The Effect of Povidone-Iodine Nasal Spray on COVID-19 Nasopharyngeal Viral Load in Patients: A Randomized Control Trial
et al., Laryngoscope, doi:10.1002/lary.29935, Nov 2021
PVP-I for COVID-19
15th treatment shown to reduce risk in
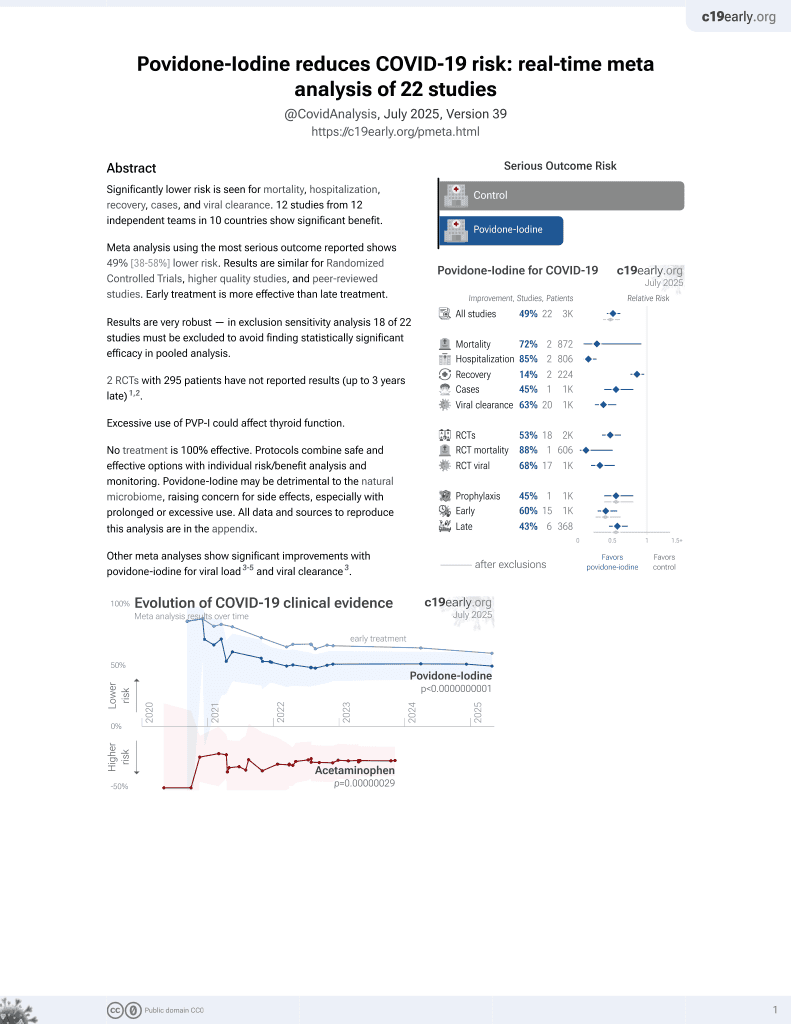
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
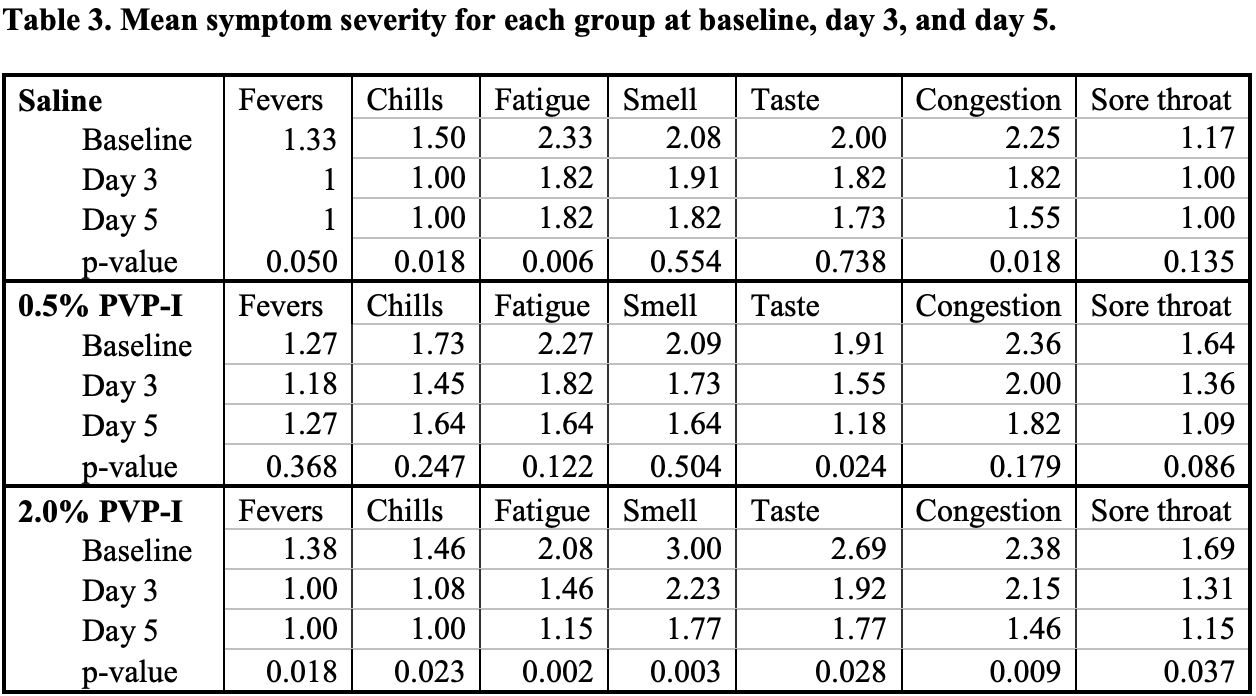
Very late treatment (7 days from onset) RCT comparing 11 & 13 PVP-I (0.5% and 2%), and 11 saline spray patients in the USA, showing no significant differences. There was no control group (saline is likely not a placebo, showing efficacy in other trials). There are large unadjusted differences between groups, e.g. 7.1 days from onset for PVP-I versus 4.8 for saline. Baseline Ct was higher for PVP-I, providing less room for improvement. Authors note that they cannot determine if earlier use is more beneficial.
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects (early treatment may be more beneficial).
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of no recovery, 26.9% higher, RR 1.27, p = 1.00, treatment 3 of 13 (23.1%), control 2 of 11 (18.2%), 2%.
|
|
risk of no recovery, 50.0% higher, RR 1.50, p = 1.00, treatment 3 of 11 (27.3%), control 2 of 11 (18.2%), 0.5%.
|
|
risk of no viral clearance, no change, RR 1.00, p = 1.00, treatment 2 of 7 (28.6%), control 2 of 7 (28.6%), day 5, minus strand PCR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zarabanda et al., 1 Nov 2021, Randomized Controlled Trial, USA, peer-reviewed, 13 authors, average treatment delay 7.0 days, this trial compares with another treatment - results may be better when compared to placebo.
The Effect of Povidone‐Iodine Nasal Spray on Nasopharyngeal SARS‐CoV‐2 Viral Load: A Randomized Control Trial
The Laryngoscope, doi:10.1002/lary.29935
Objectives To determine the effect of povidone-iodine (PVP-I) nasal sprays on nasopharyngeal (NP) viral load as assessed by cycle threshold on quantitative polymerase chain reaction (qPCR) of SARS-CoV-2 in outpatients.
Study Design Three arm, triple blinded, randomized, placebo-controlled clinical trial.
