Cetylpyridinium Chloride Mouthwash to Reduce Shedding of Infectious SARS-CoV-2: A Double-Blind Randomized Clinical Trial
et al., Journal of Dental Research, doi:10.1177/00220345221102310, NCT04757818, Jun 2022
58th treatment shown to reduce risk in
September 2025, now with p = 0.0035 from 4 studies.
Lower risk for recovery and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
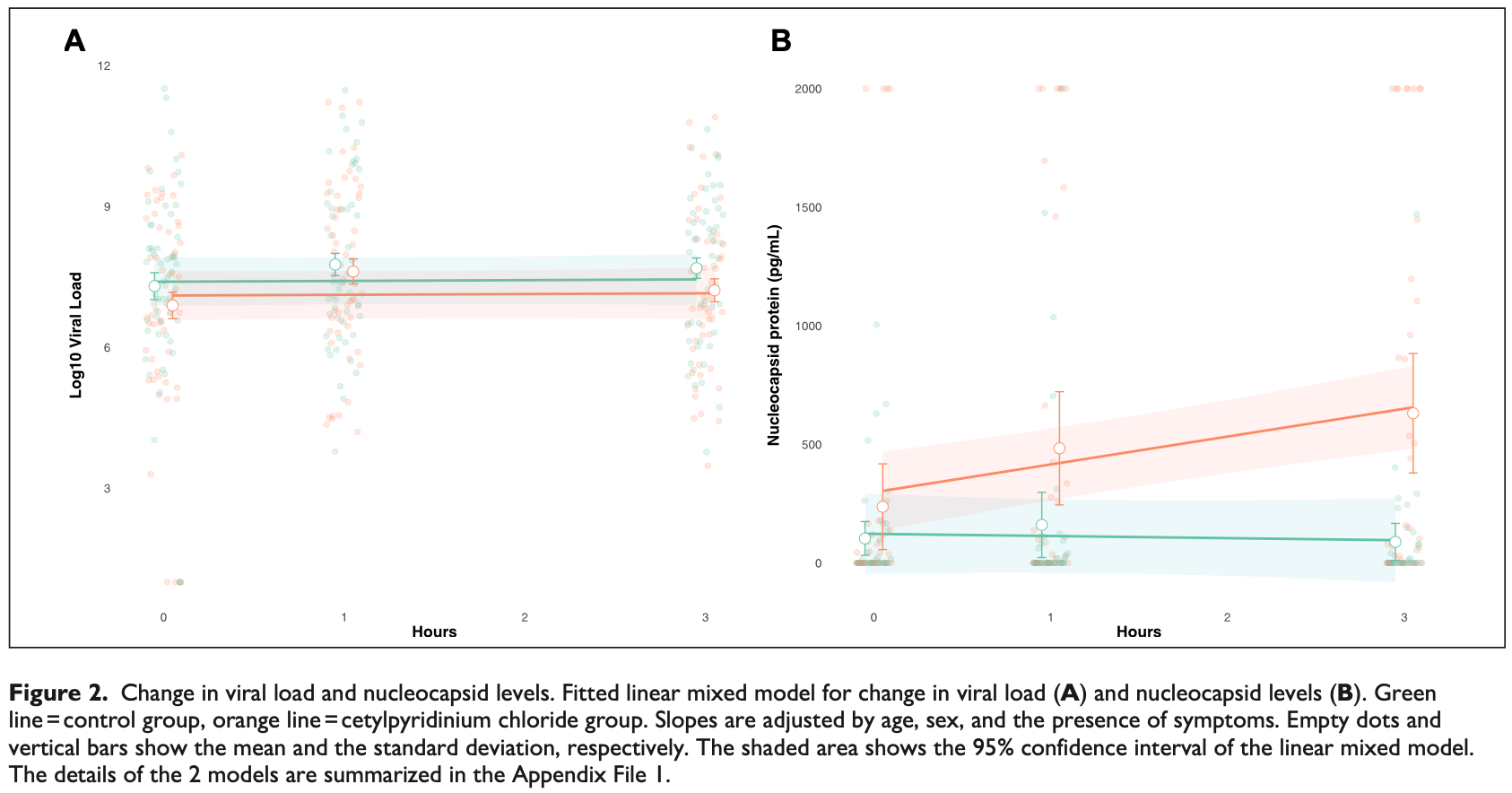
118 patient RCT testing cetylpyridinium chloride (CPC) mouthwash, showing significantly increased detection of SARS-CoV-2 nucleocapsid protein, indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane, exposing the nucleocapsid of the virus. Notably, there was no significant difference in viral load detected with PCR, highlighting the limitations of PCR, which is unable to differentiate between intact infectious virus and non-infectious or destroyed virus particles. PCR viral load may not correlate well with actual remaining infectivity after treatments like mouthwashes.
