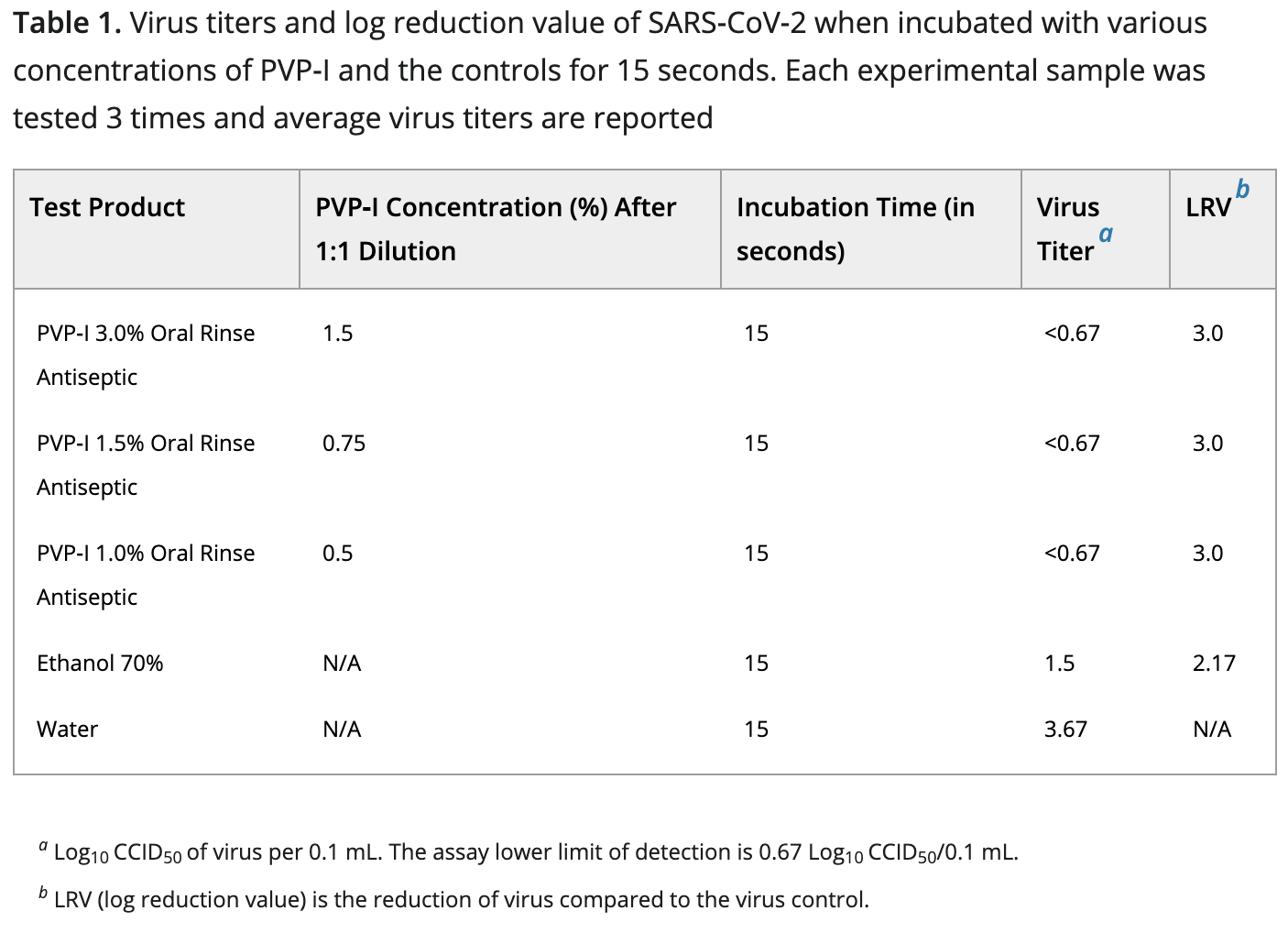
Rapid In-Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Using Povidone-Iodine Oral Antiseptic Rinse
et al., Journal of Prosthodontics, doi:10.1111/jopr.13209, Jun 2020
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing PVP-I rapidly inactivated SARS-CoV-2. Viricidal activity was present at the lowest concentration and contact time tested (0.5% PVP-I and 15 seconds).
