Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2)
et al., Ear, Nose & Throat Journal, doi:10.1177/0145561320957237, Sep 2020
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
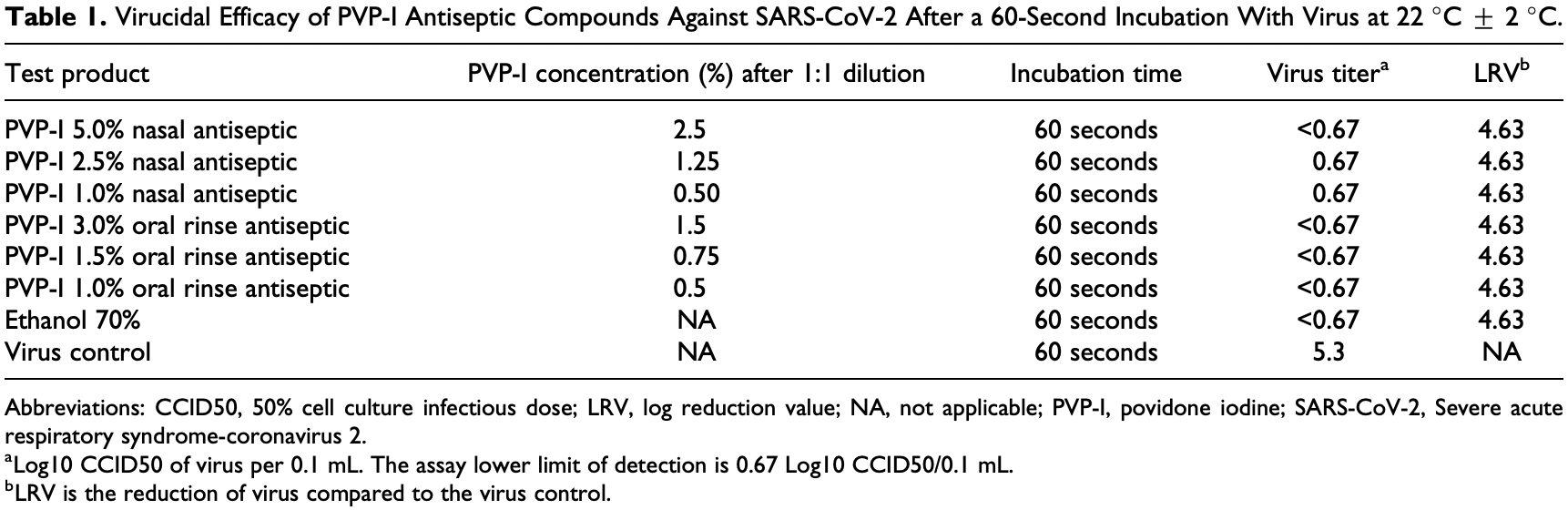
In vitro study testing nasal and oral PVP-I formulations with 60 second exposure time, showing complete inactivation of SARS-CoV-2 with all concentrations (1% to 5% PVP-I).
9 preclinical studies support the efficacy of povidone-iodine for COVID-19:
1.
Xu et al., Differential Effects of Antiseptic Mouth Rinses on SARS-CoV-2 Infectivity In Vitro, Pathogens, doi:10.3390/pathogens10030272.
2.
Tucker et al., In vitro inactivation of SARS-CoV-2 with 0.5% povidone iodine nasal spray (Nasodine) at clinically relevant concentrations and timeframes using tissue culture and PCR based assays, bioRxiv, doi:10.1101/2021.01.31.426979.
3.
Pelletier et al., Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2), Ear, Nose & Throat Journal, doi:10.1177/0145561320957237.
4.
Frank et al., In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.3053.
5.
Meister et al., Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaa471.
6.
Anderson et al., Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease, Infectious Diseases and Therapy, doi:10.1007/s40121-020-00316-3.
7.
Hassandarvish et al., Povidone iodine gargle and mouthwash, British Dental Journal volume, doi:10.1038/s41415-020-1794-1.
