Efficacy of povidone-iodine nasal rinse and mouth wash in COVID-19 management: a prospective, randomized pilot clinical trial (povidone-iodine in COVID-19 management)
et al., BMC Infectious Diseases, doi:10.1186/s12879-024-09137-y, NCT04449965, Dec 2023 (preprint)
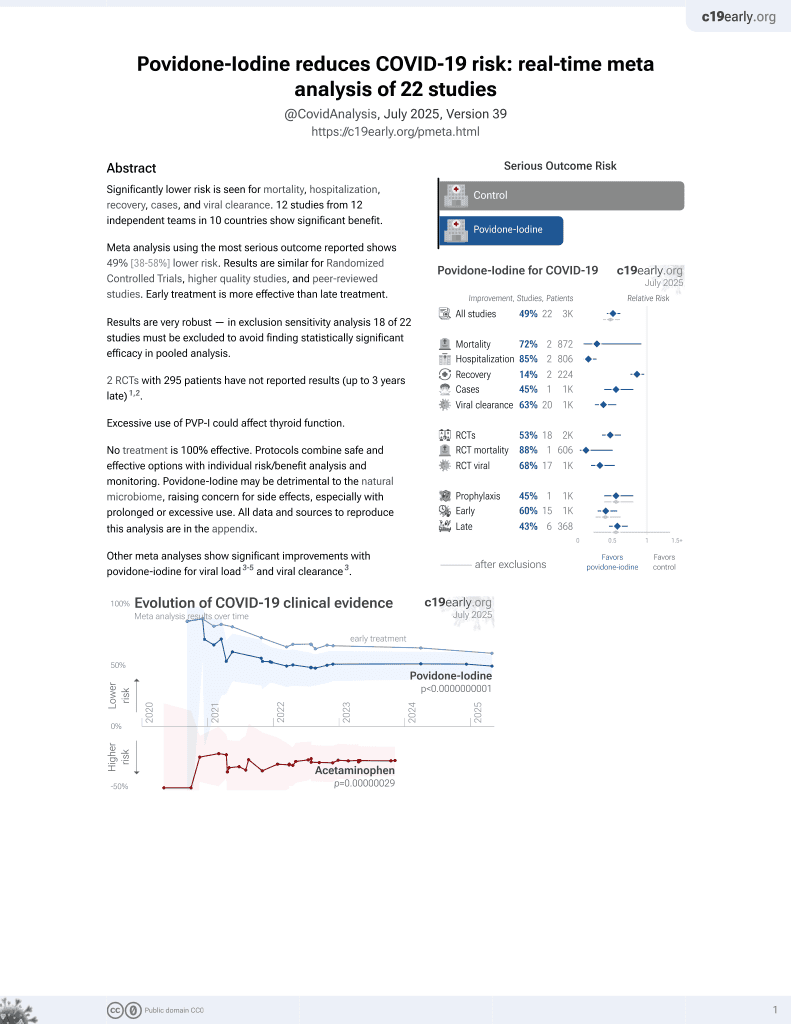
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Tiny RCT with 5 PVP-I patients, 6 saline patients, and 8 control patients, showing improved viral clearance with 0.23% povidone-iodine (PVP-I) nasal rinses and mouthwashes. No significant differences were found for viral load by PCR cycle threshold values or patient-reported symptom scales.
Authors claim the trial is placebo controlled, however they state that the control group "did not receive any intervention".
WURSS-11 was higher in the PVP-I group vs. control at baseline (29 vs. 24).
Authors report that patients developed symptoms within the last three days, however the mean baseline Ct for the PVP-I group was 23, indicating high viral load and suggesting later treatment.
