Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease
et al., Infectious Diseases and Therapy, doi:10.1007/s40121-020-00316-3, Jul 2020
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
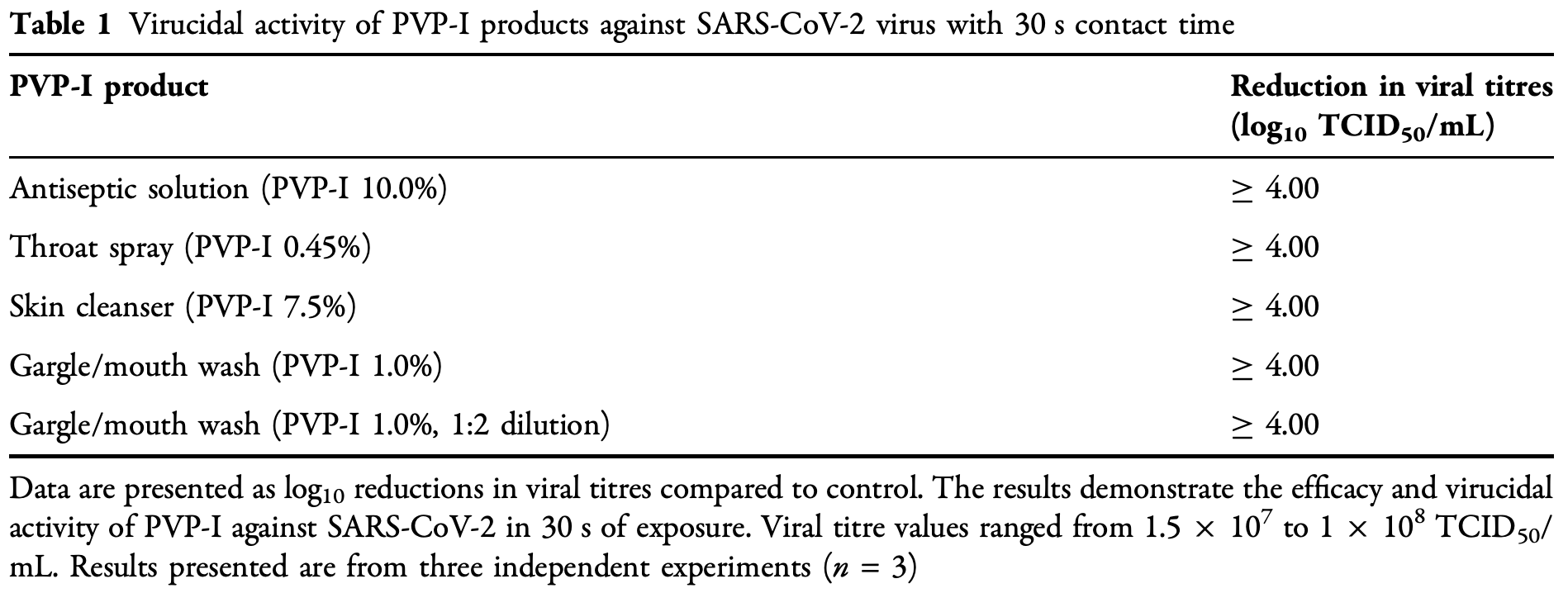
In vitro study showing rapid and effective virucidal activity of PVP-I against SARS-CoV-2. All four products tested [antiseptic solution (PVP-I 10%), skin cleanser (PVP-I 7.5%), gargle and mouth wash (PVP-I 1%) and throat spray (PVP-I 0.45%)] achieved C 99.99% virucidal activity against SARS-CoV-2, corresponding to C 4 log10 reduction of virus titre, within 30s of contact.
9 preclinical studies support the efficacy of povidone-iodine for COVID-19:
1.
Xu et al., Differential Effects of Antiseptic Mouth Rinses on SARS-CoV-2 Infectivity In Vitro, Pathogens, doi:10.3390/pathogens10030272.
2.
Tucker et al., In vitro inactivation of SARS-CoV-2 with 0.5% povidone iodine nasal spray (Nasodine) at clinically relevant concentrations and timeframes using tissue culture and PCR based assays, bioRxiv, doi:10.1101/2021.01.31.426979.
3.