Methods Participants were randomized within 5 days of testing positive for COVID-19 to receive nasal sprays containing either placebo (0.9% saline), 0.5% PVP-I, or 2.0% PVP-I. NP swabs for qPCR analysis were taken at baseline, 1-hour post-PVP-I spray (2 sprays/nostril),
References
Anderson, David, Scholz, Efficacy of skin and nasal povidone-iodine preparation against mupirocin-resistant methicillin-resistant Staphylococcus aureus and S. aureus within the anterior nares, Antimicrob Agents Chemother, doi:10.1128/AAC.04624-14
Anderson, Sivalingam, Kang, Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease, Infect Dis Ther, doi:10.1007/s40121-020-00316-3
Baden, Sahly, Essink, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, N Engl J Med, doi:10.1056/NEJMoa2035389
Cevik, Tate, Lloyd, Maraolo, Schafers et al., SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis, The Lancet Microbe, doi:10.1016/S2666-5247(20)30172-5
Corman, Landt, Kaiser, Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Eurosurveillance, doi:10.2807/1560-7917.ES.2020.25.3.2000045
Doty, Shaman, Dann, Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function, Physiol Behav, doi:10.1016/0031-9384(84)90269-5
Fajnzylber, Regan, Coxen, SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat Commun, doi:10.1038/s41467-020-19057-5
Forum, More on Betadine from JAMA
Frank, Brown, Capriotti, Westover, Pelletier et al., In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2, JAMA Otolaryngol -Head Neck Surg, doi:10.1001/jamaoto.2020.3053
Gluck, Martin, Bosse, Reimer, Mueller, A clinical study on the tolerability of a liposomal povidone-iodine nasal spray: Implications for further development, ORL, doi:10.1159/000097758
Guenezan, Garcia, Strasters, Povidone Iodine Mouthwash, Gargle, and Nasal Spray to Reduce Nasopharyngeal Viral Load in Patients With COVID-19, JAMA Otolaryngol Neck Surg, doi:10.1001/jamaoto.2020.5490
Hogan, Huang, Sahoo, Strand-Specific Reverse Transcription PCR for Detection of Replicating SARS-CoV-2, Emerg Infect Dis, doi:10.3201/eid2702.204168
Hogan, Sahoo, Huang, Comparison of Panther Fusion and a laboratory-developed test targeting the envelope gene for detection of SARS-CoV-2, J Clin Virol, doi:10.1016/j.jcv.2020.104383
Kirk-Bayley, Challacombe, Sunkaraneni, Combes, The Use of Povidone Iodine Nasal Spray and Mouthwash During the Current COVID-19 Pandemic May Protect Healthcare Workers and Reduce Cross Infection, SSRN Electron J, doi:10.2139/ssrn.3563092
Lee, Nakayama, Wu, ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs, Nat Commun, doi:10.1038/s41467-020-19145-6
Mangram, Horan, Pearson, Silver, Jarvis, Guideline for Prevention of Surgical Site Infection, 1999, Infect Control Hosp Epidemiol, doi:10.1086/501620
Polack, Thomas, Kitchin, Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577
Sungnak, Huang, Bécavin, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med, doi:10.1038/s41591-020-0868-6
Urbaniak, Plous, Research Randomizer
Yan, Faraji, Prajapati, Boone, Deconde, Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms, Int Forum Allergy Rhinol, doi:10.1002/alr.22579
Zacharioudakis, Prasad, Zervou, Association of SARS-CoV-2 Genomic Load with COVID-19 Patient Outcomes, Ann Am Thorac Soc, doi:10.1513/AnnalsATS.202008-931RL
DOI record:
{
"DOI": "10.1002/lary.29935",
"ISSN": [
"0023-852X",
"1531-4995"
],
"URL": "http://dx.doi.org/10.1002/lary.29935",
"abstract": "<jats:sec><jats:title>Objectives/Hypothesis</jats:title><jats:p>To determine the effect of povidone‐iodine (PVP‐I) nasal sprays on nasopharyngeal (NP) viral load as assessed by cycle threshold (Ct) on quantitative polymerase chain reaction (qPCR) of SARS‐CoV‐2 in outpatients.</jats:p></jats:sec><jats:sec><jats:title>Study Design</jats:title><jats:p>Three arm, triple blinded, randomized, placebo‐controlled clinical trial.