A Randomized trial on the regular use of potent mouthwash in COVID-19 treatment
et al., medRxiv, doi:10.1101/2020.11.27.20234997, ISRCTN10197987, Nov 2020
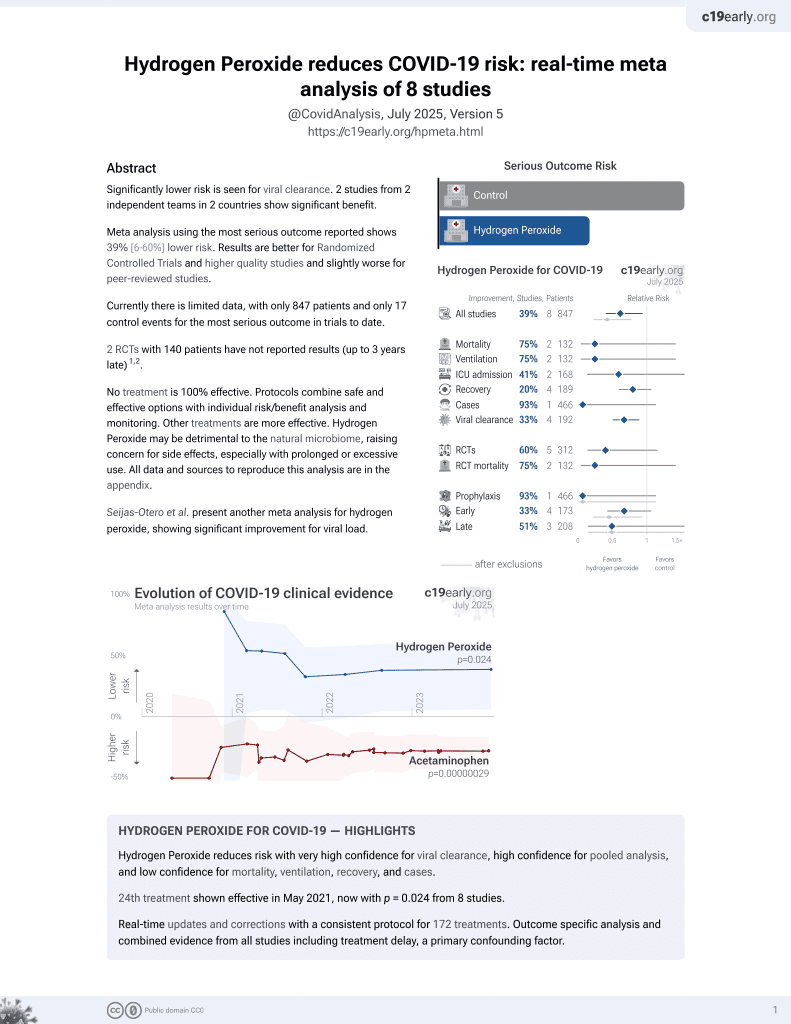
24th treatment shown to reduce risk in
May 2021, now with p = 0.024 from 8 studies.
Lower risk for viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT for mouthwash containing hydrogen peroxide 2% and chlorhexidine gluconate, showing higher discharge, shorter hospital stay, less intubation, and lower mortality with treatment.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
|
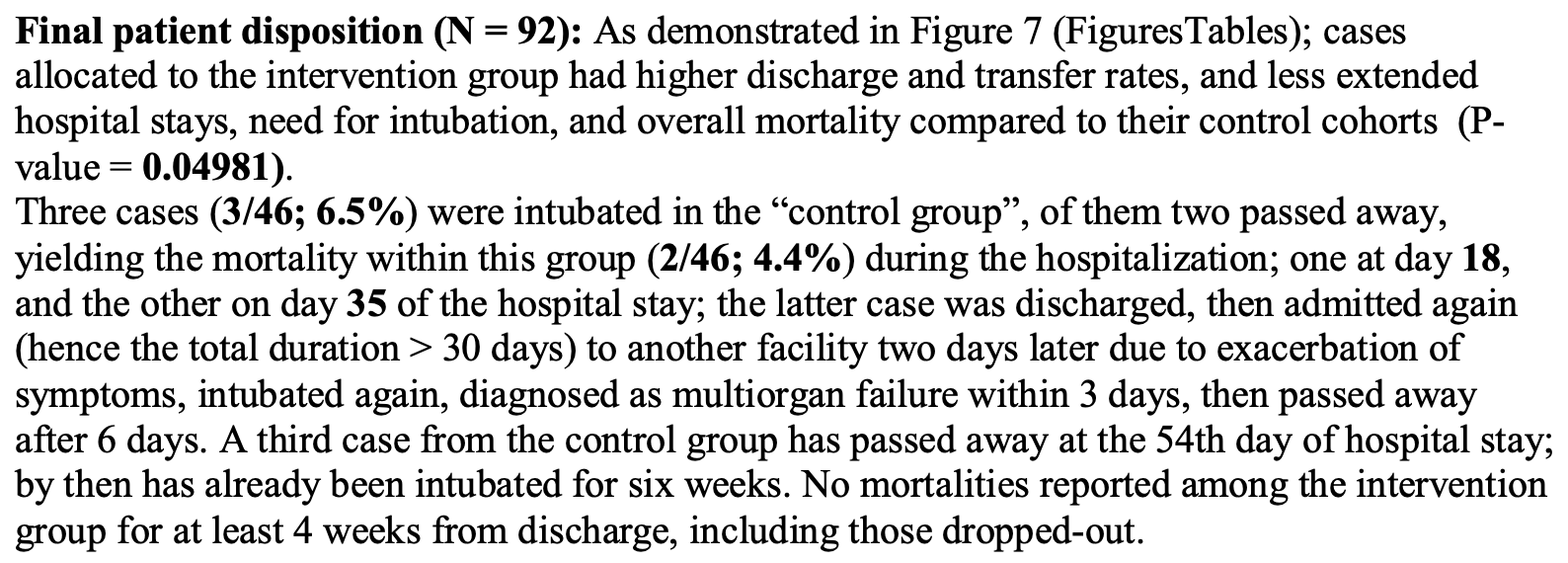
risk of death, 85.7% lower, RR 0.14, p = 0.24, treatment 0 of 46 (0.0%), control 3 of 46 (6.5%), NNT 15, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), including third control death on day 54.
|
|
risk of mechanical ventilation, 85.7% lower, RR 0.14, p = 0.24, treatment 0 of 46 (0.0%), control 3 of 46 (6.5%), NNT 15, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of no viral clearance, 18.1% lower, RR 0.82, p = 0.16, treatment 28 of 43 (65.1%), control 35 of 44 (79.5%), NNT 6.9, day 15.
|
|
risk of no viral clearance, 14.0% lower, RR 0.86, p = 0.01, treatment 37 of 43 (86.0%), control 44 of 44 (100.0%), NNT 7.2, day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mukhtar et al., 30 Nov 2020, Randomized Controlled Trial, Qatar, preprint, 16 authors, this trial uses multiple treatments in the treatment arm (combined with chlorhexidine) - results of individual treatments may vary, trial ISRCTN10197987.