9 preclinical studies support the efficacy of povidone-iodine for COVID-19:
1.
Xu et al., Differential Effects of Antiseptic Mouth Rinses on SARS-CoV-2 Infectivity In Vitro, Pathogens, doi:10.3390/pathogens10030272.
2.
Tucker et al., In vitro inactivation of SARS-CoV-2 with 0.5% povidone iodine nasal spray (Nasodine) at clinically relevant concentrations and timeframes using tissue culture and PCR based assays, bioRxiv, doi:10.1101/2021.01.31.426979.
3.
Pelletier et al., Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2), Ear, Nose & Throat Journal, doi:10.1177/0145561320957237.
4.
Frank et al., In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.3053.
5.
Meister et al., Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaa471.
6.
Anderson et al., Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease, Infectious Diseases and Therapy, doi:10.1007/s40121-020-00316-3.
7.
Hassandarvish et al., Povidone iodine gargle and mouthwash, British Dental Journal volume, doi:10.1038/s41415-020-1794-1.
Bidra et al., 8 Jun 2020, peer-reviewed, 6 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Rapid In‐Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) Using Povidone‐Iodine Oral Antiseptic Rinse
Journal of Prosthodontics, doi:10.1111/jopr.13209
Purpose: To investigate the optimal contact time and concentration for viricidal activity of oral preparation of povidone-iodine (PVP-I) against SARS-CoV-2 ('corona virus') to mitigate the risk and transmission of the virus in the dental practice.
Materials and Methods: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) USA-WA1/2020 strain, virus stock was tested against oral antiseptic solutions consisting of aqueous povidone-iodine (PVP-I) as the sole active ingredient. The PVP-I was tested at diluted concentrations of 0.5%, 1%, and 1.5%. Test media without any virus was added to 2 tubes of the compounds to serve as toxicity and neutralization controls. Ethanol (70%) was tested in parallel as a positive control, and water only as a negative control. The test solutions and virus were incubated at room temperature (22 ± 2 °C) for time periods of 15 and 30 seconds. The solution was then neutralized by a 1/10 dilution in minimum essential medium (MEM) 2% fetal bovine serum (FBS), 50 µg/mL gentamicin. Surviving virus from each sample was quantified by standard end-point dilution assay and the log reduction value (LRV) of each compound compared to the negative (water) control was calculated. Results: PVP-I oral antiseptics at all tested concentrations of 0.5%, 1%, and 1.5%, completely inactivated SARS-CoV-2 within 15 seconds of contact. The 70% ethanol control group was unable to completely inactivate SARS-CoV-2 after 15 seconds of contact, but was able to inactivate the virus at 30 seconds of contact. Conclusions: PVP-I oral antiseptic preparations rapidly inactivated SARS-CoV-2 virus in vitro. The viricidal activity was present at the lowest concentration of 0.5 % PVP-I and at the lowest contact time of 15 seconds. This important finding can justify the use of preprocedural oral rinsing with PVP-I (for patients and health care providers) may be useful as an adjunct to personal protective equipment, for dental and surgical specialties during the COVID-19 pandemic.
