Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2
et al., Scientific Reports, doi:10.1038/s41598-021-03461-y, Dec 2021
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
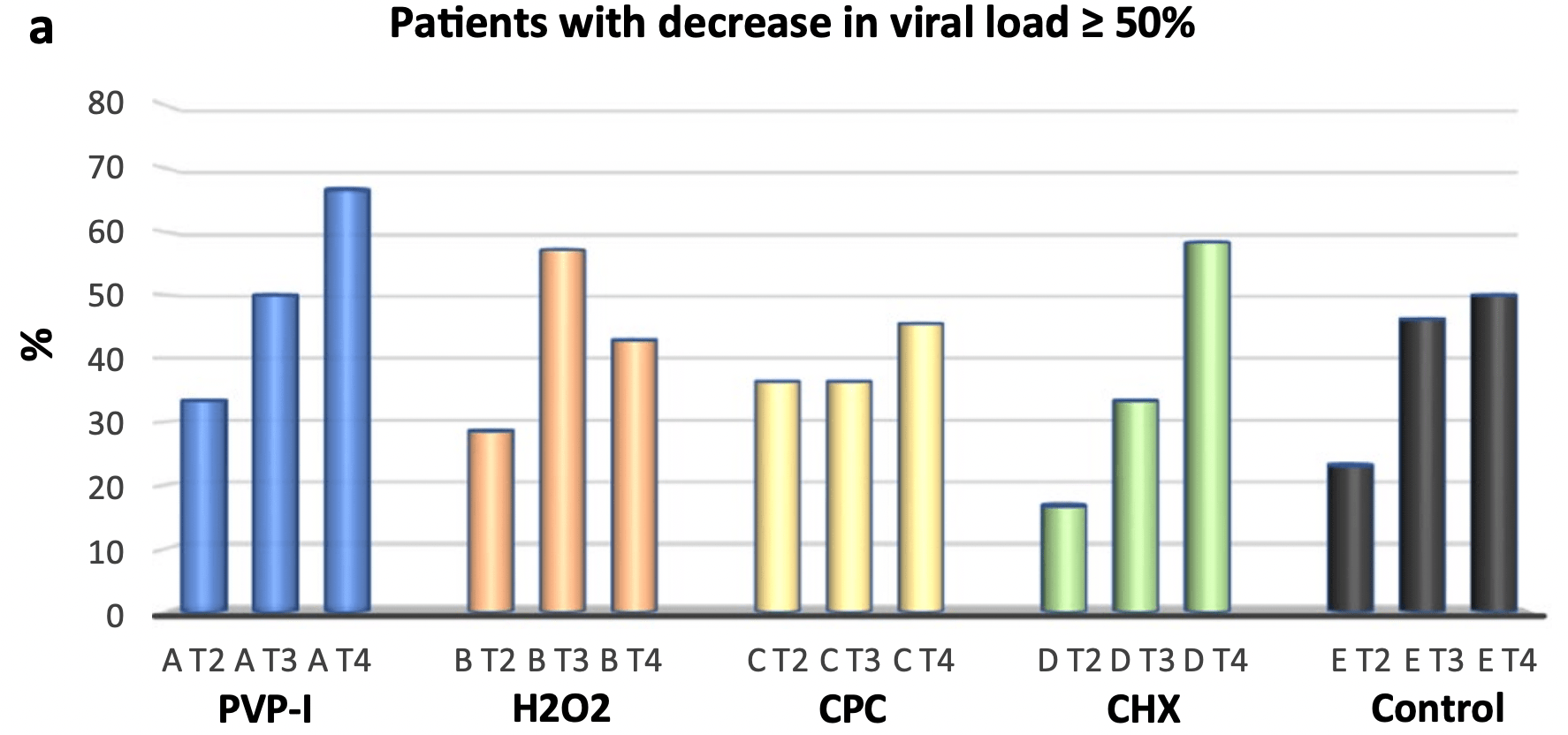
Small very late (>50% 7+ days from symptom onset, 9 PVP-I patients) RCT testing mouthwashing with cetylpyridinium chloride, chlorhexidine, povidone-iodine, hydrogen peroxide, and distilled water, showing no significant differences. Over 30% of patients show >90% decrease in viral load @2 hrs with all 5. Authors note that a trend was observed for viral load decrease with PVP-I @2h for patients <6 days from onset (p=0.06, Wilcox test).
