Use of mouthwash and dentifrice containing antimicrobial phthalocyanine derivative for the reduction of clinical symptoms of COVID-19: A randomized triple-blinded clinical trial
et al., Journal of Evidence-Based Dental Practice, doi:10.1016/j.jebdp.2022.101777, RBR-8x8g36, Dec 2021 (preprint)
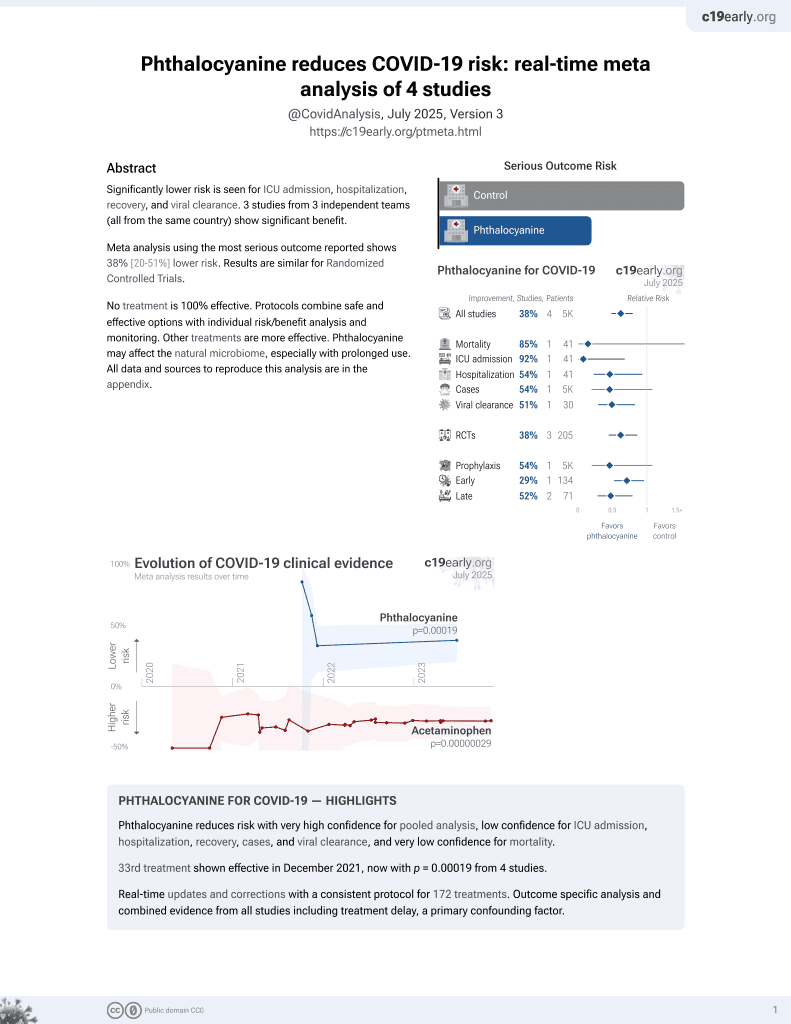
34th treatment shown to reduce risk in
December 2021, now with p = 0.00019 from 4 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 500 patients in Brazil, showing improved recovery with a phthalocyanine derivative mouthwash and toothpaste. Toothbrushing for 2 minutes, three times per day, and gargling/rising (5ml) for one minute, three times a day, for 7 days.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
|
risk of no recovery, 29.1% lower, RR 0.71, p = 0.02, treatment 29 of 59 (49.2%), control 52 of 75 (69.3%), NNT 5.0, day 7.
|
|
risk of no recovery, 22.1% lower, RR 0.78, p = 0.02, treatment 38 of 59 (64.4%), control 62 of 75 (82.7%), NNT 5.5, day 3.
|
|
risk of no recovery, 45.5% lower, RR 0.54, p = 0.04, treatment 12 of 59 (20.3%), control 28 of 75 (37.3%), NNT 5.9, day 7, dyspnea.
|
|
risk of no recovery, 32.5% lower, RR 0.68, p = 0.11, treatment 17 of 59 (28.8%), control 32 of 75 (42.7%), NNT 7.2, day 3, dyspnea.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Poleti et al., Use of mouthwash and dentifrice containing antimicrobial phthalocyanine derivative for the reduction of clinical symptoms of COVID-19: A randomized triple-blinded clinical trial, Journal of Evidence-Based Dental Practice, doi:10.1016/j.jebdp.2022.101777.
