Population-based virucidal phthalocyanine gargling/rinsing protocol to reduce the risk of coronavirus disease-2019: a community trial
et al., German Medical Science GMS Publishing House, doi:10.3205/dgkh000426, RBR-6c9xnw3, Nov 2021 (preprint)
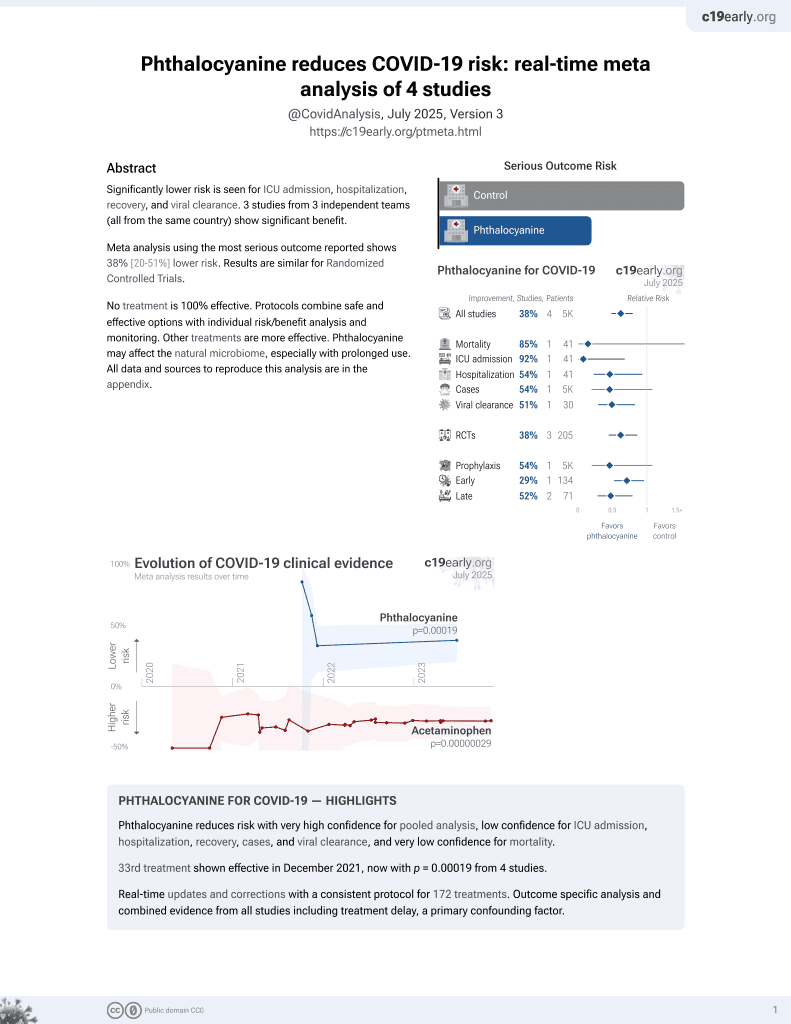
33rd treatment shown to reduce risk in
December 2021, now with p = 0.00019 from 4 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
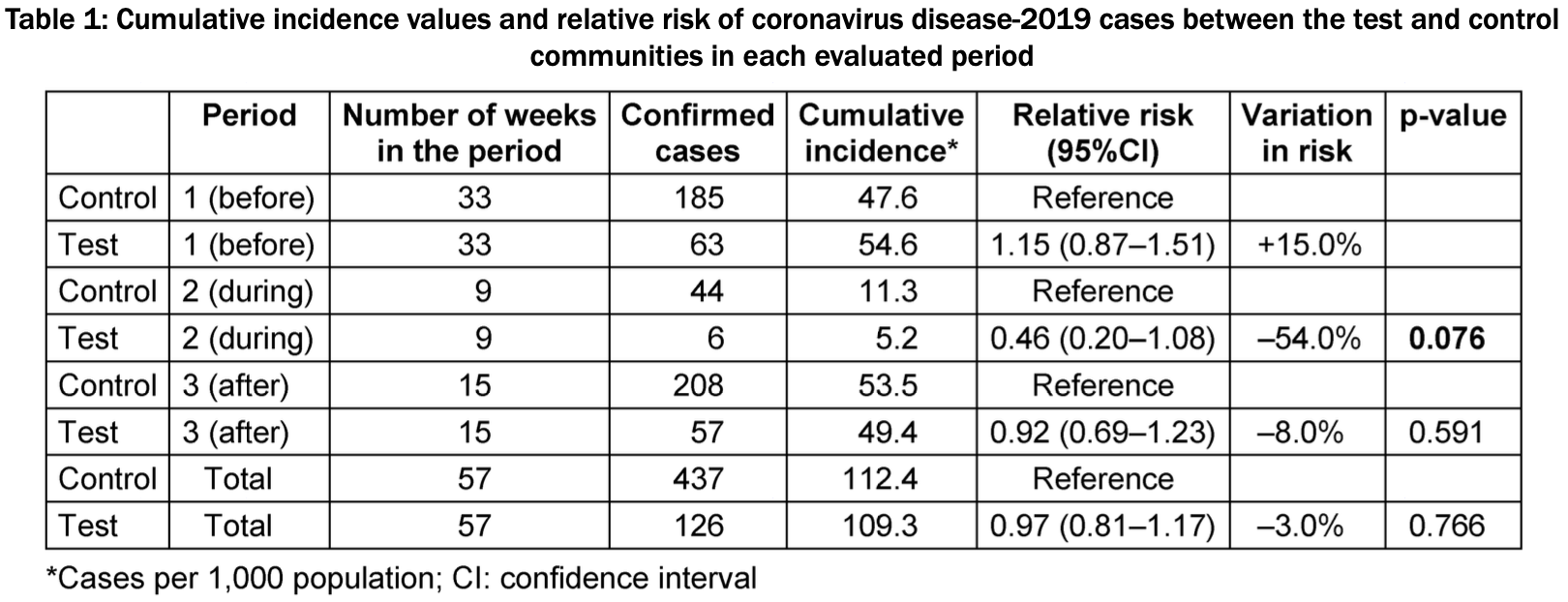
Comparison of two similar communities in Brazil, with one using a phthalocyanine derivative mouthwash, suggesting efficacy of the treatment in lowering COVID-19 cases. There was 54% lower risk of confirmed cases during the intervention in the treatment community, compared with 15% higher and 8% lower risk before and after the intervention. Gargle/rinse with 5mL of mouthwash containing phthalocyanine derivative for 1 minute, 3 to 5 times per day.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
|
risk of case, 54.0% lower, RR 0.46, p = 0.08, treatment 6 of 1,153 (0.5%), control 44 of 3,887 (1.1%), NNT 164.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Poleti et al., Use of mouthwash and dentifrice containing antimicrobial phthalocyanine derivative for the reduction of clinical symptoms of COVID-19: A randomized triple-blinded clinical trial, Journal of Evidence-Based Dental Practice, doi:10.1016/j.jebdp.2022.101777.
Brito-Reia et al., 15 Nov 2021, prospective, Brazil, peer-reviewed, 7 authors, trial RBR-6c9xnw3.
Contact: veronicabr@usp.br.
Population-based virucidal phthalocyanine gargling/rinsing protocol to reduce the risk of coronavirus disease-2019: a community trial Ein viruzides Phthalocyanin-basiertes Mundspül-/Gurgelprotokoll zur Reduzierung des Erkrankungsrisikos durch COVID-19: eine Bevölkerungsstudie
doi:10.3205/dgkh000426
Aim: In this community trial, the objective was to evaluate the incidence of coronavirus disease-2019 (COVID-19) cases in two similar communi-
Competing interests The authors declare that they have no competing interests.