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
This study is excluded in the after exclusion results of meta-analysis:
study only provides short-term viral load results.
Study covers hydrogen peroxide and povidone-iodine.
|
relative viral load reduction, 34.0% better, RR 0.66, p = 0.82, treatment 9, control 12, PVP-I vs. water, data from Table S1.
|
|
relative viral load T4 vs. T1, 93.0% better, RR 0.07, p = 0.35, treatment 9, control 9, data from Table S1.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ferrer et al., 22 Dec 2021, Randomized Controlled Trial, Spain, peer-reviewed, 19 authors.
Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2
Scientific Reports, doi:10.1038/s41598-021-03461-y
Most public health measures to contain the COVID-19 pandemic are based on preventing the pathogen spread, and the use of oral antiseptics has been proposed as a strategy to reduce transmission risk. The aim of this manuscript is to test the efficacy of mouthwashes to reduce salivary viral load in vivo. This is a multi-centre, blinded, parallel-group, placebo-controlled randomised clinical trial that tests the effect of four mouthwashes (cetylpyridinium chloride, chlorhexidine, povidone-iodine and hydrogen peroxide) in SARS-CoV-2 salivary load measured by qPCR at baseline and 30, 60 and 120 min after the mouthrinse. A fifth group of patients used distilled water mouthrinse as a control. Eighty-four participants were recruited and divided into 12-15 per group. There were no statistically significant changes in salivary viral load after the use of the different mouthwashes. Although oral antiseptics have shown virucidal effects in vitro, our data show that salivary viral load in COVID-19 patients was not affected by the tested treatments. This could reflect that those mouthwashes are not effective in vivo, or that viral particles are not infective but viral RNA is still detected by PCR. Viral infectivity studies after the use of mouthwashes are therefore required. (https:// clini caltr ials. gov/ ct2/ show/ NCT04 707742; Identifier: NCT04707742) The coronavirus disease 2019 (COVID-19) outbreak was quickly declared by the World Health Organization (WHO) a public health emergency of international concern and has given rise to one of the most dramatic pandemics in recent human history 1 . The disease is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) virus, a single-stranded enveloped RNA virus which belongs to the betacoronavirus genus from the Coronaviridae family 2 . Because no effective treatment for COVID-19 is currently available, most public health measures to contain the pandemic are based on preventing the spread of the pathogen. The virus is transmitted by the respiratory route (respiratory droplets and aerosols) and by direct contact with contaminated surfaces and subsequent contact with nasal, oral or ocular mucosa 3 . Although patients with symptomatic COVID-19 have been the main source of transmission, asymptomatic and pre-symptomatic patients also have the ability to transmit SARS-CoV-2 4 . Higher viral loads are detected after the onset of COVID-19 symptoms, being significantly higher in the nose compared to the throat 5 . Angiotensin-converting Enzyme 2 (ACE2) is the main cellular receptor for SARS-CoV-2, which interacts with the spike protein to facilitate its entry. ACE2 receptors are highly expressed in the oral cavity and present at high levels in oral epithelial cells 6 . The mean expression of ACE2 was higher in the tongue compared to that in other oral tissues and it has been found to be higher in the minor salivary glands than in the lungs 6 . These
Author contributions
Competing interests The authors declare no competing interests.