Poleti et al., 8 Dec 2021, Double Blind Randomized Controlled Trial, Brazil, peer-reviewed, 10 authors, study period 6 November, 2020 - 19 November, 2020, trial RBR-8x8g36.
Contact: marcelo_poleti@yahoo.com.br.
USE OF MOUTHWASH AND DENTIFRICE CONTAINING AN ANTIMICROBIAL PHTHALOCYANINE DERIVATIVE FOR THE REDUCTION OF CLINICAL SYMPTOMS OF COVID-19: A RANDOMIZED TRIPLE-BLIND CLINICAL TRIAL
Journal of Evidence-Based Dental Practice, doi:10.1016/j.jebdp.2022.101777
Purpose This clinical trial aimed to evaluate the use of mouthwash and dentifrice containing an antimicrobial phthalocyanine derivative (APD) to reduce the clinical symptoms in patients with COVID-19.
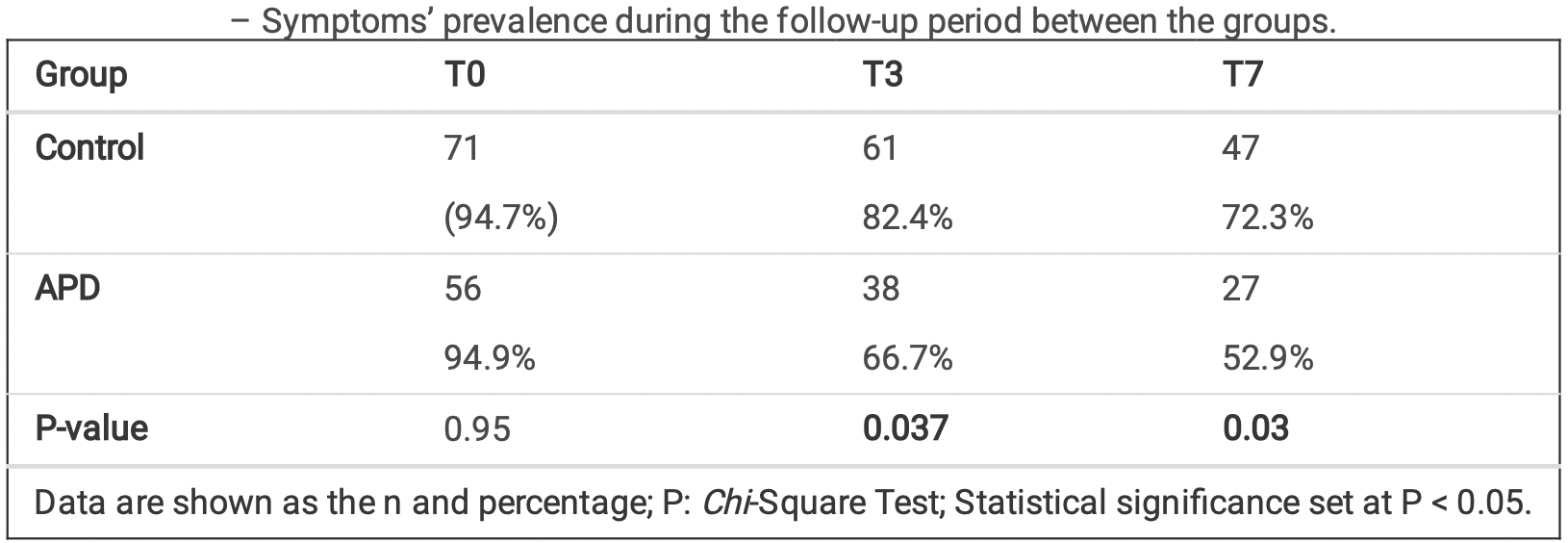
Methods This randomized, triple-blind clinical trial enrolled 134 patients aged 18 years or older who underwent COVID-19 testing through the use of nasopharyngeal swab RT-qPCR in a reference center for the diagnosis of COVID-19, had no clinical contraindications to mouthwash and gargle, and had access to cell phones with communication applications. According to the use of a mouthwash and dentifrice containing antimicrobial phthalocyanine derivatives (APD), patients were randomly assigned (1:1) to the APD or non-APD (control) group. All participants were instructed to floss twice a day, brush teeth for 2 minutes 3 times a day, and gargle/rinse (5 mL) for 1 min/3 times a day for 7 days. An online questionnaire was sent to collect data on the clinical symptoms of COVID-19 3 times: T0 (baseline before using the oral hygiene products), T3 (3 days after), and T7 (7 days after). The investigators, patients, and outcome assessors were blinded to group assignment. The Mann-Whitney, Chi -Square, Fisher's exact, and Cochran's tests were used according to the nature of the variables studied, with the level of significance set at P < .05.