Pelletier et al., 21 Sep 2020, peer-reviewed, 6 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2)
Ear, Nose & Throat Journal, doi:10.1177/0145561320957237
Introduction: Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) is the pathogen responsible for the global pandemic of coronavirus disease 2019 (COVID-19). From the first reported cases in December 2019, the virus has spread to over 4 million people worldwide. Human-to-human transmission occurs mainly through the aerosolization of respiratory droplets. Transmission also occurs through contact with contaminated surfaces and other fomites. Improved antisepsis of human and nonhuman surfaces has been identified as a key feature of transmission reduction. There are no previous studies of povidone iodine (PVP-I) against SARS-CoV-2. This study evaluated nasal and oral antiseptic formulations of PVP-I for virucidal activity against SARS-CoV-2. This is the first report on the efficacy of PVP-I against the virus that causes COVID-19. Methods: Povidone iodine nasal antiseptic formulations and PVP-I oral rinse antiseptic formulations from 1% to 5% concentrations as well as controls were studied for virucidal efficacy against the SARS-CoV-2. Test compounds were evaluated for ability to inactivate SARS-CoV-2 as measured in a virucidal assay. SARS-CoV-2 was exposed directly to the test compound for 60 seconds, compounds were then neutralized, and surviving virus was quantified. Results: All concentrations of nasal antiseptics and oral rinse antiseptics evaluated completely inactivated the SARS-CoV-2. Conclusions: Nasal and oral PVP-I antiseptic solutions are effective at inactivating the SARS-CoV-2 at a variety of concentrations after 60-second exposure times. The formulations tested may help to reduce the transmission of SARS-CoV-2 if used for nasal decontamination, oral decontamination, or surface decontamination in known or suspected cases of COVID-19.
References
Berkelman, Holland, Anderson, Increased bactericidal activity of dilute preparations of povidone-iodine solutions, J Clin Microbiol
Dexter, Parra, Brown, Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management, Anesth analg
Eggers, Eickmann, Zorn, Rapid and effective virucidal activity of povidone-iodine products against middle east respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus Ankara (MVA), Infectious diseases Therapy
Eggers, Infectious disease management and control with povidone iodine, Infect Dis Ther
Frank, Capriotti, Brown, Tessema, Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 era
Gocke, Ponticas, Pollack, In vitro studies of the killing of clinical isolates by povidone-iodine solutions, J Hosp Infect
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lan, doi:10.1016/S0140-6736(20)30183-5
Kariwa, Fujii, Takashima, Inactivation of SARS coronavirus by means of povidone iodine, physical conditions and chemical reagents, Dermatology
Kawana, Kitamura, Nakagomi, Inactivation of human viruses by povidone iodine in comparison with other antiseptics, Dermatology
Khan, Parab, Paranjape, Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in Covid 19 pandemic, Am J Otolaryngol
Kim, Rimmer, Mrad, Ahmadzada, Harvey, Betadine has a ciliotoxic effect on ciliated human respiratory cells, J Laryngol Otol
Krajewska, Krajewski, Zub, COVID-19 in otolaryngologist practice: a review of current knowledge, Eur Arch Otorhinol, doi:10.1007/s00405-020-05968-y
Leung, Chu, Shiu, Respiratory virus shedding in exhaled breath and efficacy of face masks, Nat Med
Mady, Kubik, Baddour, Consideration of povidoneiodine as a public healthy intervention for COVID-19: utilization as ''personal protective equipment'' for frontline providers exposed in high-risk head and neck and skull base oncology care, Oral Oncol
Parhar, Tasche, Brody, Topical preparations to reduce SARS-CoV-2 aerosolization in head and neck mucosal surgery, Head Neck
Ramezanpour, Smith, Psaltis, In vitro safety evaluation of a povidone-iodine solution applied to human nasal epithelial cells. [Online ahead of print, Int Forum Allergy Rhinol, doi:10.1002/alr.22575
Reimer, Wichelhaus, Schafer, Antimicrobial effectiveness of povidone-iodine and consequences for new application areas, Dermatology
Sapkota, Wyatt, Alcohol, aldehydes, adducts and airways, Biomolecules
Sato, Miyake, Hazama, Povidone-iodine-induced cell death in cultured human epithelial HeLa cells and rat oral mucosal tissue, Drug Chem Toxicol
Sriwilaijaroen, Wilairat, Hiramatsu, Mechanisms of the action of povidone-iodine against human and avian influenza a viruses: its effects on hemagglutination and sialidase activities, Virol J