Figure 1 shows patients lost to followup in both the PVP-I and NS groups. For PVP-I, Figure 2 indicates a patient censored at day 4 and only 4 patients with further data, but Table 2 shows a mean viral clearance of 9.8 days from 5 patients.
|
risk of no recovery, 16.4% lower, RR 0.84, p = 0.76, treatment mean 4.6 (±4.0) n=4, control mean 5.5 (±5.0) n=8, mid-recovery, day 4.
|
|
risk of no recovery, 40.0% higher, RR 1.40, p = 0.73, treatment mean 2.8 (±4.0) n=4, control mean 2.0 (±3.5) n=8, day 18.
|
|
risk of no recovery, 13.0% lower, RR 0.87, p = 0.89, treatment mean 2.0 (±1.0) n=4, control mean 2.3 (±4.0) n=8, day 12.
|
|
risk of no recovery, 61.4% lower, RR 0.39, p = 0.28, treatment mean 2.7 (±3.0) n=4, control mean 7.0 (±7.1) n=8, day 10.
|
|
risk of no recovery, 66.7% lower, RR 0.33, p = 0.36, treatment mean 1.0 (±1.0) n=4, control mean 3.0 (±4.0) n=8, inverted to make RR<1 favor treatment, day 8.
|
|
risk of no recovery, 3.1% higher, RR 1.03, p = 0.96, treatment mean 3.3 (±3.0) n=4, control mean 3.2 (±3.0) n=8, day 6.
|
|
risk of no recovery, 16.4% lower, RR 0.84, p = 0.76, treatment mean 4.6 (±4.0) n=4, control mean 5.5 (±5.0) n=8, day 4.
|
|
risk of no recovery, 68.0% higher, RR 1.68, p = 0.07, treatment mean 16.3 (±6.0) n=4, control mean 9.7 (±5.0) n=8, day 2.
|
|
relative improvement in viral load, 72.7% better, RR 0.27, p = 0.72, treatment mean 2.2 (±4.88) n=5, control mean 0.6 (±8.7) n=8, day 4.
|
|
time to viral-, 22.2% lower, relative time 0.78, p = 0.02, treatment mean 9.8 (±1.6) n=4, control mean 12.6 (±1.68) n=8.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Alsaleh et al., 6 Dec 2023, Randomized Controlled Trial, Saudi Arabia, peer-reviewed, median age 38.0, 9 authors, study period August 2021 - July 2022, trial NCT04449965 (history).
Contact: alhussienah@gmail.com.
Efficacy of povidone-iodine nasal rinse and mouth wash in COVID-19 management: a prospective, randomized pilot clinical trial (povidone-iodine in COVID-19 management)
BMC Infectious Diseases, doi:10.1186/s12879-024-09137-y
Objectives/Hypothesis To assess the efficacy of 0.23% povidone-iodine (PVP-I) nasal rinses and mouth washes on detectability of the coronavirus disease 2019 (COVID-19) virus and cycle threshold (Ct) values in nasopharyngeal swabs.
Study design This was an open-label, prospective, randomized, placebo-controlled clinical trial.
Supplementary Information The online version contains supplementary material available at https://doi. org/10.1186/s12879-024-09137-y.
Supplementary Material 1
Author contributions Saad Alsaleh (SA) and Amin Javer (AJ) conceptualized the study. Ahmed Alhussien (AH) curated the data and performed formal analysis. SA secured funding for the project. AH, Abduljabbar Alyamani (AY), Fahad Alhussain (FA), Ali Alhijji (AJ), and Khalifa Binkhamis (KB) contributed to the investigation. SA, AJ, KB, Anas Khan (AK), and Fatimah Al-Shahrani (FA) contributed to the methodology. AH, AJ, and KB were involved in project administration. SA, KB, AK, and FA provided resources. AH developed the software. SA supervised the project. KB validated the findings. SA visualized the data. AH, AY, and FA drafted the original manuscript. SA, KB, and AJ reviewed and edited the manuscript. All authors reviewed the final manuscript.
Declarations Ethics approval and consent to participate The study was approved by the King Saud University Institutional Review Board and the Saudi Food and Drug Authority Clinical Trial Unit. All patients who met the inclusion criteria signed a written informed consent form that mentioned possible risks and benefits associated with the intervention.
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and..