Alemany et al., 21 Jun 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Spain, peer-reviewed, 31 authors, study period 15 February, 2021 - 2 June, 2021, trial NCT04757818 (history).
Contact: aalemany@lluita.org.
Cetylpyridinium Chloride Mouthwash to Reduce Shedding of Infectious SARS-CoV-2: A Double-Blind Randomized Clinical Trial
Journal of Dental Research, doi:10.1177/00220345221102310
The airborne transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) via respiratory fluids and droplets suggests that mouthwashes containing substances with virucidal activity can help reduce viral spread. We conducted a multicenter, double-blind, placebo-controlled, randomized trial to assess the virucidal activity of cetylpyridinium chloride (CPC) mouthwashes. Outpatients who tested positive for SARS-CoV-2 infection with or without symptoms were randomized to perform washes and gargles for 1 min with 15 mL of either colored distilled water or 0.07% CPC (Vitis CPC Protect) mouthwash. The study outcomes were the SARS-CoV-2 log 10 viral RNA load and the nucleocapsid protein levels, both in saliva at 1 and 3 h after the intervention. In total, 118 patients were enrolled and randomized (mean [SD], age 46 [14] y). Thirteen of 118 participants (11%) did not complete follow-up or had insufficient sample volume for testing and were excluded from the analysis. The assessment of the viral load showed no significant differences between groups at any of the investigated points. However, the levels of SARS-CoV-2 nucleocapsid protein of lysed viruses were significantly higher in the CPC group compared with the control group at 1 h (adjusted difference 269.3 pg/mL; 95% confidence interval [CI], 97.1-441.5) and at 3 h postintervention (561.1 pg/mL; 95% CI, 380.0-742.2). In nonhospitalized patients with asymptomatic or mild symptomatic SARS-CoV-2 infection, a 0.07% CPC mouthwash, compared to placebo, was associated with a significant increase of nucleocapsid protein levels in saliva, indicating enhanced disruption of viral particles.
Author Contributions A. Alemany, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; D. Perez-Zsolt, D. Raïch-Regué, J. Muñoz-Basagoiti, D. Ouchi, J. Ara, M.A. Rodriguez-Arias, J. Puig, I. Blanco, C. Casañ Lopez, A. Hernández, A.E. Bordoy, C. Esteban Redondo, V. González Soler, M. Giménez, contributed to data analysis and interpretation, critically revised the manuscript; C. Laporte-Villar, M. Ochoa Gianinetto, M. Viaplana Gutiérrez, M. Garcia Sánchez-Paniagua, N. Larrosa Henríquez, J. Moreno Vicente, contributed to data acquisition, critically revised the manuscript; B. Baro, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; N. Henríquez, N. Prat, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; V. Blanc, R. León, J. Gispert, contributed to conception and design, critically revised the manuscript; B. Clotet, N. Izquierdo-Useros, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript; O. Mitjà, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Declaration of Conflicting Interests The authors declared the following potential conflicts of interest with respect to the..