References
Anderson, Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease, Infect. Dis. Ther
Chan, A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster, Lancet
Core, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing
Corman, Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Euro Surveill
Duke, Forward, The conditions occurring in vivo when brushing with toothpastes, Br. Dent. J
Eggers, Eickmann, Zorn, Rapid and effective virucidal activity of povidone-iodine products against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA), Infect. Dis. Ther
Eggers, Koburger-Janssen, Eickmann, Zorn, In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/ mouthwash against respiratory and oral tract pathogens, Infect. Dis. Ther
Eggers, Koburger-Janssen, Ward, Newby, Müller, Bactericidal and virucidal activity of povidone-iodine and chlorhexidine gluconate cleansers in an in vivo hand hygiene clinical simulation study, Infect. Dis. Ther
Gottsauner, A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2, Clin. Oral Investig
Herrera, Serrano, Roldán, Sanz, Is the oral cavity relevant in SARS-CoV-2 pandemic?, Clin. Oral Investig, doi:10.1007/s00784-020-03413-2
Kampf, Todt, Pfaender, Steinmann, Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents, J. Hosp. Infect
Kariwa, Fujii, Takashima, Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents, Dermatology
Martínez, Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests, Oral Dis, doi:10.1111/odi.13526
Meister, Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2, J. Infect. Dis
Meng, Hua, Bian, Coronavirus Disease 2019 (COVID-19): Emerging and future challenges for dental and oral medicine, J. Dent. Res
Meyers, Lowering the transmission and spread of human coronavirus, J. Med. Virol, doi:10.1002/jmv.26514
Mohan, Hemalatha, Kopperi, Ranjith, Kumar, SARS-CoV-2 in environmental perspective: Occurrence, persistence, surveillance, inactivation and challenges, Chem. Eng. J
O'donnell, Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection, Function (Oxf)
Paz, Mauer, Ritchie, Robishaw, Caputi, A simplified SARS-CoV-2 detection protocol for research laboratories, PLoS One, doi:10.1371/journal.pone.0244271.PMID:33338082;PMCID:PMC7748277
Popkin, Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo, Pathog. Immun
Riva, Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing, Nature
Sabino-Silva, Jardim, Siqueira, Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis, Clin. Oral Investig
Seneviratne, Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: Randomized control trial in Singapore, Infection
Statkute, Rubina, O'donnell, Stanton, The virucidal efficacy of oral rinse components against SARS-CoV-2, Vitro, doi:10.1101/2020.11.13.381079
Steinhauer, Comparison of the in vitro-efficacy of different mouthwash solutions targeting SARS-CoV-2 based on the European Standard EN 14476, doi:10.1101/2020.10.25.354571
Sun, The kinetics of viral load and antibodies to SARS-CoV-2, Clin. Microbiol. Infect
Wang, Detection of SARS-CoV-2 in different types of clinical specimens, JAMA J. Am. Med. Assoc, doi:10.1001/jama.2020.3786
Whitworth, COVID-19: A fast evolving pandemic, Trans. R. Soc. Trop. Med. Hyg, doi:10.1038/s41598-021-03461-ywww.nature.com/scientificreports/
Who, Molecular assays to diagnose COVID-19: Summary table of available protocols
Wood, Payne, The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses, J. Hosp. Infect
Xu, Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding, Nat. Med
Xu, High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa, Int. J. Oral Sci
Xu, Li, Gan, Du, Yao, Salivary glands: Potential reservoirs for COVID-19 asymptomatic infection, J. Dent. Res
Yoon, Clinical significance of a high SARS-CoV-2 viral load in the saliva, J. Korean Med. Sci
DOI record:
{