Abstract: medRxiv preprint doi: https://doi.org/10.1101/2020.11.27.20234997; this version posted April 25, 2021. The copyright holder for this preprint
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
It is made available under a CC-BY-NC-ND 4.0 International license .
Title:
A randomized trial on the regular use of potent mouthwash in COVID-19 treatment
Authors list:
Khalid Mukhtar 1 (First & corresponding Author), kmukhter@hamad.qa
Suelen Qassim 2, sq1513914@student.qu.edu.qa
Shaikha Ali Al Qahtani 3, SALQAHTANI4@hamad.qa
Mohamed Ibn-Masud Danjuma 4, mdanjuma@hamad.qa
Mohamed Mohamedali 5, mmohamedali@hamad.qa
Housamaddeen Al Farhan 6, halfarhan1@hamad.qa
Mohammed F. Khudair 7, mkhudhair@hamad.qa
Abdel Rehim El Tayeh 8, aeltayeh@hamad.qa
Mohammed Al-Dosari 9, maldosari1@hamad.qa
Mohamed Elhassan Babiker 10, mbabiker@hamad.qa
Ahmed Hassib 11, ahassib1@hamad.qa
Rumaisa Mohamed Elmustafa 12, rmohamed31@hamad.qa
Wesal Elhadary 13, welhadary@hamad.qa
Morwan Abdulkarim 14, mabdulkrim@hamad.qa
Rajvir Singh 15, rsingh@hamad.qa
Muna Al.Maslamani 16, malmaslamani@hamad.qa
Authors affiliation:
Hamad Medical Corporation, Doha, QATAR (1, 3, 4, 5, 6, 7, 8,9, 10, 11, 12, 13, 14, 15, 16)
Qatar University – Public Health program, Doha, Qatar (2)
NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice.
medRxiv preprint doi: https://doi.org/10.1101/2020.11.27.20234997; this version posted April 25, 2021. The copyright holder for this preprint
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
It is made available under a CC-BY-NC-ND 4.0 International license .