References
Alemany, Millat-Martinez, Ouchi, Corbacho-Monné, Bordoy et al., Selfcollected mid-nasal swabs and saliva specimens, compared with nasopharyngeal swabs, for SARS-CoV-2 detection in mild COVID-19 patients, J Infect
Bai, Cao, Liu, Li, The SARS-CoV-2 nucleocapsid protein and its role in viral structure, biological functions, and a potential target for drug or vaccine mitigation, Viruses
Burton, Clarkson, Goulao, Glenny, Mcbain et al., Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them, Cochrane Database Syst Rev
Carrouel, Gonçalves, Conte, Campus, Fisher et al., Antiviral activity of reagents in mouth rinses against SARS-CoV-2, J Dent Res
Carrouel, Valette, Gadea, Esparcieux, Illes et al., Use of an antiviral mouthwash as a barrier measure in the SARS-CoV-2 transmission in adults with asymptomatic to mild COVID-19: a multicentre, randomized, double-blind controlled trial, Clin Microbiol Infect
Eduardo, Corrêa, Heller, Daep, Benitez et al., Salivary SARS-CoV-2 load reduction with mouthwash use: a randomized pilot clinical trial
Gottsauner, Michaelides, Schmidt, Scholz, Buchalla et al., A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2, Clin Oral Investig
Guenezan, Garcia, Strasters, Jousselin, Lévêque et al., Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: a randomized clinical trial, JAMA Otolaryngol Head Neck Surg
Herrera, Serrano, Roldán, Sanz, Is the oral cavity relevant in SARS-CoV-2 pandemic?, Clin Oral Investig
Hsieh, Goldsmith, Schaub, Divenere, Kuo et al., Structure-based design of prefusion-stabilized SARS-CoV-2 spikes, Science
Huang, Huang, Use of chlorhexidine to eradicate oropharyngeal SARS-CoV-2 in COVID-19 patients, J Med Virol
Lamas, Dios, Rodríguez, Campo, Alvargonzalez et al., Is povidone-iodine mouthwash effective against SARS-CoV-2? First in vivo tests, Oral Dis
Mao, Auer, Buchalla, Hiller, Maisch et al., Cetylpyridinium chloride: mechanism of action, antimicrobial efficacy in biofilms, and potential risks of resistance, Antimicrob Agents Chemother
Meister, Brüggemann, Todt, Conzelmann, Müller et al., Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2, J Infect Dis
Meselson, Droplets and aerosols in the transmission of SARS-CoV-2, N Engl J Med
Muñoz-Basagoiti, Perez-Zsolt, León, Blanc, Raïch-Regué et al., Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in vitro, J Dent Res
O'donnell, Thomas, Stanton, Maillard, Murphy et al., Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection
Park, Li, Yu, Brinkman, Wong, Characterization of RNA in saliva, Clin Chem
Popkin, Zilka, Dimaano, Fujioka, Rackley et al., Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo, Pathog Immun
Seneviratne, Balan, Ko, Udawatte, Lai et al., Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore, Infection
Torres, Collins, Corbit, Ramirez, Winters et al., Comparison of saliva and nasopharyngeal swab SARS-CoV-2 RT-qPCR testing in a community setting
Wyllie, Fournier, Casanovas-Massana, Campbell, Tokuyama et al., Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2, N Engl J Med
Wölfel, Corman, Guggemos, Seilmaier, Zange et al., Virological assessment of hospitalized patients with COVID-2019
DOI record:
{
"DOI": "10.1177/00220345221102310",
"ISSN": [
"0022-0345",
"1544-0591"
],
"URL": "http://dx.doi.org/10.1177/00220345221102310",
"abstract": "<jats:p> The airborne transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) via respiratory fluids and droplets suggests that mouthwashes containing substances with virucidal activity can help reduce viral spread. We conducted a multicenter, double-blind, placebo-controlled, randomized trial to assess the virucidal activity of cetylpyridinium chloride (CPC) mouthwashes. Outpatients who tested positive for SARS-CoV-2 infection with or without symptoms were randomized to perform washes and gargles for 1 min with 15 mL of either colored distilled water or 0.07% CPC (Vitis CPC Protect) mouthwash. The study outcomes were the SARS-CoV-2 log<jats:sub>10</jats:sub> viral RNA load and the nucleocapsid protein levels, both in saliva at 1 and 3 h after the intervention. In total, 118 patients were enrolled and randomized (mean [SD], age 46 [14] y). Thirteen of 118 participants (11%) did not complete