References
Ausina-Márquez, Ferrer, Oral antiseptics against coronavirus: in-vitro and clinical evidence, J Hosp Infect, doi:10.1016/j.jhin.2021.04.004
Brito-Reia, Population-based virucidal phthalocyanine gargling/rinsing
Brito-Reia, Population-based virucidal phthalocyanine gargling/rinsing
Burton, Clarkson, Goulao, Glenny, Mcbain et al., Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them, Cochrane Database Syst Rev, doi:10.1002/14651858.CD013627.pub2
Carrouel, Conte, Fisher, Gonçalves, Dussart et al., COVID-19: A Recommendation to Examine the Effect of Mouthrinses with β-Cyclodextrin Combined with Citrox in Preventing Infection and Progression, J Clin Med, doi:10.3390/jcm9041126
Carrouel, Gonçalves, Conte, Campus, Fisher et al., Antiviral Activity of Reagents in Mouth Rinses against SARS-CoV-2, J Dent Res, doi:10.1177/0022034520967933
Carrouel, Valette, Gadea, Esparcieux, Illes et al., Use of an antiviral mouthwash as a barrier measure in the SARS-CoV-2 transmission in adults with asymptomatic to mild COVID-19: a multicentre, randomized, double-blind controlled trial, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.05.028
Casale, Rinaldi, Sabatino, Moffa, Ciccozzi, Could nasal irrigation and oral rinse reduce the risk for COVID-19 infection?, Int J Immunopathol Pharmacol, doi:10.1177/2058738420941757
Chaudhary, Melkonyan, Meethil, Saraswat, Hall et al., Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load: A randomized controlled trial, J Am Dent Assoc, doi:10.1016/j.adaj.2021.05.021
Choudhury, Shabnam, Ahsan, Kabir, Khan et al., Effect of 1?% povidone iodine mouthwash/gargle, nasal and eye drop in COVID -19 patient, Biores Comm, doi:10.3329/brc.v7i1.54245
Da Fonseca Orcina, Vilhena, De Oliveira, Da, Alves et al., A Phthalocyanine Derivate Mouthwash to Gargling/Rinsing as an Option to Reduce Clinical Symptoms of COVID-19: Case Series, Clin Cosmet Investig Dent, doi:10.2147/CCIDE.S295423
Da, Santos, Da Fonseca Orcina, Da, Alves, A recommendation of PHTALOX® mouthwash for preventing infection and progression of COVID-19, Acta Scient Dent Sci, doi:10.31080/ASDS.2020.04.0991
Da, Santos, Da Fonseca Orcina, Machado, Vilhena et al., Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: randomised trial, Sci Rep, doi:10.1038/s41598-021-99013-5
De Almeida, Pronunciate, Grotto, Pugliesi, Guimarães et al., Two hundred days of COVID-19 in São Paulo State, Brazil, Epidemiol Infect, doi:10.1017/S0950268820002927
Eduardo, Corrêa, Heller, Daep, Benitez et al., Salivary SARS-CoV-2 load reduction with mouthwash use: A randomized pilot clinical trial, Heliyon, doi:10.1016/j.heliyon.2021.e07346
Encinar, Menendez, Potential Drugs Targeting Early Innate Immune Evasion of SARS-Coronavirus 2 via 2'-O-Methylation of Viral RNA, Viruses, doi:10.3390/v12050525
Fernandes Matuck, Dolhnikoff, Maia, Sendyk, Zarpellon et al., Periodontal tissues are targets for Sars-Cov-2: a post-mortem study, J Oral Microbiol, doi:10.1080/20002297.2020.18481357/8
Fortaleza, Guimarães, Catão, Ferreira, Berg De Almeida et al., The use of health geography modeling to understand early dispersion of COVID-19 in São Paulo, Brazil, PLoS One, doi:10.1371/journal.pone.0245051
Gao, Xu, Sun, Wang, Guo et al., A systematic review of asymptomatic infections with COVID-19, J Microbiol Immunol Infect, doi:10.1016/j.jmii.2020.05.001
Justo, Bueno, Barbosa, Perosa, Carvalho et al., Comparison of viral load between saliva and nasopharyngeal swabs for SARS-CoV2: the role of days of symptoms onset on diagnosis, Mem Inst Oswaldo Cruz, doi:10.1590/0074-02760210018
Konishi, Saito, Ishikawa, Kanai, Igei, How Did Japan Cope with COVID-19? Big Data and Purchasing Behavior, Asian Econ Papers, doi:10.1162/asep_a_00797
Kramer, Eggers, Hübner, Walger, Steinmann et al., Virucidal gargling and virucidal nasal spray, GMS Hyg Infect Control, doi:10.3205/dgkh000373
Lamas, Dios, Rodríguez, Campo Pérez, Alvargonzalez et al., Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests, Oral Dis, doi:10.1111/odi.13526
Matuck, Dolhnikoff, Duarte-Neto, Maia, Gomes et al., Salivary glands are a target for SARS-CoV-2: a source for saliva contamination, J Pathol, doi:10.1002/path.5679