Pelletier et al., Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2), Ear, Nose & Throat Journal, doi:10.1177/0145561320957237.
4.
Frank et al., In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.3053.
5.
Meister et al., Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaa471.
6.
Anderson et al., Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease, Infectious Diseases and Therapy, doi:10.1007/s40121-020-00316-3.
7.
Hassandarvish et al., Povidone iodine gargle and mouthwash, British Dental Journal volume, doi:10.1038/s41415-020-1794-1.
Anderson et al., 8 Jul 2020, peer-reviewed, 8 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease
Infectious Diseases and Therapy, doi:10.1007/s40121-020-00316-3
Introduction: As of 22 June 2020, Severe Acute Respiratory Syndrome (SARS)-coronavirus (CoV)-2 has infected more than 8.95 million people worldwide, causing [ 468,000 deaths. The virus is transmitted through respiratory droplets and physical contact from contaminated surfaces to the mucosa. Hand hygiene and oral decontamination among other measures are key to preventing the spread of the virus. We report the in vitro virucidal activity of topical and oral povidone-iodine (PVP-I) products against SARS-CoV-2. Methods: Suspension assays were used to assess the virucidal activity of PVP-I against SARS-CoV-2. Products were tested at a contact time of 30 s for virucidal activity. Viral titres were calculated using the Spearman-Ka ¨rber method and reported as median tissue culture infectious dose (TCID 50 )/mL. Results: All four products [antiseptic solution (PVP-I 10%), skin cleanser (PVP-I 7.5%), gargle and mouth wash (PVP-I 1%) and throat spray (PVP-I 0.45%)] achieved C 99.99% virucidal activity against SARS-CoV-2, corresponding to C 4 log 10 reduction of virus titre, within 30 s of contact.
Conclusion: This study provides evidence of rapid and effective virucidal activity of PVP-I against SARS-CoV-2. PVP-I-based products are widely available for medical and personal use for hand hygiene and oral decontamination, and could be readily integrated into coronavirus disease, COVID-19, infection control measures in hospital and community settings.
References
Bai, Yao, Wei, Presumed asymptomatic carrier of COVID-19, JAMA
Cascella, Rajnik, Cuomo, Dulebohn, Napoli, Features, evaluation and treatment coronavirus (COVID-19)
Chin, Chu, Perera, Stability of SARS-CoV-2 in different environmental conditions, Lancet Microbe
Comite ´europe ´en, ? A1:2015: Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of virucidal activity in the medical area. Test method and requirements
Corman, Muth, Niemeyer, Drosten, Hosts and sources of endemic human coronaviruses, Adv Virus Res
Eggers, Eickmann, Zorn, Rapid and effective virucidal activity of povidone-iodine products against middle east respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus Ankara (MVA), Infect Dis Ther
Eggers, Koburger-Janssen, Eickmann, Zorn, In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens, Infect Dis Ther
Eggers, Koburger-Janssen, Ward, Newby, Mu ¨ller S, Bactericidal and virucidal activity of povidone-iodine and chlorhexidine gluconate cleansers in an in vivo hand hygiene clinical simulation study, Infect Dis Ther
Gui, Pepe, Magalini, Just one more hygiene practice in COVID-19, Eur Rev Med Pharmacol Sci
Kariwa, Fujii, Takashima, Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents, Dermatology
Kawana, Kitamura, Nakagomi, Inactivation of human viruses by povidone-iodine in comparison with other antiseptics, Dermatology
Kirk-Bayley, Challacombe, Sunkaraneni, Combes, The use of povidone iodine nasal spray and mouthwash during the current COVID-19 pandemic may reduce cross infection and protect healthcare workers, doi:10.2139/ssrn.3563092
Mady, Kubik, Baddour, Snyderman, Rowan, Consideration of povidone-iodine as a public health intervention for COVID-19: Utilization as ''Personal Protective Equipment'' for frontline providers exposed in high-risk head and neck and skull base oncology care, Oral Oncol
Nagatake, Ahmed, Oishi, Prevention of respiratory lnfections by povidone-lodine gargle, Dermatology
O'donnell, Thomas, Stanton, Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection, Function
Sanche, Lin, Xu, Romero-Severson, Hengartner et al., High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2, Emerg Infect Dis
Spearman, The method of 'right and wrong cases' ('constant stimuli') without Gauss's formulae, Br J Psychol
To, Tsang, Leung, Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study, Lancet Infect Dis