References
Aderaw, Reinhardt, Safran, Pino, Sam, Effect of mouth rinsing with two polyvinylpyrrolidone-iodine mixtures on iodine absorption and thyroid function, J Clin Endocrinol Metab
Alanazy, Dousary, Albosaily, Aldriweesh, Alsaleh et al., Psychometric arabic sino-nasal outcome Test-22: validation and translation in chronic rhinosinusitis patients, Ann Saudi Med
Amin, Harrison, Benton, Roberts, Weinstein, Effect of povidoneiodine on Streptococcus mutans in children with extensive dental caries, Pediatr Dent
Arakeri, Brennan, Povidone-iodine: an anti-oedematous agent?, Int J Oral Maxillofac Surg
Bustin, Mueller, Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis, Clin Sci
Chaudhary, Melkonyan, Meethil, Saraswat, Hall et al., Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load: a randomized controlled trial, J Am Dent Association
Domingo, García-Crespo, Vega, Perales, Mutation rates, mutation frequencies, and proofreading-repair activities in RNA virus genetics, Viruses
Eggers, Koburger, Janssen, Eickmann, H Diro bactericidal and virucidal efficacy of povidone 一 iodine gargle/mouthwashagainstrespiratoryand oraltract pathogens, J. Infect DisTher
Elzein, Sater, Fakhreddine, Hanna, Feghali et al., In vivo evaluation of the virucidal efficacy of chlorhexidine and povidoneiodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial, J Evid Based Dent Pract
Fan, Chan, Hung, Safety and efficacy of COVID-19 vaccines: a systematic review and meta-analysis of different vaccines at phase 3, Vaccines
Fantozzi, Pampena, Pierangeli, Oliveto, Sorrentino et al., Efficacy of antiseptic mouthrinses against SARS-CoV-2: a prospective randomized placebo-controlled pilot study, Am J Otolaryngol
Farahat, Mesallam, Alrasheed, Malki, Validity and reliability of the arabic version of the wisconsin upper respiratory symptom survey (AWURSS-11), Saudi Journal of Otorhinolaryngology Head and Neck Surgery
Frank, Brown, Capriotti, Westover, Pelletier et al., In vitro efficacy of a povidone-iodine nasal antiseptic for rapid inactivation of SARS-CoV-2, JAMA Otolaryngology-Head Neck Surg
Frank, Capriotti, Brown, Tessema, Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 era, Ear Nose Throat J
Guenezan, Garcia, Strasters, Jousselin, Lévêque et al., Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: a randomized clinical trial, JAMA Otolaryngology-Head Neck Surg
Hasan, Rumi, Banu, Uddin, Islam et al., Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: a structured summary of a study protocol for an open-label randomized clinical trial, Trials
Idrees, Mcgowan, Fawzy, Abuderman, Balasubramaniam et al., Efficacy of Mouth rinses and nasal spray in the inactivation of SARS-CoV-2: a systematic review and Meta-analysis of in Vitro and in vivo studies, Int J Environ Res Public Health
Joynt, Wu, Understanding. COVID-19: what does viral RNA load really mean?, Lancet Infect Dis
Julious, Sample size of 12 per group rule of thumb for a pilot study, Pharm Statistics: J Appl Stat Pharm Ind
Lee, Pottinger, Davis, Tolerability and effectiveness of povidoneiodine or mupirocin versus saline sinus irrigations for chronic rhinosinusitis, Am J Otolaryngol
Levy, Thorpe, Scherer, Scherer, Drews et al., Misrepresentation and Nonadherence Regarding COVID-19 Public Health Measures, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.35837
Liang, Yuan, Wei, Wang, Zhang et al., In-vivo toxicity studies and in-vitro inactivation of SARS-CoV-2 by povidone-iodine in-situ gel forming formulations, BioRxiv
Moher, Schulz, Altman, Group, The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials, Ann Intern Med
Natto, Bakhrebah, Afeef, Al-Harbi, Nassar et al., The short-term effect of different chlorhexidine forms versus povidone iodine mouth rinse in minimizing the oral SARS-CoV-2 viral load: an open label randomized controlled clinical trial study, Medicine
Ni, Yang, Yang, Bao, Li et al., Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19, Crit Care
Pelletier, Tessema, Frank, Westover, Brown et al., Efficacy of povidone-iodine nasal and oral antiseptic preparations against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), Ear Nose Throat J
Rao, Manissero, Steele, Pareja, A systematic review of the clinical utility of cycle threshold values in the context of COVID-19, Infect Dis Ther
Rothan, Byrareddy, The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak, J Autoimmun
Saggers, Stewart, Polyvinyl-pyrrolidone-iodine: an assessment of antibacterial activity, Epidemiol Infect
Seneviratne, Balan, Ko, Udawatte, Lai et al., Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore, Infection
Wu, Cheng, Chang, Lo, Cheng, Efficacy of povidone-iodine nasal irrigation solution after sinonasal surgery: a randomized controlled study, Laryngoscope
Wölfel, Corman, Guggemos, Seilmaier, Zange et al., Virological assessment of hospitalized patients with COVID-2019, Nature
Zarabanda, Vukkadala, Phillips, Qian, Mfuh et al., The Effect of Povidone-Iodine nasal spray on nasopharyngeal SARS-CoV-2 viral load: a Randomized Control Trial, Laryngoscope, doi:10.1002/lary.29935
Zou, Ruan, Huang, Liang, Huang et al., SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med