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Participants were randomized within 5 days of testing positive for COVID‐19 to receive nasal sprays containing placebo (0.9% saline), 0.5% PVP‐I, or 2.0% PVP‐I. NP swabs for qPCR analysis were taken at baseline, 1‐hour post‐PVP‐I spray (two sprays/nostril), and 3 days post‐PVP‐I spray (20 sprays/nostril). Symptom and adverse event questionnaires were completed at baseline, day 3, and day 5. University of Pennsylvania Smell Identification Tests (UPSIT) were completed at baseline and day 30.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Mean Ct values increased over time in all groups, indicating declining viral loads, with no statistically significant difference noted in the rate of change between placebo and PVP‐I groups. The 2.0% PVP‐I group showed statistically significant improvement in all symptom categories; however, it also reported a high rate of nasal burning. Olfaction via UPSIT showed improvement by at least one category in all groups. There were no hospitalizations or mortalities within 30 days of study enrollment.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Saline and low concentration PVP‐I nasal sprays are well tolerated. Similar reductions in SARS‐CoV‐2 NP viral load were seen over time in all groups. All treatment groups showed improvement in olfaction over 30 days. These data suggest that dilute versions of PVP‐I nasal spray are safe for topical use in the nasal cavity, but that PVP‐I does not demonstrate virucidal activity in COVID‐19 positive outpatients.</jats:p></jats:sec><jats:sec><jats:title>Level of Evidence</jats:title><jats:p>2 <jats:italic>Laryngoscope</jats:italic>, 132:2089–2095, 2022</jats:p></jats:sec>",
"alternative-id": [
"10.1002/lary.29935"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-08-31"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-10-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-11-09"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Otolaryngology‐Head and Neck Surgery Stanford University School of Medicine Stanford California U.S.A."
}
],
"family": "Zarabanda",
"given": "David",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-1787-935X",
"affiliation": [
{
"name": "Department of Otolaryngology‐Head and Neck Surgery Stanford University School of Medicine Stanford California U.S.A."
}
],
"authenticated-orcid": false,
"family": "Vukkadala",
"given": "Neelaysh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Otolaryngology‐Head and Neck Surgery University of Cincinnati School of Medicine Cincinnati Ohio U.S.A."
}
],
"family": "Phillips",
"given": "Katie M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4150-1023",
"affiliation": [
{
"name": "Department of Otolaryngology‐Head and Neck Surgery Stanford University School of Medicine Stanford California U.S.A."
}
],
"authenticated-orcid": false,
"family": "Qian",
"given": "Z. Jason",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Virology Laboratory Stanford Health Care Stanford California U.S.A."
}
],
"family": "Mfuh",
"given": "Kenji O.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Otolaryngology‐Head and Neck Surgery Stanford University School of Medicine Stanford California U.S.A."
}
],
"family": "Hatter",
"given": "Matthew J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Otolaryngology‐Head and Neck Surgery Stanford University School of Medicine Stanford California U.S.A."
},
{
"name": "Division of Allergy and Immunology, Department of Pediatrics Stanford University School of Medicine Stanford California U.S.A."
}
],
"family": "Lee",
"given": "Ivan T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Divisions of Cardiothoracic Anesthesiology and Critical Medicine, Department of Anesthesiology, Perioperative and Pain Medicine Stanford University School of Medicine Stanford California U.S.A."
}
],
"family": "Rao",
"given": "Vidya K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Otolaryngology‐Head and Neck Surgery Stanford University School of Medicine Stanford California U.S.A."
}
],
"family": "Hwang",
"given": "Peter H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Shasta‐ENT Medical Group Redding California U.S.A."