Sungnak, Huang, Bécavin, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med
Tessema, Frank, Bidra, SARS-CoV-2 viral inactivation using low dose povidone-iodine oral rinse-immediate application for the prosthodontic practice, J Prosthodont
Van Doremalen, Bushmaker, Morris, Aerosol and surface stability of SARS-CoV-2 compared with SARS-CoV-1, N Engl J Med
Vetter, Augustijns, Bernkop-Schnurch, Solubilizing agents in nasal formulations and their effect on ciliary beat frequency, Toxicology in Vitro
Wei, Li, Chiew, Presymptomatic transmission of SARS-CoV-2-Singapore, January 23, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm6914e1
Workman, Cohen, The effect of drugs and other compounds on the ciliary beat frequency of human respiratory epithelium, Am J Rhinol Allergy
Wu, Mcgoogan, of 72314 cases from the Chinese center for disease control and prevention
Xu, Zhang, Jin, Persistent shedding of viable SARS-CoV in urine and stool of SARS patients during the convalescent phase, Eur J Clin Microbiol Infect Dis
Zou, Ruan, Huang, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med
DOI record:
{
"DOI": "10.1177/0145561320957237",
"ISSN": [
"0145-5613",
"1942-7522"
],
"URL": "http://dx.doi.org/10.1177/0145561320957237",
"abstract": "<jats:sec><jats:title>Introduction:</jats:title><jats:p> Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) is the pathogen responsible for the global pandemic of coronavirus disease 2019 (COVID-19). From the first reported cases in December 2019, the virus has spread to over 4 million people worldwide. Human-to-human transmission occurs mainly through the aerosolization of respiratory droplets. Transmission also occurs through contact with contaminated surfaces and other fomites. Improved antisepsis of human and nonhuman surfaces has been identified as a key feature of transmission reduction. There are no previous studies of povidone iodine (PVP-I) against SARS-CoV-2. This study evaluated nasal and oral antiseptic formulations of PVP-I for virucidal activity against SARS-CoV-2. This is the first report on the efficacy of PVP-I against the virus that causes COVID-19. </jats:p></jats:sec><jats:sec><jats:title>Methods:</jats:title><jats:p> Povidone iodine nasal antiseptic formulations and PVP-I oral rinse antiseptic formulations from 1% to 5% concentrations as well as controls were studied for virucidal efficacy against the SARS-CoV-2. Test compounds were evaluated for ability to inactivate SARS-CoV-2 as measured in a virucidal assay. SARS-CoV-2 was exposed directly to the test compound for 60 seconds, compounds were then neutralized, and surviving virus was quantified. </jats:p></jats:sec><jats:sec><jats:title>Results:</jats:title><jats:p> All concentrations of nasal antiseptics and oral rinse antiseptics evaluated completely inactivated the SARS-CoV-2. </jats:p></jats:sec><jats:sec><jats:title>Conclusions:</jats:title><jats:p> Nasal and oral PVP-I antiseptic solutions are effective at inactivating the SARS-CoV-2 at a variety of concentrations after 60-second exposure times. The formulations tested may help to reduce the transmission of SARS-CoV-2 if used for nasal decontamination, oral decontamination, or surface decontamination in known or suspected cases of COVID-19. </jats:p></jats:sec>",
"alternative-id": [
"10.1177/0145561320957237"
],
"author": [
{
"affiliation": [
{
"name": "Ocean Ophthalmology Group, Miami, FL, USA"
}
],
"family": "Pelletier",
"given": "Jesse S.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-7418-6034",
"affiliation": [
{
"name": "ProHealth Physicians Ear, Nose, and Throat, Farmington, CT, USA"
},
{
"name": "Department of Otolaryngology, University of Connecticut, Farmington, CT, USA"
}
],
"authenticated-orcid": false,
"family": "Tessema",
"given": "Belachew",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5068-1065",
"affiliation": [
{
"name": "Department of Otolaryngology, University of Connecticut, Farmington, CT, USA"
}
],
"authenticated-orcid": false,
"family": "Frank",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Institute for Antiviral Research at Utah State University, Logan, UT, USA"
}
],
"family": "Westover",
"given": "Jonna B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ProHealth Physicians Ear, Nose, and Throat, Farmington, CT, USA"
},
{
"name": "Department of Otolaryngology, University of Connecticut, Farmington, CT, USA"
}
],
"family": "Brown",
"given": "Seth M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Veloce BioPharma, Fort Lauderdale, FL, USA"
}
],
"family": "Capriotti",