References
Ader, Paul, Reinhardt, Effect of mouth rinsing with two polyvinylpyrrolidone-iodine mixtures on iodine absorption and thyroid function, J Clin Endocrinol Metab
Domingo, Farrales, Loya, The effect of 1% povidone iodine as a pre-procedural mouth rinse in 20 patients with varying degrees of oral hygiene, J Philipp Dent Assoc
Eggers, Koburger-Janssen, Eickmann, In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens, Infect Dis Ther
Foley, The relationship between autoimmune thyroid disease and iodine intake: a review, Endokrynol Pol
Frank, Capriotti, Brown, Povidone-iodine use in sinonasal and oral cavities: A review of safety in the COVID-19 era, Ear Nose Throat J
Furudate, Nishimaki, Muto, 125I uptake competing with iodine absorption by the thyroid gland following povidone-iodine skin application, Exp. Anim
Gray, Katelaris, Lipson, Recurrent anaphylaxis caused by topical povidone iodine (Betadine), J Paediatr Child Health
Harrel, Molinari, Aerosols and splatter in dentistry: a brief review of the literature and infection control implications, J Am Dent Assoc
Kampf, Todt, Pfaender, Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents, J Hosp Infect
Kariwa, Fujii, Takashima, Inactivation of SARS coronavirus by means of povidoneiodine, physical conditions and chemical reagents, Dermatology
Kovesi, The use of betadine antiseptic in the treatment of oral surgical, paradontological and oral mucosal diseases, Fogorvosi szemle
Lu, Stratton, Tang, Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle, J Med Virol
Madan, Sequeira, Shenoy, The effect of three mouthwashes on radiation-induced oral mucositis in patients with head and neck malignancies: a randomized control trial, J Cancer Res Ther
Managutti, Managutti, Patel, Evaluation of Post-surgical Bacteremia with Use of Povidone-Iodine and Chlorhexidine During Mandibular Third Molar Surgery, J Maxillofac Oral Surg
Micik, Miller, Leong, Studies on dental aerobiology, III: efficacy of surgical masks in protecting dental personnel from airborne bacterial particles, J Dent Res
Micik, Miller, Mazzarella, Studies on dental aerobiology, I: bacterial aerosols generated during dental procedures, J Dent Res
Miller, Micik, Abel, Studies of dental aerobiology, II: microbial splatter discharged from the oral cavity of dental patients, J Dent Res
Panchmatia, Payandeh, Al-Salman, The efficacy of diluted topical povidone-iodine rinses in the management of recalcitrant chronic rhinosinusitis: a prospective cohort study, Eur Arch Otorhinolaryngol
Perrella, Rovai, Marco, Clinical and microbiological evaluation of povidone-iodine 10% as an adjunct to nonsurgical periodontal therapy in chronic periodontitis: a randomized clinical trial, J Int Acad Periodontol
Reed, Muench, A simple method of estimating fifty percent endpoints, Am J Epidemiol
Reimer, Wichelhaus, Schafer, Antimicrobial effectiveness of povidone-iodine and consequences for new application areas, Dermatology
Stein, Hammächer, Michael, Combination of ultrasonic decontamination, soft tissue curettage, and submucosal air polishing with povidone-iodine application for non-surgical therapy of peri-implantitis: 12 Month clinical outcomes, J Periodontol
Sungnak, Huang, Bécavin, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med
Tessema, Frank, Bidra, SARS-CoV-2 viral inactivation using low dose povidone-iodine oral rinse-immediate application for the prosthodontic practice. Letter to the Editor, J Prosthodont, doi:10.1111/jopr.13207
Tsuda, Soutome, Hayashida, Topical povidone iodine inhibits bacterial growth in the oral cavity of patients on mechanical ventilation: a randomized controlled study, BMC Oral Health
Zou, Ruan, Huang, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med
DOI record:
{
"DOI": "10.1111/jopr.13209",
"ISSN": [
"1059-941X",
"1532-849X"
],