DOI record:
{
"DOI": "10.1186/s12879-024-09137-y",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-024-09137-y",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Objectives/Hypothesis</jats:title>\n <jats:p>To assess the efficacy of 0.23% povidone-iodine (PVP-I) nasal rinses and mouth washes on detectability of the coronavirus disease 2019 (COVID-19) virus and cycle threshold (Ct) values in nasopharyngeal swabs.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Study design</jats:title>\n <jats:p>This was an open-label, prospective, randomized, placebo-controlled clinical trial.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Setting</jats:title>\n <jats:p>The study was conducted in King Saud University Medical City, Riyadh, Saudi Arabia, from August 2021 to July 2022.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Participants diagnosed with SARS-CoV-2 were randomly assigned to one of three groups, with participants receiving either 0.23% PVP-I, 0.9% normal saline (NS) nasal rinses and mouth washes, or no intervention (control group). Nasopharyngeal swabs were taken 4, 8, 12, and 18 days after the first swab to measure the detectability of the virus and the Ct.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 19 participants were involved in this study. The mean viral survival was 9.8, 12, and 12.6 days for the PVP-I, NS, and control groups, respectively, with a statistically significant difference (<jats:italic>p</jats:italic> = 0.046). The Ct mean values were 23 ± 3.4, 23.5 ± 6.3, and 26.3 ± 5.9 at the time of recruitment and 25.2 ± 3.5, 15 ± 11.7, and 26.9 ± 6.4 after 4 days for the PVP-I, NS, and control groups, respectively.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>When used continuously at a concentration of 0.23%, PVP-I showed promising results in terms of decreasing the pandemic burden by reducing the period of infectiousness and viral load. However, the use of PVP-I did not result in significantly different changes in the quality-of-life parameters in recently vaccinated and mild COVID-19 patients.</jats:p>\n </jats:sec>",
"alternative-id": [
"9137"
],
"article-number": "271",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "6 October 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "14 February 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "1 March 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The study was approved by the King Saud University Institutional Review Board and the Saudi Food and Drug Authority Clinical Trial Unit. All patients who met the inclusion criteria signed a written informed consent form that mentioned possible risks and benefits associated with the intervention."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1236-2098",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alsaleh",
"given": "Saad",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-4754-0293",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alhussien",
"given": "Ahmed",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2495-7523",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alyamani",
"given": "Abduljabbar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9655-9698",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alhussain",
"given": "Fahad",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2074-8916",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alhijji",
"given": "Ali",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5425-9531",
"affiliation": [],
"authenticated-orcid": false,
"family": "Binkhamis",
"given": "Khalifa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5075-7392",
"affiliation": [],
"authenticated-orcid": false,
"family": "Khan",
"given": "Anas",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3116-6559",
"affiliation": [],
"authenticated-orcid": false,
"family": "Javer",
"given": "Amin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4646-7488",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alshahrani",
"given": "Fatimah S.",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T10:02:24Z",
"timestamp": 1709287344000
},
"deposited": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T10:02:52Z",
"timestamp": 1709287372000
},
"indexed": {
"date-parts": [
[
2024,
3,
2
]
],
"date-time": "2024-03-02T00:28:52Z",
"timestamp": 1709339332222
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
3,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T00:00:00Z",
"timestamp": 1709251200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T00:00:00Z",
"timestamp": 1709251200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-09137-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-024-09137-y/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-09137-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
3,
1
]
]
},
"published-online": {
"date-parts": [
[
2024,
3,
1
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "9137_CR1",
"unstructured": "WHO Director-General’s remarks at the media briefing. on 2019-nCoV on 11 February 2020 [Internet]. [cited 2022 Oct 1]. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020."