"DOI": "10.1038/s41598-021-03461-y",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-021-03461-y",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Most public health measures to contain the COVID-19 pandemic are based on preventing the pathogen spread, and the use of oral antiseptics has been proposed as a strategy to reduce transmission risk. The aim of this manuscript is to test the efficacy of mouthwashes to reduce salivary viral load in vivo. This is a multi-centre, blinded, parallel-group, placebo-controlled randomised clinical trial that tests the effect of four mouthwashes (cetylpyridinium chloride, chlorhexidine, povidone-iodine and hydrogen peroxide) in SARS-CoV-2 salivary load measured by qPCR at baseline and 30, 60 and 120 min after the mouthrinse. A fifth group of patients used distilled water mouthrinse as a control. Eighty-four participants were recruited and divided into 12–15 per group. There were no statistically significant changes in salivary viral load after the use of the different mouthwashes. Although oral antiseptics have shown virucidal effects in vitro, our data show that salivary viral load in COVID-19 patients was not affected by the tested treatments. This could reflect that those mouthwashes are not effective in vivo, or that viral particles are not infective but viral RNA is still detected by PCR. Viral infectivity studies after the use of mouthwashes are therefore required. (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04707742\">https://clinicaltrials.gov/ct2/show/NCT04707742</jats:ext-link>; Identifier: NCT04707742)</jats:p>",
"alternative-id": [
"3461"
],
"article-number": "24392",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "28 June 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2 December 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "22 December 2021"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4521-1632",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ferrer",
"given": "Maria D.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-7919-5765",
"affiliation": [],
"authenticated-orcid": false,
"family": "Barrueco",
"given": "Álvaro Sánchez",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1523-9415",
"affiliation": [],
"authenticated-orcid": false,
"family": "Martinez-Beneyto",
"given": "Yolanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mateos-Moreno",
"given": "María V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ausina-Márquez",
"given": "Verónica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García-Vázquez",
"given": "Elisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puche-Torres",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giner",
"given": "Maria J. Forner",
"sequence": "additional"
},
{
"affiliation": [],
"family": "González",
"given": "Alfonso Campos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coello",
"given": "Jessica M. Santillán",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rueda",
"given": "Ignacio Alcalá",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aubá",
"given": "José M. Villacampa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Español",
"given": "Carlos Cenjor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Velasco",
"given": "Ana López",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abad",
"given": "Diego Santolaya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García-Esteban",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Artacho",
"given": "Alejandro",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9403-8258",
"affiliation": [],
"authenticated-orcid": false,
"family": "López-Labrador",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mira",
"given": "Alex",
"sequence": "additional"
}
],
"container-title": [
"Scientific Reports"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T11:08:57Z",
"timestamp": 1640171337000
},
"deposited": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T11:14:22Z",
"timestamp": 1640171662000
},
"indexed": {
"date-parts": [
[
2021,
12,
23
]
],
"date-time": "2021-12-23T06:50:54Z",
"timestamp": 1640242254865
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "2045-2322"
}
],
"issue": "1",
"issued": {
"date-parts": [
[
2021,
12
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 21,
"start": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T00:00:00Z",