"URL": "http://dx.doi.org/10.1111/jopr.13209",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Purpose</jats:title><jats:p>To investigate the optimal contact time and concentration for viricidal activity of oral preparation of povidone‐iodine (PVP‐I) against SARS‐CoV‐2 (‘corona virus’) to mitigate the risk and transmission of the virus in the dental practice.</jats:p></jats:sec><jats:sec><jats:title>Materials and Methods</jats:title><jats:p>The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) USA‐WA1/2020 strain, virus stock was tested against oral antiseptic solutions consisting of aqueous povidone‐iodine (PVP‐I) as the sole active ingredient. The PVP‐I was tested at diluted concentrations of 0.5%, 1%, and 1.5%. Test media without any virus was added to 2 tubes of the compounds to serve as toxicity and neutralization controls. Ethanol (70%) was tested in parallel as a positive control, and water only as a negative control. The test solutions and virus were incubated at room temperature (22 ± 2 °C) for time periods of 15 and 30 seconds. The solution was then neutralized by a 1/10 dilution in minimum essential medium (MEM) 2% fetal bovine serum (FBS), 50 µg/mL gentamicin. Surviving virus from each sample was quantified by standard end‐point dilution assay and the log reduction value (LRV) of each compound compared to the negative (water) control was calculated.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>PVP‐I oral antiseptics at all tested concentrations of 0.5%, 1%, and 1.5%, completely inactivated SARS‐CoV‐2 within 15 seconds of contact. The 70% ethanol control group was unable to completely inactivate SARS‐CoV‐2 after 15 seconds of contact, but was able to inactivate the virus at 30 seconds of contact.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>PVP‐I oral antiseptic preparations rapidly inactivated SARS‐CoV‐2 virus in vitro. The viricidal activity was present at the lowest concentration of 0.5 % PVP‐I and at the lowest contact time of 15 seconds. This important finding can justify the use of preprocedural oral rinsing with PVP‐I (for patients and health care providers) may be useful as an adjunct to personal protective equipment, for dental and surgical specialties during the COVID‐19 pandemic.</jats:p></jats:sec>",
"alternative-id": [
"10.1111/jopr.13209"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2020-06-04"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2020-06-16"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8831-8096",
"affiliation": [
{
"name": "Department of Reconstructive Sciences University of Connecticut Health Center Farmington CT"
}
],
"authenticated-orcid": false,
"family": "Bidra",
"given": "Avinash S.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Ocean Ophthalmology Group Miami FL"
}
],
"family": "Pelletier",
"given": "Jesse S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Institute for Antiviral Research at Utah State University Logan UT"
}
],
"family": "Westover",
"given": "Jonna B",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Otolaryngology University of Connecticut Health Center Farmington CT"
}
],
"family": "Frank",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Otolaryngology University of Connecticut Health Center Farmington CT"
},
{
"name": "ProHealth Physicians Ear, Nose and Throat Farmington CT"
}
],
"family": "Brown",
"given": "Seth M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Otolaryngology University of Connecticut Health Center Farmington CT"
},
{
"name": "ProHealth Physicians Ear, Nose and Throat Farmington CT"
}
],
"family": "Tessema",
"given": "Belachew",
"sequence": "additional"
}
],
"container-title": "Journal of Prosthodontics",
"container-title-short": "Journal of Prosthodontics",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2020,
6,
8
]
],
"date-time": "2020-06-08T17:27:52Z",
"timestamp": 1591637272000
},
"deposited": {
"date-parts": [
[
2023,
9,
5
]
],
"date-time": "2023-09-05T19:24:11Z",
"timestamp": 1693941851000
},
"indexed": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T14:31:31Z",
"timestamp": 1714573891718
},
"is-referenced-by-count": 116,
"issue": "6",
"issued": {
"date-parts": [
[
2020,
6,
16
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2020,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
6,
16
]
],
"date-time": "2020-06-16T00:00:00Z",
"timestamp": 1592265600000
}
}
],
"link": [
{
"URL": "https://api.wiley.com/onlinelibrary/tdm/v1/articles/10.1111%2Fjopr.13209",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jopr.13209",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/jopr.13209",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jopr.13209",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "529-533",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2020,