1. Abstract
In this work we tried to study the effect of the regular use of potent mouthwash in COVID19 cases,
on the premise that it may speedup the recovery, through the repeated reduction of microbial load,
of both, the 2019-nCOV and oral microbiota; thus slowing the disease progression and lowering the
incidence of superinfections.
Through a randomized controlled trial, a mixed solution of Hydrogen peroxide 2% and
chlorhexidine gluconate, to be used for oral rinsing and gargling three times daily, was tested in
cases admitted to COVID treatment facility, versus the standard (only) COVID19-treatment
protocol, starting with 46 cases in each group, matched in terms of disease severity, of symptoms,
and average cycle threshold value (CT-value) for the COVID PCR test on diagnosis.
Our findings showed statistically significant improvement in terms of a higher conversion rate to
"COVID19-negative PCR" by five days of treatment (6/46 Vs 0/46), improvement in “symptoms
severity” after two days of treatment, and less intubation and mortality (0/46 Vs 3/46) with all Pvalue < 0.05. There was also a trend of improvement in other outcome variables, though with no
statistically significant difference; namely “shorter hospital stays," "less progression in Oxygen
requirements”, “less rate of plasma transfusion”, and better "gross extent of improvement”.
Our findings support a beneficial role in treating active cases (Disease) and anticipates better
outcome should implemented earlier in course of the disease; thus, suggest a role in limiting the
spread (Pandemic), as an additional preventive method. Additionally, we think the repeated
reduction in the microbial load..
DOI record:
{
"DOI": "10.1101/2020.11.27.20234997",
"URL": "http://dx.doi.org/10.1101/2020.11.27.20234997",
"abstract": "<jats:label>1.</jats:label><jats:title>Abstract</jats:title><jats:p>In this work we tried to study the effect of the regular use of potent mouthwash in COVID19 cases, on the premise that it may speedup the recovery, through the repeated reduction of microbial load, of both, the 2019-nCOV and oral microbiota; thus slowing the disease progression and lowering the incidence of superinfections.</jats:p><jats:p>Through a randomized controlled trial, a mixed solution of Hydrogen peroxide 2% and chlorhexidine gluconate, to be used for oral rinsing and gargling three times daily, was tested in cases admitted to COVID treatment facility, versus the standard (only) COVID19-treatment protocol, starting with 46 cases in each group, matched in terms of disease severity, of symptoms, and average cycle threshold value (CT-value) for the COVID PCR test on diagnosis.</jats:p><jats:p>Our findings showed statistically significant improvement in terms of a higher conversion rate to “COVID19-negative PCR” by five days of treatment (6/46 Vs 0/46), improvement in “symptoms severity” after two days of treatment, and less intubation and mortality (0/46 Vs 3/46) with all P-value < 0.05. There was also a trend of improvement in other outcome variables, though with no statistically significant difference; namely “shorter hospital stays,” “less progression in Oxygen requirements”, “less rate of plasma transfusion”, and better “gross extent of improvement”.</jats:p><jats:p>Our findings support a beneficial role in treating active cases (Disease) and anticipates better outcome should implemented earlier in course of the disease; thus, suggest a role in limiting the spread (Pandemic), as an additional preventive method. Additionally, we think the repeated reduction in the microbial load might have been sufficient to induce a strain in a possible viral-microbial interaction, resulting in slowing down of the disease progress.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
4,
25
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9261-731X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mukhtar",
"given": "Khalid",
"sequence": "first"
},
{
"affiliation": [],
"family": "Qassim",
"given": "Suelen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Qahtani",
"given": "Shaikha Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Danjuma",
"given": "Mohamed Ibn-Masud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mohamedali",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farhan",
"given": "Housamaddeen Al",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khudair",
"given": "Mohammed F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "El Tayeh",
"given": "Abdel Rehim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al-Dosari",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Babiker",
"given": "Mohamed Elhassan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hassib",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elmustafa",
"given": "Rumaisa Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elhadary",
"given": "Wesal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdulkarim",