Mohamed, Baharom, Sulaiman, Rashid, Early viral clearance among covid-19 patients when gargling with povidone-iodine and essential oils: a pilot clinical trial
Nagatake, Ahmed, Oishi, Prevention of respiratory infections by povidone-iodine gargle, Dermatology, doi:10.1159/000057722
Orcina, Santos, Oral manifestation COVID-19 and the rapid resolution of symptoms post-phtalox treatment: a case series, Int J Odontostomat
Peng, Xu, Li, Cheng, Zhou et al., Transmission routes of 2019-nCoV and controls in dental practice, Int J Oral Sci, doi:10.1038/s41368-020-0075-9
Santos, Da Fonseca Orcina, Reia, Ribeiro, Grotto et al., Virucidal Activity of the Antiseptic Mouthwash and Dental Gel Containing Anionic Phthalocyanine Derivative: In vitro Study, GMS Hygiene and Infection Control, doi:10.2147/CCIDE.S3154196/8
Tashiro, Shaw, COVID-19 Pandemic response in Japan: what is behind the initial flattening of the curve? Sustainability, doi:10.3390/su12135250
Teodoro, Santos, Campos, Koga-Ito, Sibelino et al., PHTALOX® antimicrobial action and cytotoxicity: in vitro study
To, Tsang, Yip, Chan, Wu et al., Consistent Detection of 2019 Novel Coronavirus in Saliva, Clin Infect Dis, doi:10.1093/cid/ciaa149
Vilhena, Reia, Da Fonseca Orcina, Santos, Zangrando et al., The use of antiviral Phthalocyanine mouthwash as a preventive measure against COVID-19, GMS Hyg Infect Control, doi:10.3205/dgkh000395
Wu, Zhao, Yu, Chen, Song et al., A new coronavirus associated with human respiratory disease in China, Nature, doi:10.1038/s41586-020-2008-3
DOI record:
{
"DOI": "10.3205/DGKH000426",
"URL": "https://www.egms.de/en/journals/dgkh/2022-17/dgkh000426.shtml",
"abstract": "Aim: In this community trial, the objective was to evaluate the incidence of coronavirus disease-2019 (COVID-19) cases in two similar communities in three distinct phases: 1 (before the intervention), 2 (during the intervention), and 3 (after the intervention). Methods: The test community received the oral antiseptic intervention (experimental), while the control community did not. The official information agency (“Statewise System for Data Analysis”) provided the number of confirmed COVID-19 cases. Data were analyzed according to the three phases per epidemiological week (epi) using the R Core Team (2021) program. The relative risk and 95% confidence intervals between the cumulative incidence values of the test and control communities were calculated for each period. In the test community, a total of 995 residents over 10 years of age received two bottles containing 600 ml of mouthwash containing antiviral phthalocyanine derivative (APD). The participants were asked to gargle/rinse with of 5 mL of the mouthwash containing ADP 3 to 5 times a day, for 1 min, until the bottles were empty. Results: In phases 1 and 3, the disease risk between the two communities did not differ significantly (p>0.05), while in phase 2, the disease risk was 54% lower in the test community than in the control community. Conclusion: The use of the APD mouthwash protocol seems to reduce the COVID-19 incidence at the population level, and further studies are needed to confirm its protective effect under more precisely controlled conditions.",
"author": [
{
"family": "Brito-Reia",
"given": "Verônica Caroline"
},
{
"family": "da Silva Bastos",
"given": "Roosevelt"
},
{
"family": "Vieira Vilhena",
"given": "Fabiano"
},
{
"family": "Marques Honório",
"given": "Heitor"
},
{
"family": "Marques da Costa Alves",
"given": "Lucas"
},
{
"family": "Frazão",
"given": "Paulo"
},
{
"family": "Sérgio da Silva Santos",
"given": "Paulo"
}
],
"categories": [
"COVID-19",
"severe acute respiratory syndrome coronavirus 2",
"antiviral mouthwash",
"population-based study",
"phthalocyanine derivate",
"COVID-19",
"Schweres Akutes Respiratorisches Syndrom-Coronavirus 2",
"antivirale Mundspülung",
"Bevölkerungsstudie",
"Phthalocyanin Derivat",
"Medicine and health"
],
"copyright": "Creative Commons Attribution 4.0 International",
"id": "https://doi.org/10.3205/dgkh000426",
"issued": {
"date-parts": [
[
2022,
12,
6
]
]
},
"language": "en",
"publisher": "German Medical Science GMS Publishing House",
"title": "Population-based virucidal phthalocyanine gargling/rinsing protocol to reduce the risk of coronavirus disease-2019: a community trial",
"type": "article-journal"
}