6,
16
]
]
},
"published-online": {
"date-parts": [
[
2020,
6,
16
]
]
},
"published-print": {
"date-parts": [
[
2020,
7
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1002/jmv.25678",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_2_1"
},
{
"DOI": "10.1056/NEJMc2001737",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_3_1"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_4_1"
},
{
"DOI": "10.14219/jada.archive.2004.0207",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_5_1"
},
{
"DOI": "10.1177/00220345690480012401",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_6_1"
},
{
"DOI": "10.1177/00220345710500031701",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_7_1"
},
{
"DOI": "10.1177/00220345710500031801",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_8_1"
},
{
"key": "e_1_2_7_9_1",
"unstructured": "ADA Interim Guidance for Minimizing Risk of COVID‐19 Transmission.https://www.ada.org/~/media/CPS/Files/COVID/ADA_COVID_Int_Guidance_Treat_Pts.pdf?utm_source=cpsorg&utm_medium=cpsalertbar&utm_content=cv-pm-ebd-interimresponse&utm_campaign=covid-19. Accessed May 28 2020"
},
{
"key": "e_1_2_7_10_1",
"unstructured": "The Centers for Disease Control and Prevention (CDC).Interim Infection Prevention and Control Guidance for Dental Settings During the COVID‐19 Response.https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.htmlAccessed May 28 2020"
},
{
"DOI": "10.1007/s40121-018-0200-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_11_1"
},
{
"DOI": "10.1159/000089211",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_12_1"
},
{
"DOI": "10.1093/oxfordjournals.aje.a118408",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_13_1"
},
{
"DOI": "10.1111/jopr.13207",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_14_1"
},
{
"DOI": "10.1186/s12903-020-1043-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_15_1"
},
{
"article-title": "Clinical and microbiological evaluation of povidone‐iodine 10% as an adjunct to nonsurgical periodontal therapy in chronic periodontitis: a randomized clinical trial",
"author": "Perrella FA",
"first-page": "109‐119",
"journal-title": "J Int Acad Periodontol",
"key": "e_1_2_7_16_1",
"volume": "18",
"year": "2016"
},
{
"DOI": "10.1902/jop.2017.170362",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_17_1"
},
{
"DOI": "10.1007/s12663-016-0976-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_18_1"
},
{
"DOI": "10.1177/0145561320932318",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_19_1"
},
{
"DOI": "10.1159/000057738",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_20_1"
},
{
"DOI": "10.4103/0973-1482.39597",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_21_1"
},
{
"DOI": "10.1007/s00405-019-05628-w",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_22_1"
},
{
"DOI": "10.1210/jcem-66-3-632",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_23_1"
},
{
"article-title": "The use of betadine antiseptic in the treatment of oral surgical, paradontological and oral mucosal diseases",
"author": "Kovesi G",
"first-page": "243",
"journal-title": "Fogorvosi szemle",
"key": "e_1_2_7_24_1",
"volume": "92",
"year": "1999"
},
{
"article-title": "The effect of 1% povidone iodine as a pre‐procedural mouth rinse in 20 patients with varying degrees of oral hygiene",
"author": "Domingo MA",
"first-page": "31",
"journal-title": "J Philipp Dent Assoc",
"key": "e_1_2_7_25_1",
"volume": "48",
"year": "1996"
},
{
"article-title": "The relationship between autoimmune thyroid disease and iodine intake: a review",
"author": "Foley TP",
"first-page": "53",
"issue": "1",
"journal-title": "Endokrynol Pol",
"key": "e_1_2_7_26_1",
"volume": "43",
"year": "1992"
},
{
"DOI": "10.1538/expanim.46.197",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_27_1"
},
{
"DOI": "10.1111/jpc.12232",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_28_1"
},
{
"DOI": "10.1016/j.jhin.2020.01.022",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_29_1"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/jopr.13209"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Rapid In‐Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) Using Povidone‐Iodine Oral Antiseptic Rinse",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "29"
}