Results No statistically significant difference was found in the prevalence of symptoms between groups at baseline. A statistically significant reduction in clinical symptoms was found in the control group (fatigue, shortness of breath, hoarse voice,
ACKNOWLEDGMENT The authors gratefully acknowledge the Londrina Municipal Health Authority for their support in this study.
CREDIT AUTHOR STATEMENT
References
Aida, Fukai, Watt, Global neglect of dental coverage in universal health coverage systems and Japan's broad coverage, Int Dent J, doi:10.1016/j.identj.2020.12.027
D'amico, Baumgart, Danese, Peyrin-Biroulet, Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management, Clin Gastroenterol Hepatol, doi:10.1016/j.cgh.2020.04.001
Da, Santos, Da Fonseca Orcina, Machado, Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: randomized trial, Sci Rep, doi:10.1038/s41598-021-99013-5
Elzein, Sater, Fakhreddine, In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial, J Evid Based Dent Pract, doi:10.1016/j.jebdp.2021.101584
Fregni, Illigens, Critical Thinking in Clinical Research: Applied Theory and Practice Using Case Studies
González-Olmo, Delgado-Ramos, Ruiz-Guillén, Romero-Maroto, Carrillo-Díaz, Oral hygiene habits and possible transmission of COVID-19 among cohabitants, BMC Oral Health, doi:10.1186/s12903-020-01274-5
Haukoos, Newgard, Advanced statistics: missing data in clinical research -part 1: an introduction and conceptual framework, Acad Emerg Med, doi:10.1111/j.1553-2712.2007.tb01855.x
Huang, Pérez, Kato, SARS-CoV-2 infection of the oral cavity and saliva, Nat Med, doi:10.1038/s41591-021-01296-8
Imai, Tanaka, SARS-CoV-2 infection and significance of oral health management in the era of "the new normal with COVID-19, Int J Mol Sci, doi:10.3390/ijms22126527
Iranmanesh, Khalili, Amiri, Zartab, Aflatoonian, Oral manifestations of COVID-19 disease: a review article, Dermatol Ther, doi:10.1111/dth.14578
Jafer, Hazazi, Mashi, COVID-19 and Periodontitis: a reality to live with, J Contemp Dent Pract
Kamel, Basuoni, Salem, Abubakr, The impact of oral health status on COVID-19 severity, recovery period and C-reactive protein values, Br Dent J, doi:10.1038/s41415-021-2656-1
Lu, Wang, Oral microbiota: a new view of body health, Food Science and Human Wellness, doi:10.1016/j.fshw.2018.12.001
Matuck, Dolhnikoff, Duarte-Neto, Salivary glands are a target for SARS-CoV-2: a source for saliva contamination, J Pathol, doi:10.1002/path.5679
Matuck, Dolhnikoff, Maia, Periodontal tissues are targets for SARS-CoV-2: a post-mortem study, J Oral Microbiol, doi:10.1080/20002297.2020.1848135
Menni, Valdes, Freidin, Real-time tracking of self-reported symptoms to predict potential COVID-19, Nat Med, doi:10.1038/s41591-020-0916-2
Moher, Hopewell, Schulz, CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials, Int J Surg, doi:10.1016/j.ijsu.2011.10.001
Moosavi, Aminishakib, Ansari, Antiviral mouthwashes: possible benefit for COVID-19 with evidence-based approach, J Oral Microbiol, doi:10.1080/20002297.2020.1794363
Nagatake, Ahmed, Oishi, Prevention of respiratory infections by povidone-iodine gargle, Dermatology, doi:10.1159/000057722
Orcina, Santos, Oral manifestation COVID-19 and the rapid resolution of symptoms post-phtalox treatment: a case series, Int J Odontostomat, doi:10.4067/S0718-381X2021000100067
Orcina, Vilhena, Oliveira, A phthalocyanine derivate mouthwash to gargling/rinsing as an option to reduce clinical symptoms of COVID-19: case series, Clin Cosmet Investig Dent, doi:10.2147/CCIDE.S295423
Pepsodent, Bangladesh research summary report 2021: attitudes, behaviors and experiences of oral health during the covid-19 pandemic
Pinzan-Vercelino, Freitas, Girão, Da Silva, Peloso et al., Does the use of face masks during the COVID-19 pandemic impact on oral hygiene habits, oral conditions, reasons to seek dental care and esthetic concerns?, J Clin Exp Dent, doi:10.4317/jced.57798