Vogt, Hauser, Mueller, Bosse, Hopp, Efficacy of conventional and liposomal povidoneiodine in infected mesh skin grafts: an exploratory study, Infect Dis Ther
Wo ¨lfel, Corman, Guggemos, Virological assessment of hospitalized patients with COVID-2019, Nature
Zhao, Lin, Ran, Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak, Int J Infect Dis
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med
¨rber, Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche, Archiv Exper Pathol Pharmakol
DOI record:
{
"DOI": "10.1007/s40121-020-00316-3",
"ISSN": [
"2193-8229",
"2193-6382"
],
"URL": "http://dx.doi.org/10.1007/s40121-020-00316-3",
"alternative-id": [
"316"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "2 June 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 2,
"value": "8 July 2020"
}
],
"author": [
{
"affiliation": [],
"family": "Anderson",
"given": "Danielle E.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sivalingam",
"given": "Velraj",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kang",
"given": "Adrian Eng Zheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ananthanarayanan",
"given": "Abhishek",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arumugam",
"given": "Harsha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jenkins",
"given": "Timothy M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hadjiat",
"given": "Yacine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eggers",
"given": "Maren",
"sequence": "additional"
}
],
"container-title": "Infectious Diseases and Therapy",
"container-title-short": "Infect Dis Ther",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2020,
7,
8
]
],
"date-time": "2020-07-08T11:03:42Z",
"timestamp": 1594206222000
},
"deposited": {
"date-parts": [
[
2021,
7,
7
]
],
"date-time": "2021-07-07T23:25:07Z",
"timestamp": 1625700307000
},
"funder": [
{
"name": "Mundipharma Singapore Holding Pte. Limited"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
7
]
],
"date-time": "2024-05-07T08:34:45Z",
"timestamp": 1715070885538
},
"is-referenced-by-count": 128,
"issue": "3",
"issued": {
"date-parts": [
[
2020,
7,
8
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2020,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
7,
8
]
],
"date-time": "2020-07-08T00:00:00Z",
"timestamp": 1594166400000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
7,
8
]
],
"date-time": "2020-07-08T00:00:00Z",
"timestamp": 1594166400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-020-00316-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40121-020-00316-3/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-020-00316-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "669-675",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2020,
7,
8
]
]
},
"published-online": {
"date-parts": [
[
2020,
7,
8
]
]
},
"published-print": {
"date-parts": [
[
2020,
9
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "316_CR1",
"unstructured": "World Health Organisation. WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020: World Health Organisation. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed 11 May 2020."
},
{
"DOI": "10.1016/j.ijid.2020.01.050",
"author": "S Zhao",
"doi-asserted-by": "publisher",
"first-page": "214",
"journal-title": "Int J Infect Dis.",
"key": "316_CR2",
"unstructured": "Zhao S, Lin Q, Ran J, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–7.",
"volume": "92",
"year": "2020"
},
{
"key": "316_CR3",
"unstructured": "Johns Hopkins University and Medicine. Coronavirus Resource Center: John Hopkins University. 2020. https://coronavirus.jhu.edu/map.html. Accessed 22 June 2020."
},
{
"DOI": "10.1016/bs.aivir.2018.01.001",
"author": "VM Corman",
"doi-asserted-by": "publisher",
"first-page": "163",
"journal-title": "Adv Virus Res.",
"key": "316_CR4",
"unstructured": "Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and sources of endemic human coronaviruses. Adv Virus Res. 2018;100:163–88.",
"volume": "100",
"year": "2018"
},
{
"DOI": "10.1056/NEJMoa2001017",
"author": "N Zhu",
"doi-asserted-by": "publisher",
"first-page": "727",
"issue": "8",
"journal-title": "N Engl J Med.",
"key": "316_CR5",
"unstructured": "Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33.",
"volume": "382",
"year": "2020"
},
{
"key": "316_CR6",
"unstructured": "World Health Organisation. Interim recommendations on obligatory hand hygiene against transmission of COVID-19: World Health Organization. 2020. https://www.who.int/who-documents-detail/interim-recommendations-on-obligatory-hand-hygiene-against-transmission-of-covid-19. Accessed 18 Apr 2020."
