Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiaa471, Jul 2020
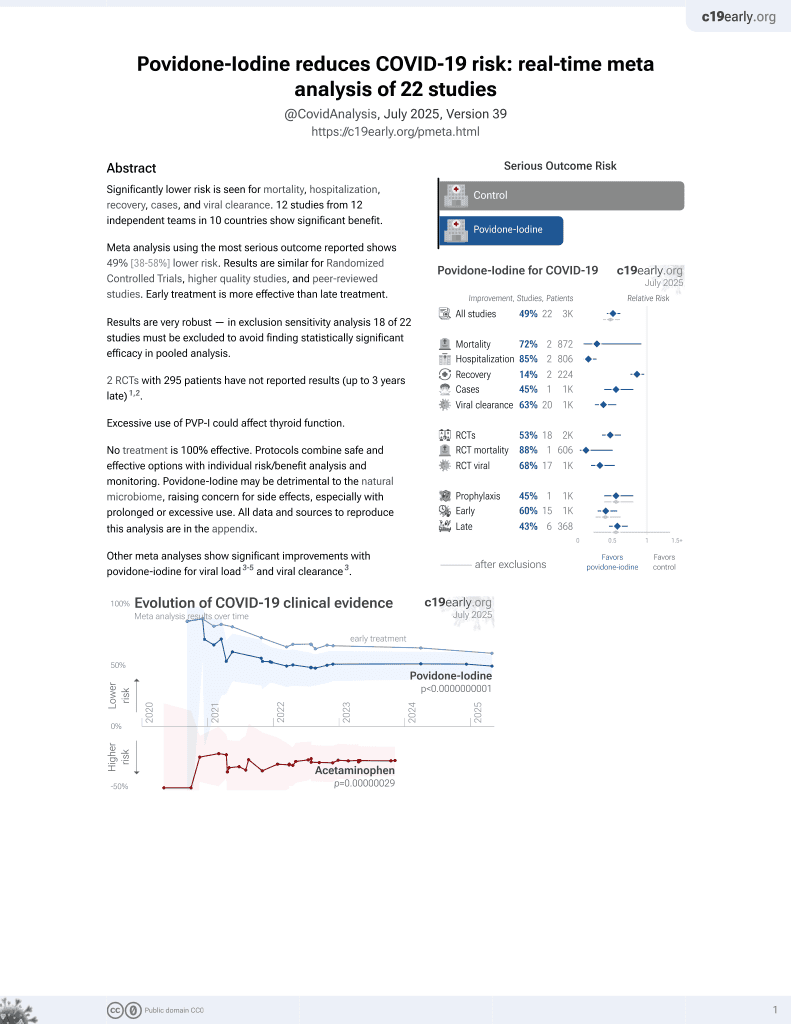
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
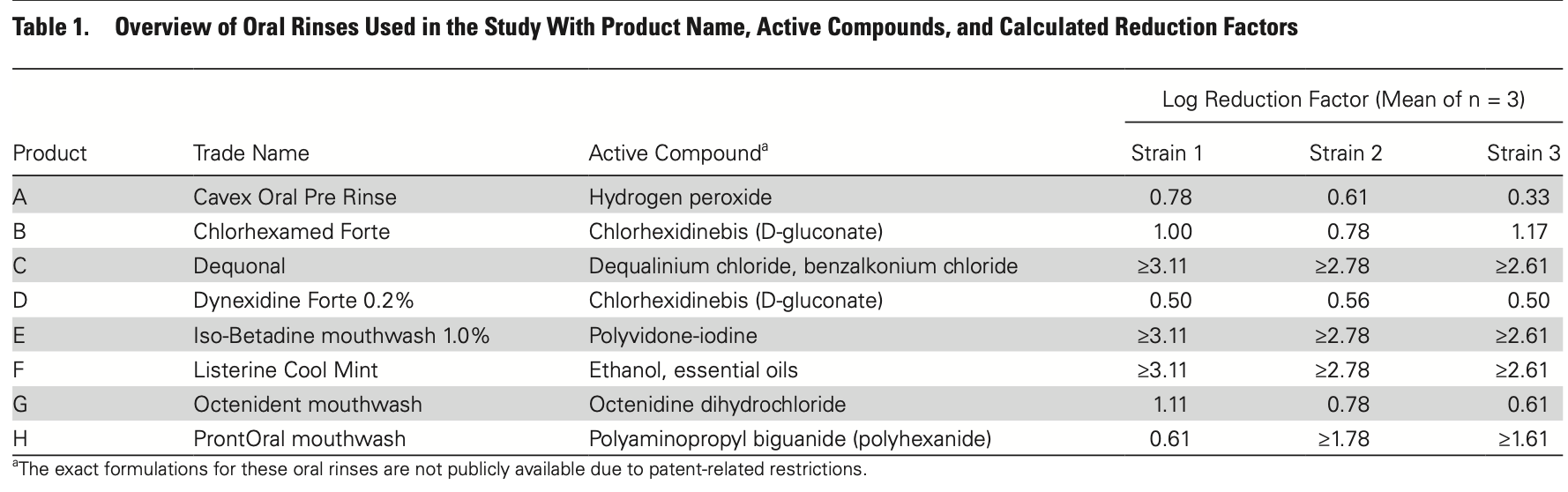
In vitro analysis of 8 oral rinses, showing reduced viral infectivity up to 3 orders of magnitude with povidone-iodine, ethanol + essential oils, and dequalinium chloride + benzalkonium chloride, and log reduction factors ranging from 0.3 to 1.78 for the other 5.