Poleti, Gregório, Bistaffa, Toothbrushing with a dentifrice containing antimicrobial phthalocyanine derivative for the intraoral reduction of viral load of SARS-CoV-2: a pilot study, Res Sq. Preprint, doi:10.21203/rs.3.rs-690819/v1
Sampson, Kamona, Sampson, Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections?, Br Dent J, doi:10.1038/s41415-020-1747-8
Santos, Da Fonseca Orcina, Reia, Virucidal activity of the antiseptic mouthwash and dental gel containing anionic phthalocyanine derivative: in vitro study, Clin Cosmet Investig Dent, doi:10.2147/CCIDE.S315419
Santos, Orcina, Alves, Oliveira, Zangrando et al., A recommendation of PHTALOX mouthwash for preventing infection and progression of COVID-19, Acta Scient Dent Sci, doi:10.31080/ASDS.2020.04.0991
Santos, Vilhena, Zangrando, Adjuvant use of phthalocyanine derivative for calculus control, Int, doi:10.5348/101220Z01CS2021CI
Satomura, Kitamura, Kawamura, Prevention of upper respiratory tract infections by gargling: a randomized trial, Am J Prev Med, doi:10.1016/j.amepre.2005.06.013
Schürmann, Aljubeh, Tiemann, Sudhoff, Mouthrinses against SARS-CoV-2: anti-inflammatory effectivity and a clinical pilot study, Eur Arch Otorhinolaryngol, doi:10.1007/s00405-021-06873-8
Schürmann, Aljubeh, Tiemann, Sudhoff, Mouthrinses against SARS-CoV-2: anti-inflammatory effectivity and a clinical pilot study, Eur Arch Otorhinolaryngol, doi:10.1007/s00405-021-06873-8
Silva, Azevedo, Sampaio-Maia, Sousa-Pinto, The effect of mouthrinses on severe acute respiratory syndrome coronavirus 2 viral load: a systematic review, J Am Dent Assoc, doi:10.1016/j.adaj.2021.12.007
Vilhena, Reia, Da Fonseca Orcina, The use of antiviral phthalocyanine mouthwash as a preventive measure against COVID-19, GMS Hyg Infect Control, doi:10.3205/dgkh000395
Zanatta, Antoniazzi, Rösing, Staining and calculus formation after 0.12% chlorhexidine rinses in plaque-free and plaque covered surfaces: a randomized trial, J Appl Oral Sci, doi:10.1590/s1678-77572010000500015
Zhang, Liu, Zhang, Jiang, Tai et al., Impact of COVID-19 on the oral health of adults in Wuhan and China: results of a nationwide online cross-sectional questionnaire survey, BMC Oral Health, doi:10.1186/s12903-021-01533-z
DOI record:
{
"DOI": "10.1016/j.jebdp.2022.101777",
"ISSN": [
"1532-3382"
],
"URL": "http://dx.doi.org/10.1016/j.jebdp.2022.101777",
"alternative-id": [
"S1532338222001002"
],
"article-number": "101777",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "USE OF MOUTHWASH AND DENTIFRICE CONTAINING AN ANTIMICROBIAL PHTHALOCYANINE DERIVATIVE FOR THE REDUCTION OF CLINICAL SYMPTOMS OF COVID-19: A RANDOMIZED TRIPLE-BLIND CLINICAL TRIAL"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Evidence-Based Dental Practice"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jebdp.2022.101777"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 Elsevier Inc. All rights reserved."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1904-5762",
"affiliation": [],
"authenticated-orcid": false,
"family": "Poleti",
"given": "Marcelo Lupion",
"sequence": "first"
},
{
"affiliation": [],
"family": "Gregório",
"given": "Danielle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bistaffa",
"given": "Alisson Gabriel Idelfonso",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernandes",
"given": "Karen Barros Parron",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vilhena",
"given": "Fabiano Vieira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "Paulo Sérgio da Silva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simão",
"given": "Andréa Name Colado",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lozovoy",
"given": "Marcell Alysson Batisti",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tatibana",
"given": "Berenice Tomoko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernandes",
"given": "Thais Maria Freire",
"sequence": "additional"
}
],
"container-title": "Journal of Evidence-Based Dental Practice",
"container-title-short": "Journal of Evidence-Based Dental Practice",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