},
{
"key": "9137_CR2",
"unstructured": "COVID-19 Map. - Johns Hopkins Coronavirus Resource Center [Internet]. [cited 2022 Oct 1]. Available from: https://coronavirus.jhu.edu/map.html."
},
{
"DOI": "10.1016/j.jaut.2020.102433",
"author": "HA Rothan",
"doi-asserted-by": "publisher",
"first-page": "102433",
"journal-title": "J Autoimmun",
"key": "9137_CR3",
"unstructured": "Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433.",
"volume": "109",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03120-0",
"author": "W Ni",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Crit Care",
"key": "9137_CR4",
"unstructured": "Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24(1):1–10.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"author": "R Wölfel",
"doi-asserted-by": "publisher",
"first-page": "465",
"issue": "7809",
"journal-title": "Nature",
"key": "9137_CR5",
"unstructured": "Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–9.",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2001737",
"author": "L Zou",
"doi-asserted-by": "publisher",
"first-page": "1177",
"issue": "12",
"journal-title": "N Engl J Med",
"key": "9137_CR6",
"unstructured": "Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–9.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1017/S0022172400040225",
"author": "BA Saggers",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "4",
"journal-title": "Epidemiol Infect",
"key": "9137_CR7",
"unstructured": "Saggers BA, Stewart GT. Polyvinyl-pyrrolidone-iodine: an assessment of antibacterial activity. Epidemiol Infect. 1964;62(4):509–18.",
"volume": "62",
"year": "1964"
},
{
"author": "MS Amin",
"first-page": "5",
"issue": "1",
"journal-title": "Pediatr Dent",
"key": "9137_CR8",
"unstructured": "Amin MS, Harrison RL, Benton TS, Roberts M, Weinstein P. Effect of povidone-iodine on Streptococcus mutans in children with extensive dental caries. Pediatr Dent. 2004;26(1):5–10.",
"volume": "26",
"year": "2004"
},
{
"DOI": "10.4103/SJOH.SJOH_36_20",
"doi-asserted-by": "crossref",
"key": "9137_CR9",
"unstructured": "Farahat M, Mesallam T, Alrasheed A, Malki K. Validity and reliability of the arabic version of the wisconsin upper respiratory symptom survey (AWURSS-11). Saudi Journal of Otorhinolaryngology Head and Neck Surgery [Internet]. 2021;23(1):26–30. Available from: https://www.sjohns.org/article.asp?issn=1319-8491."