"timestamp": 1640131200000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-021-03461-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-021-03461-y",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-021-03461-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2021,
12
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
22
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1177/0022034520914246",
"author": "L Meng",
"doi-asserted-by": "publisher",
"first-page": "481",
"journal-title": "J. Dent. Res.",
"key": "3461_CR1",
"unstructured": "Meng, L., Hua, F. & Bian, Z. Coronavirus Disease 2019 (COVID-19): Emerging and future challenges for dental and oral medicine. J. Dent. Res. 99, 481–487 (2020).",
"volume": "99",
"year": "2020"
},
{
"DOI": "10.1016/j.cej.2020.126893",
"author": "SV Mohan",
"doi-asserted-by": "publisher",
"journal-title": "Chem. Eng. J.",
"key": "3461_CR2",
"unstructured": "Mohan, S. V., Hemalatha, M., Kopperi, H., Ranjith, I. & Kumar, A. K. SARS-CoV-2 in environmental perspective: Occurrence, persistence, surveillance, inactivation and challenges. Chem. Eng. J. 405, 126893 (2021).",
"volume": "405",
"year": "2021"
},
{
"DOI": "10.1093/trstmh/traa025",
"author": "J Whitworth",
"doi-asserted-by": "publisher",
"first-page": "227",
"journal-title": "Trans. R. Soc. Trop. Med. Hyg.",
"key": "3461_CR3",
"unstructured": "Whitworth, J. COVID-19: A fast evolving pandemic. Trans. R. Soc. Trop. Med. Hyg. 114, 227–228 (2020).",
"volume": "114",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30154-9",
"author": "JF Chan",
"doi-asserted-by": "publisher",
"first-page": "514",
"issue": "10223",
"journal-title": "Lancet",
"key": "3461_CR4",
"unstructured": "Chan, J. F. et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 395(10223), 514–523 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.3786",
"author": "W Wang",
"doi-asserted-by": "publisher",
"journal-title": "JAMA J. Am. Med. Assoc.",
"key": "3461_CR5",
"unstructured": "Wang, W. et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA J. Am. Med. Assoc. https://doi.org/10.1001/jama.2020.3786 (2020).",
"year": "2020"
},
{
"DOI": "10.1038/s41368-019-0067-9",
"author": "H Xu",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Int. J. Oral Sci.",
"key": "3461_CR6",
"unstructured": "Xu, H. et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 12(1), 1–5 (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1177/0022034520918518",
"author": "J Xu",
"doi-asserted-by": "publisher",
"first-page": "989",
"issue": "8",
"journal-title": "J. Dent. Res.",
"key": "3461_CR7",
"unstructured": "Xu, J., Li, Y., Gan, F., Du, Y. & Yao, Y. Salivary glands: Potential reservoirs for COVID-19 asymptomatic infection. J. Dent. Res. 99(8), 989 (2020).",
"volume": "99",
"year": "2020"
},
{
"DOI": "10.1007/s00784-020-03248-x",
"author": "R Sabino-Silva",
"doi-asserted-by": "publisher",
"first-page": "1619",
"issue": "4",
"journal-title": "Clin. Oral Investig.",
"key": "3461_CR8",
"unstructured": "Sabino-Silva, R., Jardim, A. C. G. & Siqueira, W. L. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin. Oral Investig. 24(4), 1619–1621 (2020).",
"volume": "24",
"year": "2020"
},
{
"author": "DL Popkin",
"first-page": "252",
"issue": "2",
"journal-title": "Pathog. Immun.",
"key": "3461_CR9",
"unstructured": "Popkin, D. L. et al. Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo. Pathog. Immun. 2(2), 252–269 (2017).",
"volume": "2",
"year": "2017"
},
{
"DOI": "10.1007/s40121-018-0202-5",
"author": "M Eggers",
"doi-asserted-by": "publisher",
"first-page": "235",
"issue": "2",
"journal-title": "Infect. Dis. Ther.",
"key": "3461_CR10",
"unstructured": "Eggers, M., Koburger-Janssen, T., Ward, L. S., Newby, C. & Müller, S. Bactericidal and virucidal activity of povidone-iodine and chlorhexidine gluconate cleansers in an in vivo hand hygiene clinical simulation study. Infect. Dis. Ther. 7(2), 235–247 (2018).",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1016/S0195-6701(98)90077-9",
"author": "A Wood",
"doi-asserted-by": "publisher",
"first-page": "283",
"journal-title": "J. Hosp. Infect.",
"key": "3461_CR11",
"unstructured": "Wood, A. & Payne, D. The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses. J. Hosp. Infect. 38, 283–295 (1998).",