9,
14
]
],
"date-time": "2022-09-14T06:29:43Z",
"timestamp": 1663136983000
},
"deposited": {
"date-parts": [
[
2023,
4,
15
]
],
"date-time": "2023-04-15T01:27:54Z",
"timestamp": 1681522074000
},
"indexed": {
"date-parts": [
[
2023,
4,
15
]
],
"date-time": "2023-04-15T04:27:39Z",
"timestamp": 1681532859771
},
"is-referenced-by-count": 1,
"issue": "4",
"issued": {
"date-parts": [
[
2022,
12
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1532338222001002?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1532338222001002?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "101777",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
12
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.identj.2020.12.027",
"article-title": "Global neglect of dental coverage in universal health coverage systems and Japan's broad coverage",
"author": "Aida",
"doi-asserted-by": "crossref",
"first-page": "454",
"journal-title": "Int Dent J",
"key": "10.1016/j.jebdp.2022.101777_bib0001",
"volume": "71",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01296-8",
"article-title": "SARS-CoV-2 infection of the oral cavity and saliva",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "892",
"journal-title": "Nat Med",
"key": "10.1016/j.jebdp.2022.101777_bib0002",
"volume": "27",
"year": "2021"
},
{
"article-title": "Periodontal tissues are targets for SARS-CoV-2: a post-mortem study",
"author": "Matuck",
"journal-title": "J Oral Microbiol",
"key": "10.1016/j.jebdp.2022.101777_bib0003",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1002/path.5679",
"article-title": "Salivary glands are a target for SARS-CoV-2: a source for saliva contamination",
"author": "Matuck",
"doi-asserted-by": "crossref",
"first-page": "239",
"journal-title": "J Pathol",
"key": "10.1016/j.jebdp.2022.101777_bib0004",
"volume": "254",
"year": "2021"
},
{
"key": "10.1016/j.jebdp.2022.101777_bib0005",
"unstructured": "Pepsodent WOHD 2021. Bangladesh research summary report 2021: attitudes, behaviors and experiences of oral health during the covid-19 pandemic. Accessed 06 September 2022. https://www.pepsodent.com/sk-eu/content/dam/brands/smile/bd/en/smile_fact_sheet_bangladesh_final-38049334.pdf."
},
{
"DOI": "10.1016/j.fshw.2018.12.001",
"article-title": "Oral microbiota: a new view of body health",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "8",
"journal-title": "Food Science and Human Wellness",
"key": "10.1016/j.jebdp.2022.101777_bib0006",
"volume": "8",
"year": "2019"
},
{
"article-title": "Impact of COVID-19 on the oral health of adults in Wuhan and China: results of a nationwide online cross-sectional questionnaire survey",
"author": "Zhang",
"first-page": "1",
"journal-title": "BMC Oral Health",
"key": "10.1016/j.jebdp.2022.101777_bib0007",
"volume": "211",
"year": "2021"
},
{
"DOI": "10.1186/s12903-020-01274-5",
"article-title": "Oral hygiene habits and possible transmission of COVID-19 among cohabitants",
"author": "González-Olmo",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "BMC Oral Health",
"key": "10.1016/j.jebdp.2022.101777_bib0008",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.4317/jced.57798",
"article-title": "Does the use of face masks during the COVID-19 pandemic impact on oral hygiene habits, oral conditions, reasons to seek dental care and esthetic concerns?",
"author": "Pinzan-Vercelino",
"doi-asserted-by": "crossref",
"first-page": "e369",
"journal-title": "J Clin Exp Dent",
"key": "10.1016/j.jebdp.2022.101777_bib0009",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.jebdp.2021.101584",
"article-title": "In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial",
"author": "Elzein",
"doi-asserted-by": "crossref",
"journal-title": "J Evid Based Dent Pract",
"key": "10.1016/j.jebdp.2022.101777_bib0010",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1007/s00405-021-06873-8",
"article-title": "Mouthrinses against SARS-CoV-2: anti-inflammatory effectivity and a clinical pilot study",
