In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2
et al., JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.3053, Sep 2020
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing povidone-iodine nasal antiseptics at concentrations (0.5%, 1.25%, and 2.5%) completely inactivated SARS-CoV-2 within 15 seconds of contact. No cytotoxic effects on cells were observed after contact with each of the nasal antiseptics tested.
9 preclinical studies support the efficacy of povidone-iodine for COVID-19:
1.
Xu et al., Differential Effects of Antiseptic Mouth Rinses on SARS-CoV-2 Infectivity In Vitro, Pathogens, doi:10.3390/pathogens10030272.
2.
Tucker et al., In vitro inactivation of SARS-CoV-2 with 0.5% povidone iodine nasal spray (Nasodine) at clinically relevant concentrations and timeframes using tissue culture and PCR based assays, bioRxiv, doi:10.1101/2021.01.31.426979.
3.
Pelletier et al., Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2), Ear, Nose & Throat Journal, doi:10.1177/0145561320957237.
4.
Frank et al., In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.3053.
5.
Meister et al., Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaa471.
6.
Anderson et al., Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease, Infectious Diseases and Therapy, doi:10.1007/s40121-020-00316-3.
7.
Hassandarvish et al., Povidone iodine gargle and mouthwash, British Dental Journal volume, doi:10.1038/s41415-020-1794-1.
Frank et al., 17 Sep 2020, peer-reviewed, 6 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2
JAMA Otolaryngology–Head & Neck Surgery, doi:10.1001/jamaoto.2020.3053
needed to demonstrate the efficacy of nasal povidone-iodine (PVP-I) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). OBJECTIVE To evaluate the in vitro efficacy of PVP-I nasal antiseptic for the inactivation of SARS-CoV-2 at clinically significant contact times of 15 and 30 seconds.
INTERVENTIONS The SARS-CoV-2, USA-WA1/2020 strain, virus stock was tested against nasal antiseptic solutions consisting of aqueous PVP-I as the sole active ingredient. Povidone-iodine was tested at diluted concentrations of 0.5%, 1.25%, and 2.5% and compared with controls. The test solutions and virus were incubated at mean (SD) room temperature of 22 (2) °C for time periods of 15 and 30 seconds. DESIGN AND SETTING This controlled