},
{
"DOI": "10.5144/0256-4947.2018.22",
"author": "F Alanazy",
"doi-asserted-by": "publisher",
"first-page": "22",
"issue": "1",
"journal-title": "Ann Saudi Med",
"key": "9137_CR10",
"unstructured": "Alanazy F, Al Dousary S, Albosaily A, Aldriweesh T, Alsaleh S, Aldrees T. Psychometric arabic sino-nasal outcome Test-22: validation and translation in chronic rhinosinusitis patients. Ann Saudi Med. 2018;38(1):22–7.",
"volume": "38",
"year": "2018"
},
{
"DOI": "10.1042/CS20050086",
"author": "SA Bustin",
"doi-asserted-by": "publisher",
"first-page": "365",
"issue": "4",
"journal-title": "Clin Sci",
"key": "9137_CR11",
"unstructured": "Bustin SA, Mueller R. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin Sci. 2005;109(4):365–79.",
"volume": "109",
"year": "2005"
},
{
"DOI": "10.1002/pst.185",
"author": "SA Julious",
"doi-asserted-by": "publisher",
"first-page": "287",
"issue": "4",
"journal-title": "Pharm Statistics: J Appl Stat Pharm Ind",
"key": "9137_CR12",
"unstructured": "Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Statistics: J Appl Stat Pharm Ind. 2005;4(4):287–91.",
"volume": "4",
"year": "2005"
},
{
"DOI": "10.7326/0003-4819-134-8-200104170-00011",
"author": "D Moher",
"doi-asserted-by": "publisher",
"first-page": "657",
"issue": "8",
"journal-title": "Ann Intern Med",
"key": "9137_CR13",
"unstructured": "Moher D, Schulz KF, Altman DG, Group* C. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134(8):657–62.",
"volume": "134",
"year": "2001"
},
{
"DOI": "10.3390/ijerph191912148",
"author": "M Idrees",
"doi-asserted-by": "publisher",
"first-page": "12148",
"issue": "19",
"journal-title": "Int J Environ Res Public Health",
"key": "9137_CR14",
"unstructured": "Idrees M, McGowan B, Fawzy A, Abuderman AA, Balasubramaniam R, Kujan O. Efficacy of Mouth rinses and nasal spray in the inactivation of SARS-CoV-2: a systematic review and Meta-analysis of in Vitro and in vivo studies. Int J Environ Res Public Health. 2022;19(19):12148.",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1177/0145561320957237",
"author": "JS Pelletier",
"doi-asserted-by": "publisher",
"first-page": "192S",
"issue": "2suppl",
"journal-title": "Ear Nose Throat J",
"key": "9137_CR15",
"unstructured": "Pelletier JS, Tessema B, Frank S, Westover JB, Brown SM, Capriotti JA. Efficacy of povidone-iodine nasal and oral antiseptic preparations against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). Ear Nose Throat J. 2021;100(2suppl):192S–6.",
"volume": "100",
"year": "2021"
},
{
"DOI": "10.1001/jamaoto.2020.3053",
"author": "S Frank",
"doi-asserted-by": "publisher",
"first-page": "1054",
"issue": "11",
"journal-title": "JAMA Otolaryngology–Head Neck Surg",
"key": "9137_CR16",
"unstructured": "Frank S, Brown SM, Capriotti JA, Westover JB, Pelletier JS, Tessema B. In vitro efficacy of a povidone-iodine nasal antiseptic for rapid inactivation of SARS-CoV-2. JAMA Otolaryngology–Head Neck Surg. 2020;146(11):1054–8.",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1101/2020.05.18.103184",
"doi-asserted-by": "crossref",
"key": "9137_CR17",
"unstructured": "Liang B, Yuan X, Wei G, Wang W, Zhang M, Peng H et al. In-vivo toxicity studies and in-vitro inactivation of SARS-CoV-2 by povidone-iodine in-situ gel forming formulations. BioRxiv. 2020."
},
{
"DOI": "10.1177/0145561320932318",
"author": "S Frank",
"doi-asserted-by": "publisher",
"first-page": "586",
"issue": "9",
"journal-title": "Ear Nose Throat J",
"key": "9137_CR18",
"unstructured": "Frank S, Capriotti J, Brown SM, Tessema B. Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 era. Ear Nose Throat J. 2020;99(9):586–93.",
"volume": "99",
"year": "2020"
},
{
"DOI": "10.1007/s15010-020-01563-9",
"author": "CJ Seneviratne",
"doi-asserted-by": "publisher",
"first-page": "305",
"issue": "2",
"journal-title": "Infection",
"key": "9137_CR19",
"unstructured": "Seneviratne CJ, Balan P, Ko KKK, Udawatte NS, Lai D, Ng DHL, et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection. 2021;49(2):305–11.",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1097/MD.0000000000028925",
"doi-asserted-by": "crossref",
"key": "9137_CR20",
"unstructured": "Natto ZS, Bakhrebah MA, Afeef M, Al-Harbi S, Nassar MS, Alhetheel AF et al. The short-term effect of different chlorhexidine forms versus povidone iodine mouth rinse in minimizing the oral SARS-CoV-2 viral load: an open label randomized controlled clinical trial study. Medicine. 2022;101(30)."