"author": "Schürmann",
"doi-asserted-by": "crossref",
"first-page": "5059",
"journal-title": "Eur Arch Otorhinolaryngol",
"key": "10.1016/j.jebdp.2022.101777_bib0011",
"volume": "278",
"year": "2021"
},
{
"DOI": "10.1080/20002297.2020.1794363",
"article-title": "Antiviral mouthwashes: possible benefit for COVID-19 with evidence-based approach",
"author": "Moosavi",
"doi-asserted-by": "crossref",
"journal-title": "J Oral Microbiol",
"key": "10.1016/j.jebdp.2022.101777_bib0012",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.adaj.2021.12.007",
"article-title": "The effect of mouthrinses on severe acute respiratory syndrome coronavirus 2 viral load: a systematic review",
"author": "Silva",
"doi-asserted-by": "crossref",
"first-page": "635",
"journal-title": "J Am Dent Assoc",
"key": "10.1016/j.jebdp.2022.101777_bib0013",
"volume": "153",
"year": "2022"
},
{
"article-title": "Toothbrushing with a dentifrice containing antimicrobial phthalocyanine derivative for the intraoral reduction of viral load of SARS-CoV-2: a pilot study",
"author": "Poleti",
"journal-title": "Res Sq. Preprint (version 1)",
"key": "10.1016/j.jebdp.2022.101777_bib0014",
"year": "2021"
},
{
"DOI": "10.2147/CCIDE.S315419",
"article-title": "Virucidal activity of the antiseptic mouthwash and dental gel containing anionic phthalocyanine derivative: in vitro study",
"author": "Santos",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Clin Cosmet Investig Dent",
"key": "10.1016/j.jebdp.2022.101777_bib0015",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.31080/ASDS.2020.04.0991",
"article-title": "A recommendation of PHTALOX mouthwash for preventing infection and progression of COVID-19",
"author": "Santos",
"doi-asserted-by": "crossref",
"first-page": "111",
"journal-title": "Acta Scient Dent Sci",
"key": "10.1016/j.jebdp.2022.101777_bib0016",
"volume": "4",
"year": "2020"
},
{
"DOI": "10.1007/s00405-021-06873-8",
"article-title": "Mouthrinses against SARS-CoV-2: anti-inflammatory effectivity and a clinical pilot study",
"author": "Schürmann",
"doi-asserted-by": "crossref",
"first-page": "5059",
"journal-title": "Eur Arch Otorhinolaryngol",
"key": "10.1016/j.jebdp.2022.101777_bib0017",
"volume": "278",
"year": "2021"
},
{
"DOI": "10.1590/S1678-77572010000500015",
"article-title": "Staining and calculus formation after 0.12% chlorhexidine rinses in plaque-free and plaque covered surfaces: a randomized trial",
"author": "Zanatta",
"doi-asserted-by": "crossref",
"first-page": "515",
"journal-title": "J Appl Oral Sci",
"key": "10.1016/j.jebdp.2022.101777_bib0018",
"volume": "18",
"year": "2010"
},
{
"article-title": "Adjuvant use of phthalocyanine derivative for calculus control",
"author": "Santos",
"first-page": "1",
"journal-title": "Int J Case Rep Images",
"key": "10.1016/j.jebdp.2022.101777_bib0019",
"volume": "12",
"year": "2021"
},
{
"article-title": "The use of antiviral phthalocyanine mouthwash as a preventive measure against COVID-19",
"author": "Vilhena",
"journal-title": "GMS Hyg Infect Control",
"key": "10.1016/j.jebdp.2022.101777_bib0020",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-99013-5",
"article-title": "Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: randomized trial",
"author": "da Silva Santos",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Sci Rep",
"key": "10.1016/j.jebdp.2022.101777_bib0021",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.2147/CCIDE.S295423",
"article-title": "A phthalocyanine derivate mouthwash to gargling/rinsing as an option to reduce clinical symptoms of COVID-19: case series",
"author": "BdF",
"doi-asserted-by": "crossref",
"first-page": "47",
"journal-title": "Clin Cosmet Investig Dent",
"key": "10.1016/j.jebdp.2022.101777_bib0022",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.4067/S0718-381X2021000100067",
"article-title": "Oral manifestation COVID-19 and the rapid resolution of symptoms post-phtalox treatment: a case series",
"author": "BdF",
"doi-asserted-by": "crossref",
