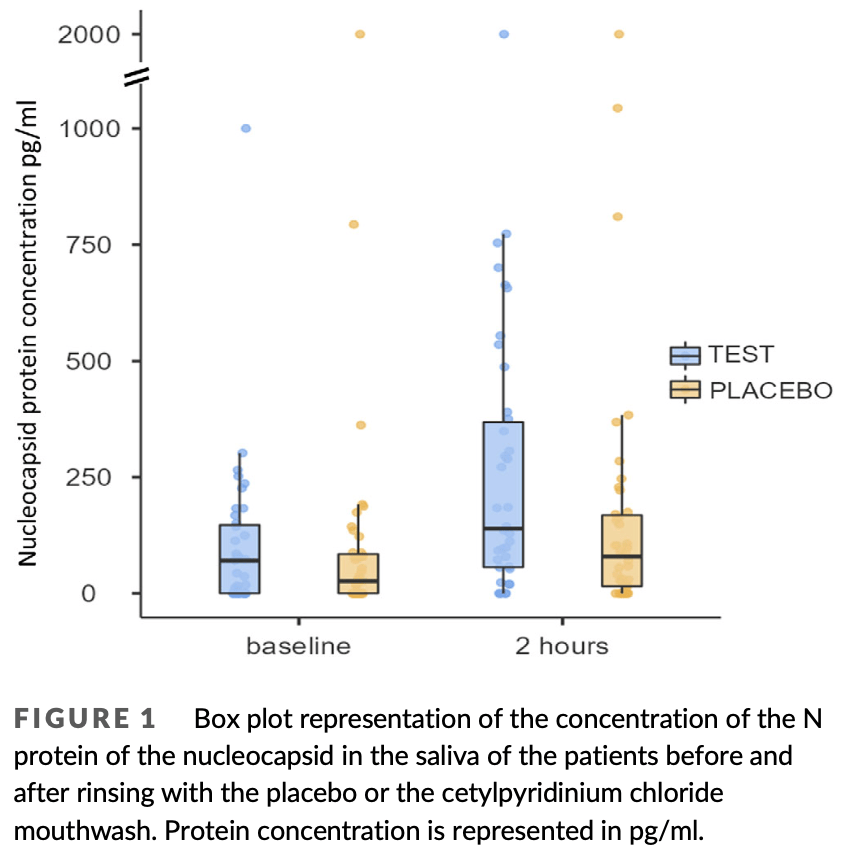
80 patient RCT testing cetylpyridinium chloride (CPC) mouthwash, showing significantly increased detection of SARS-CoV-2 nucleocapsid protein, indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane, exposing the nucleocapsid of the virus. Notably, there was no significant difference in viral load detected with PCR, highlighting the limitations of PCR, which is unable to differentiate between intact infectious virus and non-infectious or destroyed virus particles. PCR viral load may not correlate well with actual remaining infectivity after treatments like mouthwashes.
Tarragó-Gil et al., 28 Nov 2022, placebo-controlled, Spain, peer-reviewed, mean age 48.6, 19 authors, trial NCT04820803 (history).
Contact: rmtarrago@salud.aragon.es, dserrano@salud.aragon.es.
Randomized clinical trial to assess the impact of oral intervention with cetylpyridinium chloride to reduce salivary SARS‐CoV‐2 viral load
Journal of Clinical Periodontology, doi:10.1111/jcpe.13746
Aim: Aerosols released from the oral cavity help spread the SARS-CoV-2 virus. The use of a mouthwash formulated with an antiviral agent could reduce the viral load in saliva, helping to lower the spread of the virus. The aim of this study was to assess the efficacy of a mouthwash with 0.07% cetylpyridinium chloride (CPC) to reduce the viral load in the saliva of Coronavirus disease 2019 (COVID-19) patients.
Materials and Methods: In this multi-centre, single-blind, randomized, parallel group clinical trial, 80 COVID-19 patients were enrolled and randomized to two groups, namely test (n = 40) and placebo (n = 40). Saliva samples were collected at baseline and 2 h after rinsing. The samples were analysed by reverse transcriptionquantitative polymerase chain reaction (RT-qPCR) and an enzyme-linked immunosorbent assay test specific for the nucleocapsid (N) protein of SARS-CoV-2. Results: With RT-qPCR, no significant differences were observed between the placebo group and the test group. However, 2 h after a single rinse, N protein concentration in saliva was significantly higher in the test group, indicating an increase in lysed virus.
Conclusions: The use of 0.07% CPC mouthwash induced a significant increase in N protein detection in the saliva of COVID-19 patients. Lysis of the virus in the mouth could help reduce the transmission of SARS-CoV-2. However, more studies are required to prove this.