"given": "Joseph A.",
"sequence": "additional"
}
],
"container-title": [
"Ear, Nose & Throat Journal"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2020,
9,
21
]
],
"date-time": "2020-09-21T07:59:58Z",
"timestamp": 1600675198000
},
"deposited": {
"date-parts": [
[
2021,
2,
25
]
],
"date-time": "2021-02-25T04:06:22Z",
"timestamp": 1614225982000
},
"funder": [
{
"name": "Veloce BioPharma"
}
],
"indexed": {
"date-parts": [
[
2021,
12,
16
]
],
"date-time": "2021-12-16T22:28:22Z",
"timestamp": 1639693702352
},
"is-referenced-by-count": 16,
"issn-type": [
{
"type": "print",
"value": "0145-5613"
},
{
"type": "electronic",
"value": "1942-7522"
}
],
"issue": "2_suppl",
"issued": {
"date-parts": [
[
2020,
9,
21
]
]
},
"journal-issue": {
"issue": "2_suppl",
"published-print": {
"date-parts": [
[
2021,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
9,
21
]
],
"date-time": "2020-09-21T00:00:00Z",
"timestamp": 1600646400000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/0145561320957237",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/0145561320957237",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/0145561320957237",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "192S-196S",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2020,
9,
21
]
]
},
"published-online": {
"date-parts": [
[
2020,
9,
21
]
]
},
"published-print": {
"date-parts": [
[
2021,
4
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1056/NEJMc2001737",
"doi-asserted-by": "publisher",
"key": "bibr1-0145561320957237"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"doi-asserted-by": "publisher",
"key": "bibr2-0145561320957237"
},
{
"DOI": "10.15585/mmwr.mm6914e1",
"doi-asserted-by": "publisher",
"key": "bibr3-0145561320957237"
},
{
"DOI": "10.1007/s10096-005-1299-5",
"doi-asserted-by": "publisher",
"key": "bibr4-0145561320957237"
},
{
"DOI": "10.1056/NEJMc2004973",
"doi-asserted-by": "publisher",
"key": "bibr7-0145561320957237"
},
{
"DOI": "10.1016/j.oraloncology.2020.104724",
"doi-asserted-by": "publisher",
"key": "bibr8-0145561320957237"
},
{
"DOI": "10.1002/hed.26200",
"doi-asserted-by": "publisher",
"key": "bibr9-0145561320957237"
},
{
"DOI": "10.1213/ANE.0000000000004829",
"doi-asserted-by": "publisher",
"key": "bibr10-0145561320957237"
},
{
"DOI": "10.1016/j.amjoto.2020.102618",
"doi-asserted-by": "publisher",
"key": "bibr11-0145561320957237"
},
{
"DOI": "10.1111/jopr.13207",
"doi-asserted-by": "publisher",
"key": "bibr12-0145561320957237"
},
{
"DOI": "10.1016/S0195-6701(85)80047-5",
"doi-asserted-by": "publisher",
"key": "bibr13-0145561320957237"
},
{
"DOI": "10.1128/JCM.15.4.635-639.1982",
"doi-asserted-by": "publisher",
"key": "bibr14-0145561320957237"
},
{
"DOI": "10.1007/s40121-019-00260-x",
"doi-asserted-by": "publisher",
"key": "bibr15-0145561320957237"
},
{
"DOI": "10.1186/1743-422X-6-124",
"doi-asserted-by": "publisher",
"key": "bibr16-0145561320957237"
},
{
"DOI": "10.1007/s40121-015-0091-9",
"doi-asserted-by": "publisher",
"key": "bibr17-0145561320957237"
},
{
"DOI": "10.1159/000246027",
"doi-asserted-by": "publisher",
"key": "bibr18-0145561320957237"
},
{
"DOI": "10.1159/000089211",
"doi-asserted-by": "publisher",
"key": "bibr19-0145561320957237"
},
{
"DOI": "10.1038/s41591-020-0843-2",
"doi-asserted-by": "publisher",
"key": "bibr20-0145561320957237"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "bibr21-0145561320957237"
},
{
"DOI": "10.1016/j.tiv.2011.10.011",
"doi-asserted-by": "publisher",
"key": "bibr22-0145561320957237"
},
{
"DOI": "10.2500/ajra.2014.28.4092",
"doi-asserted-by": "publisher",
"key": "bibr23-0145561320957237"
},
{
"DOI": "10.3390/biom5042987",
"doi-asserted-by": "publisher",
"key": "bibr24-0145561320957237"
},
{
"DOI": "10.1159/000057738",
"doi-asserted-by": "publisher",
"key": "bibr25-0145561320957237"
},
{
"DOI": "10.1002/alr.22575",
"doi-asserted-by": "publisher",
"key": "bibr26-0145561320957237"
},
{
"DOI": "10.1017/S0022215114002746",
"doi-asserted-by": "publisher",
"key": "bibr27-0145561320957237"
},
{
"DOI": "10.3109/01480545.2013.846364",
"doi-asserted-by": "publisher",
"key": "bibr28-0145561320957237"
},
{
"author": "Frank S",
"first-page": "2020",
"journal-title": "Ear Nose Throat J",
"key": "bibr29-0145561320957237"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2020.05.25.20110239",
"id-type": "doi"
}
]
},
"score": 1,
"short-container-title": [
"Ear Nose Throat J"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Otorhinolaryngology"
],
"subtitle": [],
"title": [
"Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2)"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "100"
}