},
{
"DOI": "10.1016/S2666-5247(20)30003-3",
"author": "AWH Chin",
"doi-asserted-by": "publisher",
"first-page": "e10",
"journal-title": "Lancet Microbe.",
"key": "316_CR7",
"unstructured": "Chin AWH, Chu JTS, Perera MRA, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1:e10.",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1007/s40121-017-0172-z",
"author": "PM Vogt",
"doi-asserted-by": "publisher",
"first-page": "545",
"issue": "4",
"journal-title": "Infect Dis Ther.",
"key": "316_CR8",
"unstructured": "Vogt PM, Hauser J, Mueller S, Bosse B, Hopp M. Efficacy of conventional and liposomal povidone-iodine in infected mesh skin grafts: an exploratory study. Infect Dis Ther. 2017;6(4):545–55.",
"volume": "6",
"year": "2017"
},
{
"DOI": "10.1007/s40121-018-0200-7",
"author": "M Eggers",
"doi-asserted-by": "publisher",
"first-page": "249",
"issue": "2",
"journal-title": "Infect Dis Ther.",
"key": "316_CR9",
"unstructured": "Eggers M, Koburger-Janssen T, Eickmann M, Zorn J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect Dis Ther. 2018;7(2):249–59.",
"volume": "7",
"year": "2018"
},
{
"key": "316_CR10",
"unstructured": "World Health Organisation. WHO R&D Blueprint COVID 19 Experimental Treatments: World Health Organisation. 2020. https://www.who.int/docs/default-source/coronaviruse/covid-classification-of-treatment-types-rev.pdf. Accessed 18 May 2020."
},
{
"DOI": "10.1159/000246027",
"author": "R Kawana",
"doi-asserted-by": "publisher",
"first-page": "29",
"issue": "Suppl 2",
"journal-title": "Dermatology",
"key": "316_CR11",
"unstructured": "Kawana R, Kitamura T, Nakagomi O, et al. Inactivation of human viruses by povidone-iodine in comparison with other antiseptics. Dermatology. 1997;195(Suppl 2):29–35.",
"volume": "195",
"year": "1997"
},
{
"DOI": "10.1159/000089211",
"author": "H Kariwa",
"doi-asserted-by": "publisher",
"first-page": "119",
"issue": "Suppl 1",
"journal-title": "Dermatology",
"key": "316_CR12",
"unstructured": "Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212(Suppl 1):119–23.",
"volume": "212",
"year": "2006"
},
{
"DOI": "10.1007/s40121-015-0091-9",
"author": "M Eggers",
"doi-asserted-by": "publisher",
"first-page": "491",
"issue": "4",
"journal-title": "Infect Dis Ther.",
"key": "316_CR13",
"unstructured": "Eggers M, Eickmann M, Zorn J. Rapid and effective virucidal activity of povidone-iodine products against middle east respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus Ankara (MVA). Infect Dis Ther. 2015;4(4):491–501.",
"volume": "4",
"year": "2015"
},
{
"author": "C Spearman",
"first-page": "227",
"journal-title": "Br J Psychol.",
"key": "316_CR14",
"unstructured": "Spearman C. The method of ‘right and wrong cases’ (‘constant stimuli’) without Gauss's formulae. Br J Psychol. 1908;2:227–42.",
"volume": "2",
"year": "1908"
},
{
"DOI": "10.1007/BF01863914",
"author": "G Kärber",
"doi-asserted-by": "publisher",
"first-page": "480",
"journal-title": "Archiv Exper Pathol Pharmakol.",
"key": "316_CR15",
"unstructured": "Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Archiv Exper Pathol Pharmakol. 1931;162:480–3.",
"volume": "162",
"year": "1931"
},
{
"key": "316_CR16",
"unstructured": "Comité Européen de Normalisation. EN14476:2013 + A1:2015: Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of virucidal activity in the medical area. Test method and requirements (Phase 2/Step 1) 2015."