References
Bañ O-Polo, Martínez-Gil, Sánchez Del Pino, Massoli, Mingarro et al., Cetylpyridinium chloride promotes disaggregation of SARS-CoV-2 virus-like particles, Journal of Oral Microbiology, doi:10.1080/20002297.2022.2030094
Bian, Gao, Zhang, He, Mao et al., Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies, Expert Review of Vaccines, doi:10.1080/14760584.2021.1903879
Burton, Clarkson, Goulao, Glenny, Mcbain et al., Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them, The Cochrane Database of Systematic Reviews, doi:10.1002/14651858.CD013627.pub2
Cohen, Statistical power analysis for the behavioral sciences
Coleman, Tay, Sen Tan, Ong, Son et al., Viral load of SARS-CoV-2 in respiratory aerosols emitted by COVID-19 patients while breathing, talking, and singing, Clinical Infectious Diseases, doi:10.1093/cid/ciab691
Deana, Seiffert, Aravena-Rivas, Alonso-Coello, Muñoz-Millán et al., Recommendations for safe dental care: A systematic review of clinical practice guidelines in the first year of the COVID-19 pandemic, International Journal of Environmental Research and Public Health, doi:10.3390/ijerph181910059
Ellinger, Bojkova, Zaliani, Cinatl, Claussen et al., A SARS-CoV-2 cytopathicity dataset generated by high-content screening of a large drug repurposing collection, Scientific data, doi:10.1038/s41597-021-00848-4
Ferrer, Barrueco, Martinez-Beneyto, Mateos-Moreno, Ausina-Márquez et al., Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2, Scientific reports, doi:10.1038/s41598-021-03461-y
Gottsauner, Michaelides, Schmidt, Scholz, Buchalla et al., A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2, Clinical Oral Investigations, doi:10.1007/s00784-020-03549-1
Hartenian, Nandakumar, Lari, Ly, Tucker et al., The molecular virology of coronaviruses, The Journal of Biological Chemistry, doi:10.1074/jbc.REV120.013930
Herrera, Serrano, Roldán, Sanz, Is the oral cavity relevant in SARS-CoV-2 pandemic?, Clinical Oral Investigations, doi:10.1007/s00784-020-03413-2
Huang, Pérez, Kato, Mikami, Okuda et al., SARS-CoV-2 infection of the oral cavity and saliva, Nature Medicine, doi:10.1038/s41591-021-01296-8
Ikegami, Benirschke, Flanagan, Tanna, Klein et al., Persistence of SARS-CoV-2 nasopharyngeal swab PCR positivity in COVID-19 convalescent plasma donors, Transfusion, doi:10.1111/trf.16015
Komine, Yamaguchi, Okamoto, Yamamoto, Virucidal activity of oral care products against SARS-CoV-2 in vitro, Journal of Oral and Maxillofacial Surgery, doi:10.1016/j.ajoms.2021.02.002
Madas, Füri, Farkas, Nagy, Czitrovszky et al., Deposition distribution of the new coronavirus (SARS-CoV-2) in the human airways upon exposure to cough-generated droplets and aerosol particles, Scientific Reports, doi:10.1038/s41598-020-79985-6
Meyers, Robison, Milici, Alam, Quillen et al., Lowering the transmission and spread of human coronavirus, Journal of Medical Virology, doi:10.1002/jmv.26514
Michalakis, Sofonea, Alizon, Bravo, SARS-CoV-2 viral RNA levels are not 'viral load', Trends in Microbiology, doi:10.1016/j.tim.2021.08.008
Muñoz-Basagoiti, Perez-Zsolt, Le On, Blanc, Raïch-Regué et al., Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in vitro, Journal of Dental Research, doi:10.1177/00220345211029269
O'donnell, Thomas, Stanton, Maillard, Murphy et al., Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection, Function, doi:10.1093/function/zqaa002
Popkin, Zilka, Dimaano, Fujioka, Rackley et al., Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo, Pathogens & immunity, doi:10.20411/pai.v2i2.200
Prada-L Opez, Quintas, Casares-De-Cal, Suárez-Quintanilla, Suárez-Quintanilla et al., Ex vivo vs. in vivo antibacterial activity of two antiseptics on oral biofilm, Frontiers in Microbiology, doi:10.3389/fmicb.2015.00655
Satarker, Nampoothiri, Structural proteins in severe acute respiratory syndrome Coronavirus-2. Archives of Medical Research, doi:10.1016/j.arcmed.2020.05.012
Shah, Kane, Elsheikh, Alfaro, Development of a rapid viability RT-PCR (RV-RT-PCR) method to detect infectious SARS-CoV-2 from swabs, Journal of Virological Methods, doi:10.1016/j.jviromet.2021.114251
Shen, Niu, Wang, Huang, Wang et al., High-throughput screening and identification of potent broad-Spectrum inhibitors of coronaviruses, Journal of Virology, doi:10.1128/JVI.00023-19
Tartof, Slezak, Fischer, Hong, Ackerson et al., Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study, Lancet, doi:10.1016/S0140-6736(21)02183-8
Wang, Prather, Sznitman, Jimenez, Lakdawala et al., Airborne transmission of respiratory viruses, Science, doi:10.1126/science.abd9149
Wyllie, Fournier, Casanovas-Massana, Campbell, Tokuyama et al., Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2, The New England Journal of Medicine, doi:10.1056/NEJMc2016359
Wölfel, Corman, Guggemos, Seilmaier, Zange et al., Virological assessment of hospitalized patients with COVID-2019, Nature, doi:10.1038/s41586-020-2196-x