9 preclinical studies support the efficacy of povidone-iodine for COVID-19:
1.
Xu et al., Differential Effects of Antiseptic Mouth Rinses on SARS-CoV-2 Infectivity In Vitro, Pathogens, doi:10.3390/pathogens10030272.
2.
Tucker et al., In vitro inactivation of SARS-CoV-2 with 0.5% povidone iodine nasal spray (Nasodine) at clinically relevant concentrations and timeframes using tissue culture and PCR based assays, bioRxiv, doi:10.1101/2021.01.31.426979.
3.
Pelletier et al., Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2), Ear, Nose & Throat Journal, doi:10.1177/0145561320957237.
4.
Frank et al., In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.3053.
5.
Meister et al., Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaa471.
6.
Anderson et al., Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease, Infectious Diseases and Therapy, doi:10.1007/s40121-020-00316-3.
7.
Hassandarvish et al., Povidone iodine gargle and mouthwash, British Dental Journal volume, doi:10.1038/s41415-020-1794-1.
Meister et al., 29 Jul 2020, Germany, peer-reviewed, 12 authors.
Contact: eike.steinmann@rub.de.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Abstract: The Journal of Infectious Diseases
BRIEF REPORT
Virucidal Efficacy of Different
Oral Rinses Against Severe Acute
Respiratory Syndrome Coronavirus 2
Toni Luise Meister,1 Yannick Brüggemann,1 Daniel Todt,1,2 Carina Conzelmann,3
Janis A. Müller,3 Rüdiger Groß,3 Jan Münch,3 Adalbert Krawczyk,4,5
Jörg Steinmann,6,7 Jochen Steinmann,8 Stephanie Pfaender,1,a and Eike Steinmann1,a
The ongoing severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) pandemic creates a significant threat to global
health. Recent studies suggested the significance of throat and
salivary glands as major sites of virus replication and transmission during early coronavirus disease 2019, thus advocating
application of oral antiseptics. However, the antiviral efficacy
of oral rinsing solutions against SARS-CoV-2 has not been
examined. Here, we evaluated the virucidal activity of different
available oral rinses against SARS-CoV-2 under conditions
mimicking nasopharyngeal secretions. Several formulations
with significant SARS-CoV-2 inactivating properties in vitro
support the idea that oral rinsing might reduce the viral load of
saliva and could thus lower the transmission of SARS-CoV-2.
Keywords. SARS-CoV-2; oral rinses; inactivation; suspension test; transmission.
The current severe acute respiratory syndrome coronavirus
2 (SARS-CoV-2) pandemic has created a significant threat to
global health. Since effective treatments and vaccines are currently not available, diligent attention on transmission-based
precautions is essential to limit viral spread. According to current
evidence, SARS-CoV-2 is mainly transmitted through respiratory droplets exhaled from infected individuals [1]. Importantly,
viral loads are high in the nasal cavity, nasopharynx, and oropharynx and viral shedding can be detected before, during, and
after the acute clinical phase of illness [2]. Aerosols produced
Received 18 June 2020; editorial decision 21 July 2020; accepted 24 July 2020; published
online July 29, 2020.
a
S.P. and E.S. are equally contributing last authors.
Correspondence: Eike Steinmann, PhD, Department of Molecular and Medical Virology, RuhrUniversity Bochum, Universitaetsstr 150, 44801 Bochum, Germany (eike.steinmann@rub.de).