}
],
"family": "Domb",
"given": "George",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2072-982X",
"affiliation": [
{
"name": "Department of Otolaryngology‐Head and Neck Surgery Stanford University School of Medicine Stanford California U.S.A."
}
],
"authenticated-orcid": false,
"family": "Patel",
"given": "Zara M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Virology Laboratory Stanford Health Care Stanford California U.S.A."
},
{
"name": "Department of Pathology Stanford University School of Medicine Stanford California U.S.A."
},
{
"name": "Division of Infectious Diseases and Geographic Medicine, Department of Medicine Stanford University School of Medicine Stanford California U.S.A."
}
],
"family": "Pinsky",
"given": "Benjamin A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4454-0861",
"affiliation": [
{
"name": "Department of Otolaryngology‐Head and Neck Surgery Stanford University School of Medicine Stanford California U.S.A."
},
{
"name": "Department of Otolaryngology‐Head and Neck Surgery Veterans Affairs Palo Alto Health Care System Palo Alto California U.S.A."
}
],
"authenticated-orcid": false,
"family": "Nayak",
"given": "Jayakar V.",
"sequence": "additional"
}
],
"container-title": "The Laryngoscope",
"container-title-short": "The Laryngoscope",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
11,
1
]
],
"date-time": "2021-11-01T14:14:45Z",
"timestamp": 1635776085000
},
"deposited": {
"date-parts": [
[
2023,
8,
22
]
],
"date-time": "2023-08-22T04:13:04Z",
"timestamp": 1692677584000
},
"indexed": {
"date-parts": [
[
2024,
3,
30
]
],
"date-time": "2024-03-30T12:27:53Z",
"timestamp": 1711801673607
},
"is-referenced-by-count": 12,
"issue": "11",
"issued": {
"date-parts": [
[
2021,
11,
9
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2022,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
9
]
],
"date-time": "2021-11-09T00:00:00Z",
"timestamp": 1636416000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/lary.29935",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/lary.29935",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/lary.29935",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "2089-2095",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2021,
11,
9
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
9
]
]
},
"published-print": {
"date-parts": [
[
2022,
11
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1056/NEJMoa2034577",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_2_1"
},
{
"DOI": "10.1056/NEJMoa2035389",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_3_1"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_4_1"
},
{
"DOI": "10.1038/s41467-020-19145-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_5_1"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_6_1"
},
{
"DOI": "10.1513/AnnalsATS.202008-931RL",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_7_1"
},
{
"DOI": "10.1128/AAC.04624-14",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_8_1"
},
{
"DOI": "10.1086/501620",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_9_1"
},
{
"DOI": "10.1007/s40121-020-00316-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_10_1"
},
{
"DOI": "10.1001/jamaoto.2020.3053",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_11_1"
},
{
"DOI": "10.1159/000097758",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_12_1"
},
{
"DOI": "10.2139/ssrn.3563092",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_13_1"
},
{
"DOI": "10.1002/alr.22579",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_14_1"
},
{
"DOI": "10.1001/jamaoto.2020.5490",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_15_1"
},
{
"key": "e_1_2_6_16_1",
"unstructured": "UrbaniakG PlousS.Research Randomizer.2013. Available at:http://www.randomizer.org/"
},
{
"DOI": "10.1016/0031-9384(84)90269-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_17_1"
},
{
"DOI": "10.1016/j.jcv.2020.104383",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_18_1"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_19_1"
},
{
"DOI": "10.3201/eid2702.204168",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_20_1"
},
{
"key": "e_1_2_6_21_1",
"unstructured": "AAO‐HNS Forum.More on betadine from JAMA.2020. Available at:https://entconnect.entnet.org"
},
{
"DOI": "10.1016/S2666-5247(20)30172-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_22_1"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/lary.29935"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Otorhinolaryngology"
],
"subtitle": [],
"title": "The Effect of <scp>Povidone‐Iodine</scp> Nasal Spray on Nasopharyngeal SARS‐CoV‐2 Viral Load: A Randomized Control Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "132"
}