in vitro laboratory research study used 3 different concentrations of study solution and ethanol, 70%, as a positive control on test media infected with SARS-CoV-2. Test media without virus were added to 2 tubes of the compounds to serve as toxicity and neutralization controls. Ethanol, 70%, was tested in parallel as a positive control and water only as a negative control.
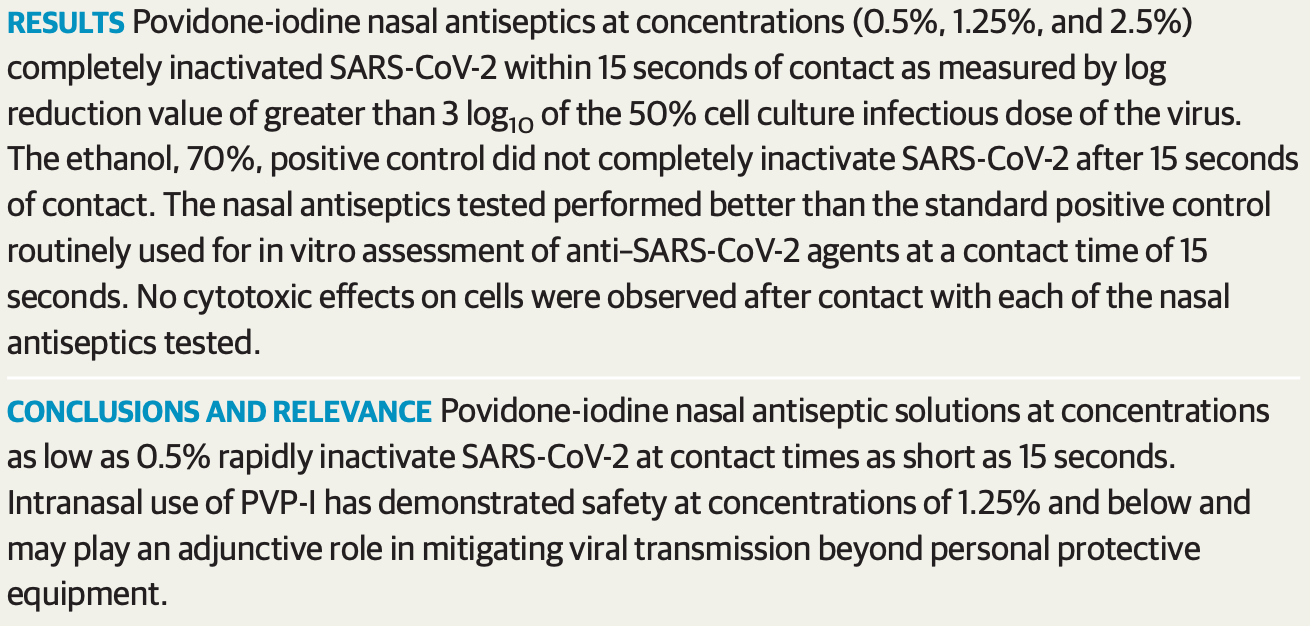
MAIN OUTCOMES AND MEASURES The primary study outcome measurement was the log reduction value after 15 seconds and 30 seconds of given treatment. Surviving virus from each sample was quantified by standard end point dilution assay, and the log reduction value of each compound was compared with the negative (water) control. RESULTS Povidone-iodine nasal antiseptics at concentrations (0.5%, 1.25%, and 2.5%) completely inactivated SARS-CoV-2 within 15 seconds of contact as measured by log reduction value of greater than 3 log 10 of the 50% cell culture infectious dose of the virus. The ethanol, 70%, positive control did not completely inactivate SARS-CoV-2 after 15 seconds of contact. The nasal antiseptics tested performed better than the standard positive control routinely used for in vitro assessment of anti-SARS-CoV-2 agents at a contact time of 15 seconds. No cytotoxic effects on cells were observed after contact with each of the nasal antiseptics tested. CONCLUSIONS AND RELEVANCE Povidone-iodine nasal antiseptic solutions at concentrations as low as 0.5% rapidly inactivate SARS-CoV-2 at contact times as short as 15 seconds. Intranasal use of PVP-I has demonstrated safety at concentrations of 1.25% and below and may play an adjunctive role in mitigating viral transmission beyond personal protective equipment.
ARTICLE INFORMATION Accepted for Publication: July 29, 2020. Published Online: September 17, 2020. doi:10.1001/jamaoto.2020.3053 Author Contributions: Drs Frank, Capriotti, and Tessema had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Funding/Support: The funding for the laboratory materials used in this study was supplied by Veloce BioPharma. Role of the Funder/Sponsor: Veloce BioPharma had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. However, the individual authors listed who are related to Veloce BioPharma did assist with design of the study and review of the manuscript.