"volume": "38",
"year": "1998"
},
{
"DOI": "10.1007/s40121-018-0200-7",
"author": "M Eggers",
"doi-asserted-by": "publisher",
"first-page": "249",
"issue": "2",
"journal-title": "Infect. Dis. Ther.",
"key": "3461_CR12",
"unstructured": "Eggers, M., Koburger-Janssen, T., Eickmann, M. & Zorn, J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect. Dis. Ther. 7(2), 249–259 (2018).",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1007/s40121-015-0091-9",
"author": "M Eggers",
"doi-asserted-by": "publisher",
"first-page": "491",
"issue": "4",
"journal-title": "Infect. Dis. Ther.",
"key": "3461_CR13",
"unstructured": "Eggers, M., Eickmann, M. & Zorn, J. Rapid and effective virucidal activity of povidone-iodine products against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA). Infect. Dis. Ther. 4(4), 491–501 (2015).",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.1016/j.jhin.2020.01.022",
"author": "G Kampf",
"doi-asserted-by": "publisher",
"first-page": "246",
"issue": "3",
"journal-title": "J. Hosp. Infect.",
"key": "3461_CR14",
"unstructured": "Kampf, G., Todt, D., Pfaender, S. & Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 104(3), 246–251 (2020).",
"volume": "104",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26514",
"author": "C Meyers",
"doi-asserted-by": "publisher",
"journal-title": "J. Med. Virol.",
"key": "3461_CR15",
"unstructured": "Meyers, C. et al. Lowering the transmission and spread of human coronavirus. J. Med. Virol. https://doi.org/10.1002/jmv.26514 (2020).",
"year": "2020"
},
{
"DOI": "10.1101/2020.10.25.354571",
"author": "K Steinhauer",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv.",
"key": "3461_CR16",
"unstructured": "Steinhauer, K. et al. Comparison of the in vitro-efficacy of different mouthwash solutions targeting SARS-CoV-2 based on the European Standard EN 14476. bioRxiv. https://doi.org/10.1101/2020.10.25.354571 (2020).",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiaa471",
"author": "TL Meister",
"doi-asserted-by": "publisher",
"first-page": "1289",
"issue": "8",
"journal-title": "J. Infect. Dis.",
"key": "3461_CR17",
"unstructured": "Meister, T. L. et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J. Infect. Dis. 222(8), 1289–1292 (2020).",
"volume": "222",
"year": "2020"
},
{
"DOI": "10.1007/s40121-020-00316-3",
"author": "DE Anderson",
"doi-asserted-by": "publisher",
"first-page": "669",
"issue": "3",
"journal-title": "Infect. Dis. Ther.",
"key": "3461_CR18",
"unstructured": "Anderson, D. E. et al. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect. Dis. Ther. 9(3), 669–675 (2020).",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1093/function/zqaa002",
"author": "VB O'Donnell",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Function (Oxf)",
"key": "3461_CR19",
"unstructured": "O’Donnell, V. B. et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function (Oxf) 1(1), zqaa002 (2020).",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0244271.PMID:33338082;PMCID:PMC7748277",
"author": "S Paz",
"doi-asserted-by": "publisher",
"issue": "12",
"journal-title": "PLoS One",
"key": "3461_CR20",
"unstructured": "Paz, S., Mauer, C., Ritchie, A., Robishaw, J. D. & Caputi, M. A simplified SARS-CoV-2 detection protocol for research laboratories. PLoS One 15(12), e0244271. https://doi.org/10.1371/journal.pone.0244271.PMID:33338082;PMCID:PMC7748277 (2020).",
"volume": "15",
"year": "2020"
},
{
"key": "3461_CR21",
"unstructured": "WHO. Molecular assays to diagnose COVID-19: Summary table of available protocols. 24 January 2020. https://www.who.int/publications/m/item/molecular-assays-to-diagnose-covid-19-summary-table-of-available-protocols"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"author": "VM Corman",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "Euro Surveill.",
"key": "3461_CR22",
"unstructured": "Corman, V. M. et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 25(3), 2000045 (2020).",
"volume": "25",
"year": "2020"
},
{
"author": "R Core Team",
"key": "3461_CR23",
"unstructured": "R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020).",