"given": "Morwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Rajvir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al.Maslamani",
"given": "Muna",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
11,
30
]
],
"date-time": "2020-11-30T21:25:19Z",
"timestamp": 1606771519000
},
"deposited": {
"date-parts": [
[
2021,
4,
27
]
],
"date-time": "2021-04-27T12:53:00Z",
"timestamp": 1619527980000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
10,
12
]
],
"date-time": "2023-10-12T23:17:01Z",
"timestamp": 1697152621874
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2020,
11,
30
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2020.11.27.20234997",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2020,
11,
30
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2020,
11,
30
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1136/thx.54.10.947",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.1"
},
{
"DOI": "10.1007/978-1-4615-9173-3_1",
"article-title": "The biochemistry of the renin-angiotensin system",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Adv Exp Med Biol",
"key": "2021042705501604000_2020.11.27.20234997v2.2",
"volume": "130",
"year": "1980"
},
{
"DOI": "10.1128/JVI.79.3.1966-1969.2005",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.3"
},
{
"DOI": "10.1097/FJC.0000000000000307",
"article-title": "ACE2 and Microbiota: Emerging Targets for Cardiopulmonary Disease Therapy",
"doi-asserted-by": "crossref",
"first-page": "540",
"issue": "6",
"journal-title": "J Cardiovasc Pharmacol",
"key": "2021042705501604000_2020.11.27.20234997v2.4",
"volume": "66",
"year": "2015"
},
{
"DOI": "10.1016/j.cell.2020.04.035",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.5"
},
{
"DOI": "10.1113/expphysiol.2007.040048",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.6"
},
{
"DOI": "10.3748/wjg.v19.i40.6794",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.7"
},
{
"DOI": "10.1128/mBio.00251-12",
"doi-asserted-by": "crossref",
"key": "2021042705501604000_2020.11.27.20234997v2.8",
"unstructured": "Madan JC , Koestler DC , Stanton BA , Davidson L , Moulton LA , Housman ML , et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. mBio. 2012;3(4)."
},
{
"DOI": "10.3389/fimmu.2019.01551",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.9"
},
{
"DOI": "10.3390/cells9071704",
"doi-asserted-by": "crossref",
"key": "2021042705501604000_2020.11.27.20234997v2.10",
"unstructured": "Zamai L. The Yin and Yang of ACE/ACE2 Pathways: The Rationale for the Use of Renin-Angiotensin System Inhibitors in COVID-19 Patients. Cells. 2020;9(7)."
},
{
"DOI": "10.1038/nature01370",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.11"
},
{
"DOI": "10.1038/s41467-020-14867-z",
"article-title": "B38-CAP is a bacteria-derived ACE2-like enzyme that suppresses hypertension and cardiac dysfunction",
"doi-asserted-by": "crossref",
"first-page": "1058",
"issue": "1",
"journal-title": "Nat Commun",
"key": "2021042705501604000_2020.11.27.20234997v2.12",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1177/00220345900690030901",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.13"
},
{
"DOI": "10.4103/jisp.jisp_414_18",
"article-title": "Hydrogen peroxide masks the bitterness of chlorhexidine mouthwash without affecting its antibacterial activity",
"doi-asserted-by": "crossref",
"first-page": "119",
"issue": "2",
"journal-title": "J Indian Soc Periodontol",
"key": "2021042705501604000_2020.11.27.20234997v2.14",
"volume": "23",
"year": "2019"
},
{
"DOI": "10.1046/j.1365-2842.1999.00343.x",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.15"
},
{
"article-title": "Antibacterial and Toxic Effect of Hydrogen Peroxide Combined with Different Concentrations of Chlorhexidine in Comparison with Sodium Hypochlorite",
"first-page": "349",
"issue": "4",
"journal-title": "J Dent (Shiraz)",
"key": "2021042705501604000_2020.11.27.20234997v2.16",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1016/j.jhin.2020.01.022",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.17"
},
{
"DOI": "10.1016/0003-9969(95)00029-O",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.18"
},
{
"DOI": "10.1111/j.1365-2958.2006.05546.x",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.19"
},
{
"article-title": "[The origin of hydrogen peroxide in oral cavity and its role in oral microecology balance]",
"first-page": "215",
"issue": "2",
"journal-title": "Hua Xi Kou Qiang Yi Xue Za Zhi",
"key": "2021042705501604000_2020.11.27.20234997v2.20",
"volume": "35",
"year": "2017"
},
{
"DOI": "10.1128/JCM.00317-06",
"doi-asserted-by": "publisher",
"key": "2021042705501604000_2020.11.27.20234997v2.21"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2020.11.27.20234997"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "A randomized trial on the regular use of potent mouthwash in COVID-19 treatment",
"type": "posted-content"
}