"first-page": "67",
"journal-title": "Int J Odontostomat",
"key": "10.1016/j.jebdp.2022.101777_bib0023",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1016/j.ijsu.2011.10.001",
"article-title": "CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials",
"author": "Moher",
"doi-asserted-by": "crossref",
"first-page": "28",
"journal-title": "Int J Surg",
"key": "10.1016/j.jebdp.2022.101777_bib0024",
"volume": "10",
"year": "2012"
},
{
"DOI": "10.1038/s41591-020-0916-2",
"article-title": "Real-time tracking of self-reported symptoms to predict potential COVID-19",
"author": "Menni",
"doi-asserted-by": "crossref",
"first-page": "1037",
"journal-title": "Nat Med",
"key": "10.1016/j.jebdp.2022.101777_bib0025",
"volume": "26",
"year": "2020"
},
{
"article-title": "Advanced statistics: missing data in clinical research – part 1: an introduction and conceptual framework",
"author": "Haukoos",
"first-page": "662",
"journal-title": "Acad Emerg Med",
"key": "10.1016/j.jebdp.2022.101777_bib0026",
"volume": "14",
"year": "2007"
},
{
"author": "Fregni",
"key": "10.1016/j.jebdp.2022.101777_bib0027",
"series-title": "Critical Thinking in Clinical Research: Applied Theory and Practice Using Case Studies",
"year": "2018"
},
{
"DOI": "10.3390/ijms22126527",
"article-title": "SARS-CoV-2 infection and significance of oral health management in the era of “the new normal with COVID-19″",
"author": "Imai",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Int J Mol Sci",
"key": "10.1016/j.jebdp.2022.101777_bib0028",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1111/dth.14578",
"article-title": "Oral manifestations of COVID-19 disease: a review article",
"author": "Iranmanesh",
"doi-asserted-by": "crossref",
"first-page": "e14578",
"journal-title": "Dermatol Ther",
"key": "10.1016/j.jebdp.2022.101777_bib0029",
"volume": "34",
"year": "2021"
},
{
"article-title": "The impact of oral health status on COVID-19 severity, recovery period and C-reactive protein values",
"author": "Kamel",
"first-page": "1",
"journal-title": "Br Dent J",
"key": "10.1016/j.jebdp.2022.101777_bib0030",
"year": "2021"
},
{
"article-title": "COVID-19 and Periodontitis: a reality to live with",
"author": "Jafer",
"first-page": "1398",
"journal-title": "J Contemp Dent Pract",
"key": "10.1016/j.jebdp.2022.101777_bib0031",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1038/s41415-020-1747-8",
"article-title": "Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections?",
"author": "Sampson",
"doi-asserted-by": "crossref",
"first-page": "971",
"journal-title": "Br Dent J",
"key": "10.1016/j.jebdp.2022.101777_bib0032",
"volume": "228",
"year": "2020"
},
{
"DOI": "10.1016/j.amepre.2005.06.013",
"article-title": "Prevention of upper respiratory tract infections by gargling: a randomized trial",
"author": "Satomura",
"doi-asserted-by": "crossref",
"first-page": "302",
"journal-title": "Am J Prev Med",
"key": "10.1016/j.jebdp.2022.101777_bib0033",
"volume": "29",
"year": "2005"
},
{
"DOI": "10.1159/000057722",
"article-title": "Prevention of respiratory infections by povidone-iodine gargle",
"author": "Nagatake",
"doi-asserted-by": "crossref",
"first-page": "32",
"journal-title": "Dermatology",
"key": "10.1016/j.jebdp.2022.101777_bib0034",
"volume": "204",
"year": "2002"
},
{
"DOI": "10.1016/j.cgh.2020.04.001",
"article-title": "Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management",
"author": "D'Amico",
"doi-asserted-by": "crossref",
"first-page": "1663",
"journal-title": "Clin Gastroenterol Hepatol",
"key": "10.1016/j.jebdp.2022.101777_bib0035",
"volume": "18",
"year": "2020"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1532338222001002"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Dentistry"
],
"subtitle": [],
"title": "USE OF MOUTHWASH AND DENTIFRICE CONTAINING AN ANTIMICROBIAL PHTHALOCYANINE DERIVATIVE FOR THE REDUCTION OF CLINICAL SYMPTOMS OF COVID-19: A RANDOMIZED TRIPLE-BLIND CLINICAL TRIAL",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "22"
}