References
Au-Duong, Vo, Lee, Bactericidal magnetic nanoparticles with iodine loaded on surface grafted poly(N-vinylpyrrolidone), J Mater Chem B, doi:10.1039/C4TB01516A
Balakrishnan, Schechtman, Hogikyan, Teoh, Mcgrath et al., COVID-19 pandemic: what every otolaryngologist-head and neck surgeon needs to know for safe airway management, Otolaryngol Head Neck Surg, doi:10.1177/0194599820919751
Bidra, Pelletier, Westover, Frank, Brown et al., Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse, Am J Epidemiol, doi:10.1093/oxfordjournals.aje.a118408
Challacombe, Kirk-Bayley, Sunkaraneni, Combes, Povidone iodine, Br Dent J, doi:10.1038/s41415-020-1589-4
Eggers, Farrales, Loya, Pura, Uy, The effect of 1% povidone iodine as a pre-procedural mouthrinse in 20 patients with varying degrees of oral hygiene, J Philipp Dent Assoc, doi:10.1007/s40121-019-00260-x
Eggers, Koburger-Janssen, Eickmann, Zorn, In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens, Infect Dis Ther, doi:10.1007/s40121-018-0200-7
Foley, The relationship between autoimmune thyroid disease and iodine intake: a review, Endokrynol Pol
Frank, Capriotti, Brown, Tessema, Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 era, Ear Nose Throat J. Published online
Furudate, Nishimaki, Muto, 125I uptake competing with iodine absorption by the thyroid gland following povidone-iodine skin application, Exp Anim, doi:10.1538/expanim.46.197
Gray, Katelaris, Lipson, Recurrent anaphylaxis caused by topical povidone-iodine (betadine), J Paediatr Child Health, doi:10.1111/jpc.12232
Hill, Casewell, The in-vitro activity of povidone-iodinecream against Staphylococcus aureus and its bioavailability in nasal secretions, J Hosp Infect, doi:10.1053/jhin.2000.0733
Hou, Okuda, Edwards, SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, Cell, doi:10.1016/j.cell.2020.05.042
Kariwa, Fujii, Takashima, Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents, Dermatology, doi:10.1159/000089211
Kim, Rimmer, Mrad, Ahmadzada, Harvey, Betadine has a ciliotoxic effect on ciliated human respiratory cells, J Laryngol Otol, doi:10.1017/S0022215114002746
Mady, Kubik, Baddour, Snyderman, Rowan, Consideration of povidone-iodine as a public health intervention for COVID-19: utilization as "personal protective equipment" for frontline providers exposed in high-risk head and neck and skull base oncology care, Oral Oncol, doi:10.1016/j.oraloncology.2020.104724
Mullings, Panchmatia, Samoy, Habib, Thamboo et al., Topical povidone-iodine as an adjunctive treatment for recalcitrant chronic rhinosinusitis, Eur J Rhinol Allergy, doi:10.5152/ejra.2019.166
Panchmatia, Payandeh, Al-Salman, The efficacy of diluted topical povidone-iodine rinses in the management of recalcitrant chronic rhinosinusitis: a prospective cohort study, Eur Arch Otorhinolaryngol, doi:10.1007/s00405-019-05628-w
Parhar, Tasche, Brody, Topical preparations to reduce SARS-CoV-2 aerosolization in head and neck mucosal surgery, Head Neck, doi:10.1002/hed.26200
Quadir, Zia, Needham, Toxicological implications of nasal formulations, Drug Deliv, doi:10.1080/107175499266823
Reimer, Wichelhaus, Schäfer, Antimicrobial effectiveness of povidone-iodine and consequences for new application areas, Dermatology, doi:10.1159/000057738
Richard, Van Den Brand, Bestebroer, Influenza A viruses are transmitted via the air from the nasal respiratory epithelium of ferrets, Nat Commun, doi:10.1038/s41467-020-14626-0
Sungnak, Huang, Bécavin, Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med, doi:10.1038/s41591-020-0868-6
Tessema, Frank, Bidra, SARS-CoV-2 viral inactivation using low dose povidone-iodine oral rinse-immediate application for the prosthodontic practice, J Prosthodont, doi:10.1111/jopr.13207
Van Doremalen, Bushmaker, Morris, Aerosol and surface stability of SARS-CoV-2 compared with SARS-CoV-1, N Engl J Med, doi:10.1056/NEJMc2004973
Workman, Cohen, The effect of drugs and other compounds on the ciliary beat frequency of human respiratory epithelium, Am J Rhinol Allergy, doi:10.2500/ajra.2014.28.4092