The Journal of Infectious Diseases® 2020;222:1289–92
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society
of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
DOI: 10.1093/infdis/jiaa471
METHODS
Virus Strains and Propagation
To isolate SARS-CoV-2 at the University Ulm Medical Center
(Ulm, Germany), 50 000 Vero E6 cells were seeded in 24-well
plates in 500 µL medium incubated overnight at 37°C. The next
day, medium was replaced by 400 µL of 2.5 µg/mL amphotericin
B–containing medium. Then, 100 µL of throat swabs that tested
positive for SARS-CoV-2 by reverse-transcription quantitative
polymerase chain reaction was titrated 5-fold on the cells and
incubated for 3–5 days. Upon visible cytopathic effect, supernatant was taken and virus expanded by inoculation of Vero
E6 cells in 75 cm2 flasks and propagated as described above.
Thereby, the viral isolates BetaCoV/Germany/Ulm/01/2020
(strain 2) and BetaCoV/Germany/Ulm/02/2020 (strain 3) were
obtained. In Essen, Germany, SARS-CoV-2 was isolated from
a nasopharyngeal swab of a patient suffering from coronavirus
disease 2019 (COVID-19) and named UKEssen strain (strain
1). The swab was taken using a Virocult vial (Sigma, Germany).
The..
DOI record:
{
"DOI": "10.1093/infdis/jiaa471",
"ISSN": [
"0022-1899",
"1537-6613"
],
"URL": "http://dx.doi.org/10.1093/infdis/jiaa471",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>The ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic creates a significant threat to global health. Recent studies suggested the significance of throat and salivary glands as major sites of virus replication and transmission during early coronavirus disease 2019, thus advocating application of oral antiseptics. However, the antiviral efficacy of oral rinsing solutions against SARS-CoV-2 has not been examined. Here, we evaluated the virucidal activity of different available oral rinses against SARS-CoV-2 under conditions mimicking nasopharyngeal secretions. Several formulations with significant SARS-CoV-2 inactivating properties in vitro support the idea that oral rinsing might reduce the viral load of saliva and could thus lower the transmission of SARS-CoV-2.</jats:p>",
"author": [
{
"affiliation": [
{
"name": "Department for Molecular and Medical Virology, Ruhr University Bochum, Bochum, Germany"
}
],
"family": "Meister",
"given": "Toni Luise",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department for Molecular and Medical Virology, Ruhr University Bochum, Bochum, Germany"
}
],
"family": "Brüggemann",
"given": "Yannick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department for Molecular and Medical Virology, Ruhr University Bochum, Bochum, Germany"
},
{
"name": "European Virus Bioinformatics Center, Jena, Germany"
}
],
"family": "Todt",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Molecular Virology, Ulm University Medical Center, Ulm, Germany"
}
],
"family": "Conzelmann",
"given": "Carina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Molecular Virology, Ulm University Medical Center, Ulm, Germany"
}
],
"family": "Müller",
"given": "Janis A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Molecular Virology, Ulm University Medical Center, Ulm, Germany"
}
],
"family": "Groß",
"given": "Rüdiger",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Molecular Virology, Ulm University Medical Center, Ulm, Germany"
}
],
"family": "Münch",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, West German Centre of Infectious Diseases, Universitätsmedizin Essen, University Duisburg-Essen, Essen, Germany"
},
{
"name": "Institute for Virology, University Hospital Essen, University of Duisburg-Essen, Essen, Germany"
}
],
"family": "Krawczyk",
"given": "Adalbert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Clinical Hygiene, Medical Microbiology and Infectiology, General Hospital Nürnberg, Paracelsus Medical University, Nuremberg, Germany"
},
{
"name": "Institute of Medical Microbiology, University Hospital of Essen, Essen, Germany"
}
],
"family": "Steinmann",
"given": "Jörg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dr. Brill + Partner GmbH Institute for Hygiene and Microbiology, Bremen, Germany"
}
],
"family": "Steinmann",
"given": "Jochen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department for Molecular and Medical Virology, Ruhr University Bochum, Bochum, Germany"
}
],
"family": "Pfaender",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department for Molecular and Medical Virology, Ruhr University Bochum, Bochum, Germany"
}
],
"family": "Steinmann",
"given": "Eike",
"sequence": "additional"
}
],
"container-title": "The Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
7,
24
]
],
"date-time": "2020-07-24T19:12:10Z",
"timestamp": 1595617930000
},
"deposited": {