follow-up or had insufficient sample volume for testing and were excluded from the analysis. The assessment of the viral load showed no significant differences between groups at any of the investigated points. However, the levels of SARS-CoV-2 nucleocapsid protein of lysed viruses were significantly higher in the CPC group compared with the control group at 1 h (adjusted difference 269.3 pg/mL; 95% confidence interval [CI], 97.1–441.5) and at 3 h postintervention (561.1 pg/mL; 95% CI, 380.0–742.2). In nonhospitalized patients with asymptomatic or mild symptomatic SARS-CoV-2 infection, a 0.07% CPC mouthwash, compared to placebo, was associated with a significant increase of nucleocapsid protein levels in saliva, indicating enhanced disruption of viral particles. </jats:p>",
"alternative-id": [
"10.1177/00220345221102310"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8379-6857",
"affiliation": [
{
"name": "Fight AIDS and Infectious Diseases Foundation, Badalona, Spain"
},
{
"name": "Hospital Universitari Germans Trias i Pujol, Badalona, Spain"
},
{
"name": "Facultat de Medicina-Universitat de Barcelona, Barcelona, Spain"
}
],
"authenticated-orcid": false,
"family": "Alemany",
"given": "A.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "IrsiCaixa AIDS Research Institute, Badalona, Spain"
}
],
"family": "Perez-Zsolt",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IrsiCaixa AIDS Research Institute, Badalona, Spain"
}
],
"family": "Raïch-Regué",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IrsiCaixa AIDS Research Institute, Badalona, Spain"
}
],
"family": "Muñoz-Basagoiti",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fight AIDS and Infectious Diseases Foundation, Badalona, Spain"
},
{
"name": "Universitat Autònoma de Barcelona, Barcelona, Spain"
}
],
"family": "Ouchi",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fight AIDS and Infectious Diseases Foundation, Badalona, Spain"
}
],
"family": "Laporte-Villar",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ISGlobal, Hospital Clinic Universitat de Barcelona, Barcelona, Spain"
}
],
"family": "Baro",
"given": "B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gerència Territorial Metropolitana Nord, Institut Català de la Salut, Barcelona, Spain"
}
],
"family": "Henríquez",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gerència Territorial Metropolitana Nord, Institut Català de la Salut, Barcelona, Spain"
}
],
"family": "Prat",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gerència Territorial Metropolitana Nord, Institut Català de la Salut, Barcelona, Spain"
}
],
"family": "Gianinetto",
"given": "M. Ochoa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gerència Territorial Metropolitana Nord, Institut Català de la Salut, Barcelona, Spain"
}
],
"family": "Gutiérrez",
"given": "M. Viaplana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gerència Territorial Metropolitana Nord, Institut Català de la Salut, Barcelona, Spain"
}
],
"family": "Sánchez-Paniagua",
"given": "M. Garcia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gerència Territorial Metropolitana Nord, Institut Català de la Salut, Barcelona, Spain"
}
],
"family": "Henríquez",
"given": "N. Larrosa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gerència Territorial Metropolitana Nord, Institut Català de la Salut, Barcelona, Spain"
}
],
"family": "Vicente",
"given": "J. Moreno",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Universitari Germans Trias i Pujol, Badalona, Spain"
},
{
"name": "Gerència Territorial Metropolitana Nord, Institut Català de la Salut, Barcelona, Spain"
}
],
"family": "Ara",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fight AIDS and Infectious Diseases Foundation, Badalona, Spain"
},
{
"name": "Hospital Universitari Germans Trias i Pujol, Badalona, Spain"
}
],
"family": "Rodriguez-Arias",
"given": "M.A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fight AIDS and Infectious Diseases Foundation, Badalona, Spain"
}
],
"family": "Puig",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Metropolitana Nord Laboratory, Institut Català de la Salut, Badalona, Spain"
}
],
"family": "Blanco",