Workman, Welling, Carter, Endonasal instrumentation and aerosolization risk in the era of COVID-19: simulation, literature review, and proposed mitigation strategies, Int Forum Allergy Rhinol, doi:10.1002/alr.22577
Wölfel, Corman, Guggemos, Virological assessment of hospitalized patients with COVID-2019, Nature, doi:10.1038/s41586-020-2196-x
Zou, Ruan, Huang, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med, doi:10.1056/NEJMc2001737
DOI record:
{
"DOI": "10.1001/jamaoto.2020.3053",
"ISSN": [
"2168-6181"
],
"URL": "http://dx.doi.org/10.1001/jamaoto.2020.3053",
"author": [
{
"affiliation": [
{
"name": "University of Connecticut School of Medicine, Farmington"
}
],
"family": "Frank",
"given": "Samantha",
"sequence": "first"
},
{
"affiliation": [
{
"name": "University of Connecticut School of Medicine, Farmington"
},
{
"name": "ProHealth, Ear, Nose and Throat, Farmington, Connecticut"
}
],
"family": "Brown",
"given": "Seth M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Veloce BioPharma, Fort Lauderdale, Florida"
}
],
"family": "Capriotti",
"given": "Joseph A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Institute for Antiviral Research at Utah State University, Logan"
}
],
"family": "Westover",
"given": "Jonna B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ocean Ophthalmology, Miami, Florida"
}
],
"family": "Pelletier",
"given": "Jesse S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Connecticut School of Medicine, Farmington"
},
{
"name": "ProHealth, Ear, Nose and Throat, Farmington, Connecticut"
}
],
"family": "Tessema",
"given": "Belachew",
"sequence": "additional"
}
],
"container-title": "JAMA Otolaryngology–Head & Neck Surgery",
"container-title-short": "JAMA Otolaryngol Head Neck Surg",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
9,
17
]
],
"date-time": "2020-09-17T16:38:23Z",
"timestamp": 1600360703000
},
"deposited": {
"date-parts": [
[
2020,
11,
19
]
],
"date-time": "2020-11-19T18:29:52Z",
"timestamp": 1605810592000
},
"indexed": {
"date-parts": [
[
2024,
5,
7
]
],
"date-time": "2024-05-07T13:14:23Z",
"timestamp": 1715087663668
},
"is-referenced-by-count": 58,
"issue": "11",
"issued": {
"date-parts": [
[
2020,
11,
1
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2020,
11,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamaotolaryngology/articlepdf/2770785/jamaotolaryngology_frank_2020_oi_200049_1605715049.50571.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "1054",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2020,
11,
1
]
]
},
"published-print": {
"date-parts": [
[
2020,
11,
1
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1056/NEJMc2001737",
"article-title": "SARS-CoV-2 viral load in upper respiratory specimens of infected patients.",
"author": "Zou",
"doi-asserted-by": "publisher",
"first-page": "1177",
"issue": "12",
"journal-title": "N Engl J Med",
"key": "ooi200049r1",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"article-title": "SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes.",
"author": "Sungnak",
"doi-asserted-by": "publisher",
"first-page": "681",
"issue": "5",
"journal-title": "Nat Med",
"key": "ooi200049r2",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2004973",
"article-title": "Aerosol and surface stability of SARS-CoV-2 compared with SARS-CoV-1.",
"author": "van Doremalen",
"doi-asserted-by": "publisher",
"first-page": "1564",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "ooi200049r3",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1177/0194599820919751",
"article-title": "COVID-19 pandemic: what every otolaryngologist–head and neck surgeon needs to know for safe airway management.",
"author": "Balakrishnan",
"doi-asserted-by": "publisher",
"first-page": "804",
"issue": "6",
"journal-title": "Otolaryngol Head Neck Surg",
"key": "ooi200049r4",
"volume": "162",
"year": "2020"
},
{
"DOI": "10.1002/alr.v10.7",
"article-title": "Endonasal instrumentation and aerosolization risk in the era of COVID-19: simulation, literature review, and proposed mitigation strategies.",
"author": "Workman",
"doi-asserted-by": "publisher",