},
{
"DOI": "10.1016/j.amjoto.2022.103549",
"author": "PJ Fantozzi",
"doi-asserted-by": "publisher",
"first-page": "103549",
"issue": "6",
"journal-title": "Am J Otolaryngol",
"key": "9137_CR21",
"unstructured": "Fantozzi PJ, Pampena E, Pierangeli A, Oliveto G, Sorrentino L, di Vanna D, et al. Efficacy of antiseptic mouthrinses against SARS-CoV-2: a prospective randomized placebo-controlled pilot study. Am J Otolaryngol. 2022;43(6):103549.",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.1016/j.jebdp.2021.101584",
"author": "R Elzein",
"doi-asserted-by": "publisher",
"first-page": "101584",
"issue": "3",
"journal-title": "J Evid Based Dent Pract",
"key": "9137_CR22",
"unstructured": "Elzein R, Abdel-Sater F, Fakhreddine S, Abi Hanna P, Feghali R, Hamad H, et al. In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial. J Evid Based Dent Pract. 2021;21(3):101584.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.adaj.2021.05.021",
"author": "P Chaudhary",
"doi-asserted-by": "publisher",
"first-page": "903",
"issue": "11",
"journal-title": "J Am Dent Association",
"key": "9137_CR23",
"unstructured": "Chaudhary P, Melkonyan A, Meethil A, Saraswat S, Hall DL, Cottle J, et al. Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load: a randomized controlled trial. J Am Dent Association. 2021;152(11):903–8.",
"volume": "152",
"year": "2021"
},
{
"DOI": "10.1186/s13063-020-04963-2",
"author": "MJ Hasan",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Trials",
"key": "9137_CR24",
"unstructured": "Hasan MJ, Rumi SK, Banu SS, Uddin AKM, Islam MS, Arefin MK. Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: a structured summary of a study protocol for an open-label randomized clinical trial. Trials. 2021;22(1):1–2.",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1002/lary.29935",
"author": "D Zarabanda",
"doi-asserted-by": "publisher",
"first-page": "2089",
"issue": "11",
"journal-title": "Laryngoscope",
"key": "9137_CR25",
"unstructured": "Zarabanda D, Vukkadala N, Phillips KM, Qian ZJ, Mfuh KO, Hatter MJ, et al. The Effect of Povidone-Iodine nasal spray on nasopharyngeal SARS‐CoV‐2 viral load: a Randomized Control Trial. Laryngoscope. 2022;132(11):2089–95. https://doi.org/10.1002/lary.29935",
"volume": "132",
"year": "2022"
},
{
"DOI": "10.1007/s40121-020-00324-3",
"author": "SN Rao",
"doi-asserted-by": "publisher",
"first-page": "573",
"issue": "3",
"journal-title": "Infect Dis Ther",
"key": "9137_CR26",
"unstructured": "Rao SN, Manissero D, Steele VR, Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther. 2020;9(3):573–86.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.3390/v13091882",
"author": "E Domingo",
"doi-asserted-by": "publisher",
"first-page": "1882",
"issue": "9",
"journal-title": "Viruses",
"key": "9137_CR27",
"unstructured": "Domingo E, García-Crespo C, Lobo-Vega R, Perales C. Mutation rates, mutation frequencies, and proofreading-repair activities in RNA virus genetics. Viruses. 2021;13(9):1882.",
"volume": "13",
"year": "2021"
},
{
"author": "M Eggers",
"first-page": "249",
"issue": "2",
"journal-title": "Infect DisTher",
"key": "9137_CR28",
"unstructured": "Eggers M, Koburger 一 Janssen T, Eickmann M, et al. H Diro bactericidal and virucidal efficacy of povidone 一 iodine gargle/mouthwashagainstrespiratoryand oraltract pathogens J. Infect DisTher. 2018;7(2):249–59.",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1210/jcem-66-3-632",
"author": "ADERAW PAUL",
"doi-asserted-by": "publisher",