"given": "I.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Microbiology Department, Clinical Laboratory Metropolitana Nord, Badalona, Barcelona, Spain"
}
],
"family": "Lopez",
"given": "C. Casañ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Universitat Autònoma de Barcelona, Barcelona, Spain"
},
{
"name": "Microbiology Department, Clinical Laboratory Metropolitana Nord, Badalona, Barcelona, Spain"
}
],
"family": "Hernández",
"given": "Á.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Microbiology Department, Clinical Laboratory Metropolitana Nord, Badalona, Barcelona, Spain"
}
],
"family": "Bordoy",
"given": "A.E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Microbiology Department, Clinical Laboratory Metropolitana Nord, Badalona, Barcelona, Spain"
}
],
"family": "Redondo",
"given": "C. Esteban",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Microbiology Department, Clinical Laboratory Metropolitana Nord, Badalona, Barcelona, Spain"
},
{
"name": "Centre of Epidemiological Studies of HIV/AIDS and STI of Catalonia (CEEISCAT), Health Department, Generalitat de Catalunya, Badalona, Spain"
},
{
"name": "CIBER Epidemiologia y Salud Pública (CIBERESP), Madrid, Spain"
}
],
"family": "Soler",
"given": "V. González",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Microbiology Department, Clinical Laboratory Metropolitana Nord, Badalona, Barcelona, Spain"
},
{
"name": "CIBER Enfermedades Respiratorias (CIBERES), Madrid, Spain"
}
],
"family": "Giménez",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "DENTAID Research Center, Cerdanyola del Vallès, Spain"
}
],
"family": "Blanc",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "DENTAID Research Center, Cerdanyola del Vallès, Spain"
}
],
"family": "León",
"given": "R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2830-8053",
"affiliation": [
{
"name": "DENTAID Research Center, Cerdanyola del Vallès, Spain"
}
],
"authenticated-orcid": false,
"family": "Gispert",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fight AIDS and Infectious Diseases Foundation, Badalona, Spain"
},
{
"name": "Hospital Universitari Germans Trias i Pujol, Badalona, Spain"
},
{
"name": "IrsiCaixa AIDS Research Institute, Badalona, Spain"
},
{
"name": "Universitat de Vic-Universitat Central de Catalunya (UVIC-UCC), Vic, Spain"
}
],
"family": "Clotet",
"given": "B.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1039-1821",
"affiliation": [
{
"name": "IrsiCaixa AIDS Research Institute, Badalona, Spain"
},
{
"name": "Germans Trias i Pujol Research Institute, Badalona, Spain"
}
],
"authenticated-orcid": false,
"family": "Izquierdo-Useros",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fight AIDS and Infectious Diseases Foundation, Badalona, Spain"
},
{
"name": "Hospital Universitari Germans Trias i Pujol, Badalona, Spain"
},
{
"name": "Universitat de Vic-Universitat Central de Catalunya (UVIC-UCC), Vic, Spain"
},
{
"name": "Lihir Medical Centre, International SOS, Lihir Island, Papua New Guinea"
}
],
"family": "Mitjà",
"given": "O.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "CPC-COVID GROUP",
"sequence": "additional"
}
],
"container-title": "Journal of Dental Research",
"container-title-short": "J Dent Res",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2022,
6,
21
]
],
"date-time": "2022-06-21T16:28:17Z",
"timestamp": 1655828897000
},
"deposited": {
"date-parts": [
[
2022,
10,
24
]
],
"date-time": "2022-10-24T11:47:11Z",
"timestamp": 1666612031000
},
"funder": [
{
"DOI": "10.13039/501100004837",
"award": [
"PID2020-117145RB-I00"
],
"doi-asserted-by": "publisher",
"name": "Ministerio de Ciencia e Innovación"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
20
]
],
"date-time": "2023-08-20T20:33:33Z",
"timestamp": 1692563613506
},
"is-referenced-by-count": 17,
"issue": "12",
"issued": {
"date-parts": [
[
2022,
6,
21
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2022,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://journals.sagepub.com/page/policies/text-and-data-mining-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
21
]
],
"date-time": "2022-06-21T00:00:00Z",