"first-page": "798",
"issue": "7",
"journal-title": "Int Forum Allergy Rhinol",
"key": "ooi200049r5",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"article-title": "SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract.",
"author": "Hou",
"doi-asserted-by": "publisher",
"first-page": "429",
"issue": "2",
"journal-title": "Cell",
"key": "ooi200049r6",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"article-title": "Virological assessment of hospitalized patients with COVID-2019.",
"author": "Wölfel",
"doi-asserted-by": "publisher",
"first-page": "465",
"issue": "7809",
"journal-title": "Nature",
"key": "ooi200049r7",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-14626-0",
"article-title": "Influenza A viruses are transmitted via the air from the nasal respiratory epithelium of ferrets.",
"author": "Richard",
"doi-asserted-by": "publisher",
"first-page": "766",
"issue": "1",
"journal-title": "Nat Commun",
"key": "ooi200049r8",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1111/jopr.13207",
"article-title": "SARS-CoV-2 viral inactivation using low dose povidone-iodine oral rinse—immediate application for the prosthodontic practice.",
"author": "Tessema",
"doi-asserted-by": "crossref",
"journal-title": "J Prosthodont",
"key": "ooi200049r9",
"year": "2020"
},
{
"DOI": "10.1016/j.oraloncology.2020.104724",
"article-title": "Consideration of povidone-iodine as a public health intervention for COVID-19: utilization as “personal protective equipment” for frontline providers exposed in high-risk head and neck and skull base oncology care.",
"author": "Mady",
"doi-asserted-by": "crossref",
"journal-title": "Oral Oncol",
"key": "ooi200049r10",
"volume": "105",
"year": "2020"
},
{
"DOI": "10.1002/hed.v42.6",
"article-title": "Topical preparations to reduce SARS-CoV-2 aerosolization in head and neck mucosal surgery.",
"author": "Parhar",
"doi-asserted-by": "publisher",
"first-page": "1268",
"issue": "6",
"journal-title": "Head Neck",
"key": "ooi200049r11",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.1038/s41415-020-1589-4",
"article-title": "Povidone iodine.",
"author": "Challacombe",
"doi-asserted-by": "publisher",
"first-page": "656",
"issue": "9",
"journal-title": "Br Dent J",
"key": "ooi200049r12",
"volume": "228",
"year": "2020"
},
{
"DOI": "10.1007/s40121-018-0200-7",
"article-title": "In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens.",
"author": "Eggers",
"doi-asserted-by": "publisher",
"first-page": "249",
"issue": "2",
"journal-title": "Infect Dis Ther",
"key": "ooi200049r13",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1159/000089211",
"article-title": "Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents.",
"author": "Kariwa",
"doi-asserted-by": "publisher",
"first-page": "119",
"journal-title": "Dermatology",
"key": "ooi200049r14",
"volume": "212",
"year": "2006"
},
{
"article-title": "Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse.",
"author": "Bidra",
"journal-title": "J Prosthodont",
"key": "ooi200049r15",
"year": "2020"
},
{
"DOI": "10.1093/oxfordjournals.aje.a118408",
"article-title": "A simple method of estimating fifty percent endpoints.",
"author": "Reed",
"doi-asserted-by": "publisher",
"first-page": "493",
"issue": "3",
"journal-title": "Am J Epidemiol",
"key": "ooi200049r16",
"volume": "27",
"year": "1938"
},
{
"DOI": "10.1159/000057738",
"article-title": "Antimicrobial effectiveness of povidone-iodine and consequences for new application areas.",
"author": "Reimer",
"doi-asserted-by": "publisher",
"first-page": "114",
"journal-title": "Dermatology",
"key": "ooi200049r17",
"volume": "204",
"year": "2002"
},
{
"DOI": "10.1017/S0022215114002746",
"article-title": "Betadine has a ciliotoxic effect on ciliated human respiratory cells.",
"author": "Kim",
"doi-asserted-by": "publisher",
"first-page": "S45",
"journal-title": "J Laryngol Otol",
"key": "ooi200049r18",
"volume": "129",
"year": "2015"
},
{
"DOI": "10.1177/0145561320932318",
"article-title": "Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 era.",
"author": "Frank",