"volume-title": "R: A Language and Environment for Statistical Computing",
"year": "2020"
},
{
"DOI": "10.1101/2020.11.13.381079",
"author": "E Statkute",
"doi-asserted-by": "publisher",
"journal-title": "In Vitro",
"key": "3461_CR24",
"unstructured": "Statkute, E., Rubina, A., O’Donnell, V. B. & Stanton, D. W. T. R. J. The virucidal efficacy of oral rinse components against SARS-CoV-2. In Vitro https://doi.org/10.1101/2020.11.13.381079 (2020).",
"year": "2020"
},
{
"DOI": "10.1159/000089211",
"author": "H Kariwa",
"doi-asserted-by": "publisher",
"first-page": "119",
"issue": "Suppl 1",
"journal-title": "Dermatology",
"key": "3461_CR25",
"unstructured": "Kariwa, H., Fujii, N. & Takashima, I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology 212(Suppl 1), 119–123 (2006).",
"volume": "212",
"year": "2006"
},
{
"DOI": "10.1038/sj.bdj.4804743",
"author": "S Duke",
"doi-asserted-by": "publisher",
"first-page": "52",
"journal-title": "Br. Dent. J.",
"key": "3461_CR26",
"unstructured": "Duke, S. & Forward, G. The conditions occurring in vivo when brushing with toothpastes. Br. Dent. J. 152, 52–54 (1982).",
"volume": "152",
"year": "1982"
},
{
"DOI": "10.1016/j.cmi.2020.08.043",
"author": "J Sun",
"doi-asserted-by": "publisher",
"first-page": "1690.e1",
"issue": "12",
"journal-title": "Clin. Microbiol. Infect.",
"key": "3461_CR27",
"unstructured": "Sun, J. et al. The kinetics of viral load and antibodies to SARS-CoV-2. Clin. Microbiol. Infect. 26(12), 1690.e1-1690.e4 (2020).",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.3346/jkms.2020.35.e195",
"author": "JG Yoon",
"doi-asserted-by": "publisher",
"issue": "20",
"journal-title": "J. Korean Med. Sci.",
"key": "3461_CR28",
"unstructured": "Yoon, J. G. et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J. Korean Med. Sci. 35(20), e195 (2020).",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1007/s00784-020-03549-1",
"author": "MJ Gottsauner",
"doi-asserted-by": "publisher",
"first-page": "3707",
"issue": "10",
"journal-title": "Clin. Oral Investig.",
"key": "3461_CR29",
"unstructured": "Gottsauner, M. J. et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin. Oral Investig. 24(10), 3707–3713 (2020).",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1111/odi.13526",
"author": "LL Martínez",
"doi-asserted-by": "publisher",
"journal-title": "Oral Dis.",
"key": "3461_CR30",
"unstructured": "Martínez, L. L. et al. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests. Oral Dis. https://doi.org/10.1111/odi.13526 (2020).",
"year": "2020"
},
{
"author": "CJ Seneviratne",
"first-page": "1",
"journal-title": "Infection",
"key": "3461_CR31",
"unstructured": "Seneviratne, C. J. et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: Randomized control trial in Singapore. Infection 14, 1–7 (2020).",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2577-1",
"author": "L Riva",
"doi-asserted-by": "publisher",
"first-page": "113",
"issue": "7827",
"journal-title": "Nature",
"key": "3461_CR32",
"unstructured": "Riva, L. et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 586(7827), 113–119 (2020).",
"volume": "586",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0817-4",
"author": "Y Xu",
"doi-asserted-by": "publisher",
"first-page": "502",
"issue": "4",
"journal-title": "Nat. Med.",
"key": "3461_CR33",
"unstructured": "Xu, Y. et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 26(4), 502–505 (2020).",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1007/s00784-020-03413-2",
"author": "D Herrera",
"doi-asserted-by": "publisher",
"first-page": "2925",
"issue": "8",
"journal-title": "Clin. Oral Investig.",
"key": "3461_CR34",
"unstructured": "Herrera, D., Serrano, J., Roldán, S. & Sanz, M. Is the oral cavity relevant in SARS-CoV-2 pandemic?. Clin. Oral Investig. 24(8), 2925–2930. https://doi.org/10.1007/s00784-020-03413-2 (2020).",
"volume": "24",
"year": "2020"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"score": 1,
"short-container-title": [
"Sci Rep"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": [
"Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "11"
}