"date-parts": [
[
2020,
10,
3
]
],
"date-time": "2020-10-03T16:23:37Z",
"timestamp": 1601742217000
},
"funder": [
{
"award": [
"101003555"
],
"name": "European Union’s Horizon 2020 research and innovation program"
},
{
"name": "International Graduate School in Molecular Medicine Ulm"
},
{
"DOI": "10.13039/501100010380",
"doi-asserted-by": "publisher",
"name": "Stiftung Universitätsmedizin Essen"
}
],
"indexed": {
"date-parts": [
[
2023,
2,
21
]
],
"date-time": "2023-02-21T21:10:58Z",
"timestamp": 1677013858453
},
"is-referenced-by-count": 117,
"issue": "8",
"issued": {
"date-parts": [
[
2020,
7,
29
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2020,
7,
29
]
]
},
"published-print": {
"date-parts": [
[
2020,
9,
14
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/journals/pages/open_access/funder_policies/chorus/standard_publication_model",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
7,
29
]
],
"date-time": "2020-07-29T00:00:00Z",
"timestamp": 1595980800000
}
}
],
"link": [
{
"URL": "http://academic.oup.com/jid/advance-article-pdf/doi/10.1093/infdis/jiaa471/33652398/jiaa471.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/jid/article-pdf/222/8/1289/33834016/jiaa471.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/jid/article-pdf/222/8/1289/33834016/jiaa471.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "1289-1292",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2020,
7,
29
]
]
},
"published-online": {
"date-parts": [
[
2020,
7,
29
]
]
},
"published-other": {
"date-parts": [
[
2020,
10,
15
]
]
},
"published-print": {
"date-parts": [
[
2020,
9,
14
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1056/NEJMc2007800",
"article-title": "Visualizing speech-generated oral fluid droplets with laser light scattering",
"author": "Anfinrud",
"doi-asserted-by": "crossref",
"first-page": "2061",
"journal-title": "N Engl J Med",
"key": "2020100312232117200_CIT0001",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"article-title": "Virological assessment of hospitalized patients with COVID-2019",
"author": "Wölfel",
"doi-asserted-by": "crossref",
"first-page": "465",
"journal-title": "Nature",
"key": "2020100312232117200_CIT0002",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2009324",
"article-title": "Droplets and aerosols in the transmission of SARS-CoV-2",
"author": "Meselson",
"doi-asserted-by": "crossref",
"first-page": "2063",
"journal-title": "N Engl J Med",
"key": "2020100312232117200_CIT0003",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.3201/eid2607.200915",
"article-title": "Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols",
"author": "Kratzel",
"doi-asserted-by": "crossref",
"first-page": "1592",
"journal-title": "Emerging Infect Dis",
"key": "2020100312232117200_CIT0004",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1093/function/zqaa002",
"article-title": "Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection",
"author": "O’Donnell",
"doi-asserted-by": "crossref",
"first-page": "zqaa002",
"journal-title": "Function",
"key": "2020100312232117200_CIT0005",
"volume": "1",
"year": "2020"
},
{
"article-title": "How effective is washing hands against influenza viruses? [in German]",
"author": "Eggers",
"first-page": "492",
"journal-title": "Hyg Med",
"key": "2020100312232117200_CIT0006",
"year": "2009"
},
{
"DOI": "10.1007/s40121-018-0200-7",
"article-title": "In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens",
"author": "Eggers",
"doi-asserted-by": "crossref",
"first-page": "249",
"journal-title": "Infect Dis Ther",
"key": "2020100312232117200_CIT0007",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1016/S1079-2104(05)80124-6",
"article-title": "The antiviral spectrum of Listerine antiseptic",
"author": "Dennison",
"doi-asserted-by": "crossref",
"first-page": "442",
"journal-title": "Oral Surg Oral Med Oral Pathol Oral Radiol Endod",
"key": "2020100312232117200_CIT0008",
"volume": "79",
"year": "1995"
},
{
"DOI": "10.1111/j.1600-051X.2005.00673.x",
"article-title": "Efficacy of Listerine antiseptic in reducing viral contamination of saliva",
"author": "Meiller",
"doi-asserted-by": "crossref",
"first-page": "341",
"journal-title": "J Clin Periodontol",
"key": "2020100312232117200_CIT0009",
"volume": "32",
"year": "2005"
},
{
"article-title": "Efficacy of Listerine antiseptic against MRSA, Candida albicans and HIV",
"author": "Yamanaka",
"first-page": "23",
"journal-title": "Bull Tokyo Dent Coll",
"key": "2020100312232117200_CIT0010",
"volume": "35",
"year": "1994"
}
],
"reference-count": 10,
"references-count": 10,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jid/article/222/8/1289/5878067"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2",
"type": "journal-article",
"volume": "222"
}