"doi-asserted-by": "crossref",
"journal-title": "Ear Nose Throat J",
"key": "ooi200049r19",
"year": "2020"
},
{
"DOI": "10.1007/s00405-019-05628-w",
"article-title": "The efficacy of diluted topical povidone-iodine rinses in the management of recalcitrant chronic rhinosinusitis: a prospective cohort study.",
"author": "Panchmatia",
"doi-asserted-by": "publisher",
"first-page": "3373",
"issue": "12",
"journal-title": "Eur Arch Otorhinolaryngol",
"key": "ooi200049r20",
"volume": "276",
"year": "2019"
},
{
"DOI": "10.5152/ejra.",
"article-title": "Topical povidone-iodine as an adjunctive treatment for recalcitrant chronic rhinosinusitis.",
"author": "Mullings",
"doi-asserted-by": "publisher",
"first-page": "45",
"issue": "2",
"journal-title": "Eur J Rhinol Allergy",
"key": "ooi200049r21",
"volume": "2",
"year": "2019"
},
{
"DOI": "10.1053/jhin.2000.0733",
"article-title": "The in-vitro activity of povidone-iodinecream against Staphylococcus aureus and its bioavailability in nasal secretions.",
"author": "Hill",
"doi-asserted-by": "publisher",
"first-page": "198",
"issue": "3",
"journal-title": "J Hosp Infect",
"key": "ooi200049r22",
"volume": "45",
"year": "2000"
},
{
"DOI": "10.1039/C4TB01516A",
"article-title": "Bactericidal magnetic nanoparticles with iodine loaded on surface grafted poly(N-vinylpyrrolidone).",
"author": "Au-Duong",
"doi-asserted-by": "publisher",
"first-page": "840",
"issue": "5",
"journal-title": "J Mater Chem B",
"key": "ooi200049r23",
"volume": "3",
"year": "2015"
},
{
"DOI": "10.1007/s40121-019-00260-x",
"article-title": "Infectious disease management and control with povidone iodine.",
"author": "Eggers",
"doi-asserted-by": "publisher",
"first-page": "581",
"issue": "4",
"journal-title": "Infect Dis Ther",
"key": "ooi200049r24",
"volume": "8",
"year": "2019"
},
{
"article-title": "The effect of 1% povidone iodine as a pre-procedural mouthrinse in 20 patients with varying degrees of oral hygiene.",
"author": "Domingo",
"first-page": "31",
"issue": "2",
"journal-title": "J Philipp Dent Assoc",
"key": "ooi200049r25",
"volume": "48",
"year": "1996"
},
{
"article-title": "The relationship between autoimmune thyroid disease and iodine intake: a review.",
"author": "Foley",
"first-page": "53",
"journal-title": "Endokrynol Pol",
"key": "ooi200049r26",
"volume": "43",
"year": "1992"
},
{
"DOI": "10.1538/expanim.46.197",
"article-title": "125I uptake competing with iodine absorption by the thyroid gland following povidone-iodine skin application.",
"author": "Furudate",
"doi-asserted-by": "publisher",
"first-page": "197",
"issue": "3",
"journal-title": "Exp Anim",
"key": "ooi200049r27",
"volume": "46",
"year": "1997"
},
{
"DOI": "10.1111/jpc.2013.49.issue-6",
"article-title": "Recurrent anaphylaxis caused by topical povidone-iodine (betadine).",
"author": "Gray",
"doi-asserted-by": "publisher",
"first-page": "506",
"issue": "6",
"journal-title": "J Paediatr Child Health",
"key": "ooi200049r28",
"volume": "49",
"year": "2013"
},
{
"DOI": "10.1080/107175499266823",
"article-title": "Toxicological implications of nasal formulations.",
"author": "Quadir",
"doi-asserted-by": "publisher",
"first-page": "227",
"issue": "4",
"journal-title": "Drug Deliv",
"key": "ooi200049r29",
"volume": "6",
"year": "1999"
},
{
"DOI": "10.2500/ajra.2014.28.4092",
"article-title": "The effect of drugs and other compounds on the ciliary beat frequency of human respiratory epithelium.",
"author": "Workman",
"doi-asserted-by": "publisher",
"first-page": "454",
"issue": "6",
"journal-title": "Am J Rhinol Allergy",
"key": "ooi200049r30",
"volume": "28",
"year": "2014"
},
{
"key": "ooi200049r31",
"unstructured": "Povidone. United States Pharmacopeia and National Formulary. United States Pharmacopeia. May 1, 2019. Accessed August 18, 2020. https://www.usp.org/sites/default/files/usp/document/harmonization/excipients/m68070.pdf"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamaotolaryngology/fullarticle/2770785"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2",
"type": "journal-article",
"volume": "146"
}