"first-page": "632",
"issue": "3",
"journal-title": "J Clin Endocrinol Metab",
"key": "9137_CR29",
"unstructured": "Paul Aderaw, Reinhardt TL, Safran W, Pino M, McARTHUR Sam. Effect of mouth rinsing with two polyvinylpyrrolidone-iodine mixtures on iodine absorption and thyroid function. J Clin Endocrinol Metab. 1988;66(3):632–5.",
"volume": "66",
"year": "1988"
},
{
"DOI": "10.1001/jamaoto.2020.5490",
"author": "J Guenezan",
"doi-asserted-by": "publisher",
"first-page": "400",
"issue": "4",
"journal-title": "JAMA Otolaryngology–Head Neck Surg",
"key": "9137_CR30",
"unstructured": "Guenezan J, Garcia M, Strasters D, Jousselin C, Lévêque N, Frasca D, et al. Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: a randomized clinical trial. JAMA Otolaryngology–Head Neck Surg. 2021;147(4):400–1.",
"volume": "147",
"year": "2021"
},
{
"DOI": "10.1016/j.ijom.2010.09.012",
"author": "G Arakeri",
"doi-asserted-by": "publisher",
"first-page": "173",
"issue": "2",
"journal-title": "Int J Oral Maxillofac Surg",
"key": "9137_CR31",
"unstructured": "Arakeri G, Brennan PA. Povidone-iodine: an anti-oedematous agent? Int J Oral Maxillofac Surg. 2011;40(2):173–6.",
"volume": "40",
"year": "2011"
},
{
"DOI": "10.1002/lary.29818",
"author": "P Wu",
"doi-asserted-by": "publisher",
"first-page": "1148",
"issue": "6",
"journal-title": "Laryngoscope",
"key": "9137_CR32",
"unstructured": "Wu P, Cheng P, Chang C, Lo W, Cheng P. Efficacy of povidone-iodine nasal irrigation solution after sinonasal surgery: a randomized controlled study. Laryngoscope. 2022;132(6):1148–52.",
"volume": "132",
"year": "2022"
},
{
"DOI": "10.1016/j.amjoto.2020.102604",
"author": "VS Lee",
"doi-asserted-by": "publisher",
"first-page": "102604",
"issue": "5",
"journal-title": "Am J Otolaryngol",
"key": "9137_CR33",
"unstructured": "Lee VS, Pottinger PS, Davis GE. Tolerability and effectiveness of povidone-iodine or mupirocin versus saline sinus irrigations for chronic rhinosinusitis. Am J Otolaryngol. 2020;41(5):102604.",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.3390/vaccines9090989",
"author": "YJ Fan",
"doi-asserted-by": "publisher",
"first-page": "989",
"issue": "9",
"journal-title": "Vaccines (Basel)",
"key": "9137_CR34",
"unstructured": "Fan YJ, Chan KH, Hung IFN. Safety and efficacy of COVID-19 vaccines: a systematic review and meta-analysis of different vaccines at phase 3. Vaccines (Basel). 2021;9(9):989.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(20)30237-1",
"author": "GM Joynt",
"doi-asserted-by": "publisher",
"first-page": "635",
"issue": "6",
"journal-title": "Lancet Infect Dis",
"key": "9137_CR35",
"unstructured": "Joynt GM, Wu WKK, Understanding. COVID-19: what does viral RNA load really mean? Lancet Infect Dis. 2020;20(6):635–6.",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2022.35837",
"doi-asserted-by": "publisher",
"key": "9137_CR36",
"unstructured": "Levy AG, Thorpe A, Scherer LD, Scherer AM, Drews FA, Butler JM et al. Misrepresentation and Nonadherence Regarding COVID-19 Public Health Measures. JAMA Netw Open [Internet]. 2022;5(10):e2235837–e2235837. https://doi.org/10.1001/jamanetworkopen.2022.35837."
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-024-09137-y"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": "Efficacy of povidone-iodine nasal rinse and mouth wash in COVID-19 management: a prospective, randomized pilot clinical trial (povidone-iodine in COVID-19 management)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}