},
{
"author": "M Cascella",
"key": "316_CR17",
"unstructured": "Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19). Treasure Island, FL: StatPearls; 2020.",
"volume-title": "Features, evaluation and treatment coronavirus (COVID-19)",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2565",
"author": "Y Bai",
"doi-asserted-by": "publisher",
"first-page": "1406",
"issue": "14",
"journal-title": "JAMA",
"key": "316_CR18",
"unstructured": "Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–7.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.3201/eid2607.200282",
"author": "S Sanche",
"doi-asserted-by": "publisher",
"first-page": "1470",
"issue": "7",
"journal-title": "Emerg Infect Dis",
"key": "316_CR19",
"unstructured": "Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1470–7.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1007/s40121-018-0202-5",
"author": "M Eggers",
"doi-asserted-by": "publisher",
"first-page": "235",
"issue": "2",
"journal-title": "Infect Dis Ther.",
"key": "316_CR20",
"unstructured": "Eggers M, Koburger-Janssen T, Ward LS, Newby C, Müller S. Bactericidal and virucidal activity of povidone-iodine and chlorhexidine gluconate cleansers in an in vivo hand hygiene clinical simulation study. Infect Dis Ther. 2018;7(2):235–47.",
"volume": "7",
"year": "2018"
},
{
"key": "316_CR21",
"unstructured": "Australian Dental Association. Managing COVID-19 Guidelines. 2020. https://www.ada.org.au/Covid-19-Portal/Cards/Dental-Profesionals/Guidelines-and-Risk-Factors/Just-an-information-Card. Accessed 21 June 2020."
},
{
"key": "316_CR22",
"unstructured": "Centers for Disease Control and Prevention. Guidance for dental settings: interim infection prevention and control guidance for dental settings during the COVID-19 response: centers for disease control and prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html. Accessed 21 June 2020."
},
{
"DOI": "10.1159/000057722",
"author": "T Nagatake",
"doi-asserted-by": "publisher",
"first-page": "32",
"issue": "Suppl 1",
"journal-title": "Dermatology",
"key": "316_CR23",
"unstructured": "Nagatake T, Ahmed K, Oishi K. Prevention of respiratory lnfections by povidone-lodine gargle. Dermatology. 2002;204(Suppl 1):32–6.",
"volume": "204",
"year": "2002"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"author": "R Wölfel",
"doi-asserted-by": "publisher",
"first-page": "465",
"issue": "7809",
"journal-title": "Nature",
"key": "316_CR24",
"unstructured": "Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–9.",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30196-1",
"author": "KK To",
"doi-asserted-by": "publisher",
"first-page": "565",
"issue": "5",
"journal-title": "Lancet Infect Dis.",
"key": "316_CR25",
"unstructured": "To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–74.",
"volume": "20",
"year": "2020"
},
{
"author": "D Gui",
"first-page": "3438",
"issue": "7",
"journal-title": "Eur Rev Med Pharmacol Sci.",
"key": "316_CR26",
"unstructured": "Gui D, Pepe G, Magalini S. Just one more hygiene practice in COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(7):3438–9.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1093/function/zqaa002",
"author": "VB O’Donnell",
"doi-asserted-by": "publisher",
"first-page": "zqaa002",
"journal-title": "Function.",
"key": "316_CR27",
"unstructured": "O’Donnell VB, Thomas D, Stanton R, et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function. 2020;1:zqaa002.",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1016/j.oraloncology.2020.104724",
"author": "LJ Mady",
"doi-asserted-by": "publisher",
"first-page": "104724",
"journal-title": "Oral Oncol.",
"key": "316_CR28",
"unstructured": "Mady LJ, Kubik MW, Baddour K, Snyderman CH, Rowan NR. Consideration of povidone-iodine as a public health intervention for COVID-19: Utilization as \"Personal Protective Equipment\" for frontline providers exposed in high-risk head and neck and skull base oncology care. Oral Oncol. 2020;105:104724.",
"volume": "105",
"year": "2020"
},
{
"DOI": "10.2139/ssrn.3563092",
"doi-asserted-by": "publisher",
"key": "316_CR29",
"unstructured": "Kirk-Bayley J, Challacombe S, Sunkaraneni S, Combes J. The use of povidone iodine nasal spray and mouthwash during the current COVID-19 pandemic may reduce cross infection and protect healthcare workers. 2020. https://doi.org/10.2139/ssrn.3563092."
}
],
"reference-count": 29,
"references-count": 29,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.21203/rs.3.rs-34544/v1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.21203/rs.3.rs-34544/v2",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40121-020-00316-3"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "9"
}