Xu, Li, Zhu, Liang, Fang et al., Randomized clinical trial to assess the impact of oral intervention with cetylpyridinium chloride to reduce salivary SARS-CoV-2 viral load
Xu, Zhong, Deng, Peng, Dan et al., High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa, International Journal of Oral Science, doi:10.1038/s41368-020-0074-x
DOI record:
{
"DOI": "10.1111/jcpe.13746",
"ISSN": [
"0303-6979",
"1600-051X"
],
"URL": "http://dx.doi.org/10.1111/jcpe.13746",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Aim</jats:title><jats:p>Aerosols released from the oral cavity help spread the SARS‐CoV‐2 virus. The use of a mouthwash formulated with an antiviral agent could reduce the viral load in saliva, helping to lower the spread of the virus. The aim of this study was to assess the efficacy of a mouthwash with 0.07% cetylpyridinium chloride (CPC) to reduce the viral load in the saliva of Coronavirus disease 2019 (COVID‐19) patients.</jats:p></jats:sec><jats:sec><jats:title>Materials and Methods</jats:title><jats:p>In this multi‐centre, single‐blind, randomized, parallel group clinical trial, 80 COVID‐19 patients were enrolled and randomized to two groups, namely test (<jats:italic>n</jats:italic> = 40) and placebo (<jats:italic>n</jats:italic> = 40). Saliva samples were collected at baseline and 2 h after rinsing. The samples were analysed by reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR) and an enzyme‐linked immunosorbent assay test specific for the nucleocapsid (N) protein of SARS‐CoV‐2.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>With RT‐qPCR, no significant differences were observed between the placebo group and the test group. However, 2 h after a single rinse, N protein concentration in saliva was significantly higher in the test group, indicating an increase in lysed virus.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>The use of 0.07% CPC mouthwash induced a significant increase in N protein detection in the saliva of COVID‐19 patients. Lysis of the virus in the mouth could help reduce the transmission of SARS‐CoV‐2. However, more studies are required to prove this.</jats:p></jats:sec>",
"alternative-id": [
"10.1111/jcpe.13746"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-04-03"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-11-03"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-11-28"
}
],
"author": [
{
"affiliation": [
{
"name": "Seminario Primary Care Health Centre Aragon Health Service Zaragoza Spain"
},
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
}
],
"family": "Tarragó‐Gil",
"given": "Rosa",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Seminario Primary Care Health Centre Aragon Health Service Zaragoza Spain"
},
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
}
],
"family": "Gil‐Mosteo",
"given": "María José",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
},
{
"name": "Zaragoza III Primary Care Health Direction Aragon Health Service Zaragoza Spain"
}
],
"family": "Aza‐Pascual‐Salcedo",
"given": "Mercedes",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
},
{
"name": "Zaragoza III Primary Care Health Direction Aragon Health Service Zaragoza Spain"
}
],
"family": "Alvarez",
"given": "María Jesús Lallana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
},
{
"name": "San José Primary Care Health Centre Aragon Health Service Zaragoza Spain"
}
],
"family": "Ainaga",
"given": "Raquel Refusta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Seminario Primary Care Health Centre Aragon Health Service Zaragoza Spain"
},
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
}
],
"family": "Gimeno",
"given": "Natalia Lázaro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
},
{
"name": "Sagasta Primary Care Health Centre Aragon Health Service Zaragoza Spain"
}
],
"family": "Viñuales",
"given": "Roberto Fuentes",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Seminario Primary Care Health Centre Aragon Health Service Zaragoza Spain"
},
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
}
],
"family": "Fernández",
"given": "Yolanda Millán",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
},
{
"name": "Lozano Blesa University Clinic Hospital Zaragoza Spain"
}
],
"family": "Marco",
"given": "Jesica Montero",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
},
{
"name": "Lozano Blesa University Clinic Hospital Zaragoza Spain"
}
],
"family": "Bolsa",
"given": "Elena Altarribas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
},
{
"name": "Lozano Blesa University Clinic Hospital Zaragoza Spain"
}
],
"family": "Sancho",
"given": "Jessica Bueno",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
},
{
"name": "Lozano Blesa University Clinic Hospital Zaragoza Spain"
}
],
"family": "Cajo",
"given": "Sonia Algarate",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IrsiCaixa AIDS Research Institute Badalona Spain"
}
],
"family": "Perez‐Zsolt",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IrsiCaixa AIDS Research Institute Badalona Spain"
}
],
"family": "Raïch‐Regué",