"timestamp": 1655769600000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/00220345221102310",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/00220345221102310",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/00220345221102310",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "1450-1456",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2022,
6,
21
]
]
},
"published-online": {
"date-parts": [
[
2022,
6,
21
]
]
},
"published-print": {
"date-parts": [
[
2022,
11
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1016/j.jinf.2021.09.012",
"doi-asserted-by": "publisher",
"key": "bibr1-00220345221102310"
},
{
"DOI": "10.3390/v13061115",
"doi-asserted-by": "publisher",
"key": "bibr2-00220345221102310"
},
{
"author": "Burton MJ",
"issue": "9",
"journal-title": "Cochrane Database Syst Rev",
"key": "bibr3-00220345221102310",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1177/0022034520967933",
"doi-asserted-by": "publisher",
"key": "bibr4-00220345221102310"
},
{
"DOI": "10.1016/j.cmi.2021.05.028",
"doi-asserted-by": "publisher",
"key": "bibr5-00220345221102310"
},
{
"DOI": "10.1016/j.heliyon.2021.e07346",
"doi-asserted-by": "publisher",
"key": "bibr6-00220345221102310"
},
{
"DOI": "10.1007/s00784-020-03549-1",
"doi-asserted-by": "publisher",
"key": "bibr7-00220345221102310"
},
{
"DOI": "10.1001/jamaoto.2020.5490",
"doi-asserted-by": "publisher",
"key": "bibr8-00220345221102310"
},
{
"DOI": "10.1007/s00784-020-03413-2",
"doi-asserted-by": "publisher",
"key": "bibr9-00220345221102310"
},
{
"DOI": "10.1126/science.abd0826",
"doi-asserted-by": "publisher",
"key": "bibr10-00220345221102310"
},
{
"DOI": "10.1002/jmv.26954",
"doi-asserted-by": "publisher",
"key": "bibr11-00220345221102310"
},
{
"DOI": "10.1128/AAC.00576-20",
"doi-asserted-by": "publisher",
"key": "bibr12-00220345221102310"
},
{
"DOI": "10.1111/odi.13526",
"doi-asserted-by": "publisher",
"key": "bibr13-00220345221102310"
},
{
"DOI": "10.1093/infdis/jiaa471",
"doi-asserted-by": "publisher",
"key": "bibr14-00220345221102310"
},
{
"DOI": "10.1056/NEJMc2009324",
"doi-asserted-by": "publisher",
"key": "bibr15-00220345221102310"
},
{
"key": "bibr16-00220345221102310",
"unstructured": "Ministerio de Sanidad. 2020. Manejo clínico del COVID-19: atención hospitalaria [accessed 2022 May 5]. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Protocolo_manejo_clinico_ah_COVID-19.pdf."
},
{
"DOI": "10.1177/00220345211029269",
"doi-asserted-by": "publisher",
"key": "bibr17-00220345221102310"
},
{
"DOI": "10.1093/function/zqaa002",
"author": "O’Donnell VB",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Function",
"key": "bibr18-00220345221102310",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1373/clinchem.2005.063206",
"doi-asserted-by": "publisher",
"key": "bibr19-00220345221102310"
},
{
"DOI": "10.20411/pai.v2i2.200",
"doi-asserted-by": "publisher",
"key": "bibr20-00220345221102310"
},
{
"key": "bibr21-00220345221102310",
"unstructured": "Scientific Committee on Consumer Safety (SCSS). 2015. Cetylpyridinium chloride—Submission II [accessed 2022 May 5]. https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_171.pdf."
},
{
"DOI": "10.1007/s15010-020-01563-9",
"doi-asserted-by": "publisher",
"key": "bibr22-00220345221102310"
},
{
"DOI": "10.1016/j.jinf.2020.11.015",
"doi-asserted-by": "publisher",
"key": "bibr23-00220345221102310"
},
{
"key": "bibr24-00220345221102310",
"unstructured": "US Department of Labor. 2020. Dentistry workers and employers. Occupational Safety and Health Administration [accessed 2022 May 5]. https://www.osha.gov/coronavirus/control-prevention/dentistry."
},
{
"DOI": "10.1038/s41586-020-2196-x",
"doi-asserted-by": "publisher",
"key": "bibr25-00220345221102310"
},
{
"key": "bibr26-00220345221102310",
"unstructured": "World Health Organization. n.d. Diagnostic testing for SARS-CoV-2 [accessed 2022 May 5]. https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2."
},
{
"DOI": "10.1056/NEJMc2016359",
"doi-asserted-by": "publisher",
"key": "bibr27-00220345221102310"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/00220345221102310"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Dentistry"
],
"subtitle": [],
"title": "Cetylpyridinium Chloride Mouthwash to Reduce Shedding of Infectious SARS-CoV-2: A Double-Blind Randomized Clinical Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "101"
}