"given": "Dàlia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IrsiCaixa AIDS Research Institute Badalona Spain"
}
],
"family": "Muñoz‐Basagoiti",
"given": "Jordana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IrsiCaixa AIDS Research Institute Badalona Spain"
},
{
"name": "Germans Trias i Pujol Research Institute (IGTP) Badalona Spain"
}
],
"family": "Izquierdo‐Useros",
"given": "Nuria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dentaid Research Center Cerdanyola del Vallès Spain"
}
],
"family": "Pociello",
"given": "Vanessa Blanc",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dentaid Research Center Cerdanyola del Vallès Spain"
}
],
"family": "León",
"given": "Rubén",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Health Research of Aragón (IIS Aragón) Zaragoza Spain"
},
{
"name": "Zaragoza III Primary Care Health Direction Aragon Health Service Zaragoza Spain"
}
],
"family": "Peris",
"given": "Diana Serrano",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Periodontology",
"container-title-short": "J Clinic Periodontology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
11,
8
]
],
"date-time": "2022-11-08T09:53:34Z",
"timestamp": 1667901214000
},
"deposited": {
"date-parts": [
[
2023,
8,
18
]
],
"date-time": "2023-08-18T18:05:03Z",
"timestamp": 1692381903000
},
"indexed": {
"date-parts": [
[
2023,
8,
19
]
],
"date-time": "2023-08-19T04:35:26Z",
"timestamp": 1692419726607
},
"is-referenced-by-count": 3,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
11,
28
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2023,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
28
]
],
"date-time": "2022-11-28T00:00:00Z",
"timestamp": 1669593600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jcpe.13746",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/jcpe.13746",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jcpe.13746",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "288-294",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2022,
11,
28
]
]
},
"published-online": {
"date-parts": [
[
2022,
11,
28
]
]
},
"published-print": {
"date-parts": [
[
2023,
3
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1080/20002297.2022.2030094",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1080/14760584.2021.1903879",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"DOI": "10.1002/14651858.CD013627.pub2",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"author": "Cohen J.",
"key": "e_1_2_10_5_1",
"volume-title": "Statistical power analysis for the behavioral sciences",
"year": "1988"
},
{
"DOI": "10.1093/cid/ciab691",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.3390/ijerph181910059",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.1038/s41597-021-00848-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1038/s41598-021-03461-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1007/s00784-020-03549-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1074/jbc.REV120.013930",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1007/s00784-020-03413-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1038/s41591-021-01296-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.1111/trf.16015",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.1016/j.ajoms.2021.02.002",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1038/s41598-020-79985-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1002/jmv.26514",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1016/j.tim.2021.08.008",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1177/00220345211029269",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1093/function/zqaa002",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"DOI": "10.20411/pai.v2i2.200",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.3389/fmicb.2015.00655",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"DOI": "10.1016/j.arcmed.2020.05.012",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_23_1"
},
{
"DOI": "10.1016/j.jviromet.2021.114251",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_24_1"
},
{
"DOI": "10.1128/JVI.00023-19",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_25_1"
},
{
"DOI": "10.1016/S0140-6736(21)02183-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_1"
},
{
"DOI": "10.1126/science.abd9149",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_1"
},
{
"DOI": "10.1056/NEJMc2016359",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_1"
},
{
"DOI": "10.1038/s41368-020-0074-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_30_1"
},
{
"DOI": "10.1038/s41591-020-0817-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_31_1"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/jcpe.13746"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Periodontics"
],
"subtitle": [],
"title": "Randomized clinical trial to assess the impact of oral intervention with cetylpyridinium chloride to reduce salivary <scp>SARS‐CoV‐2</scp> viral load",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "50"
}
