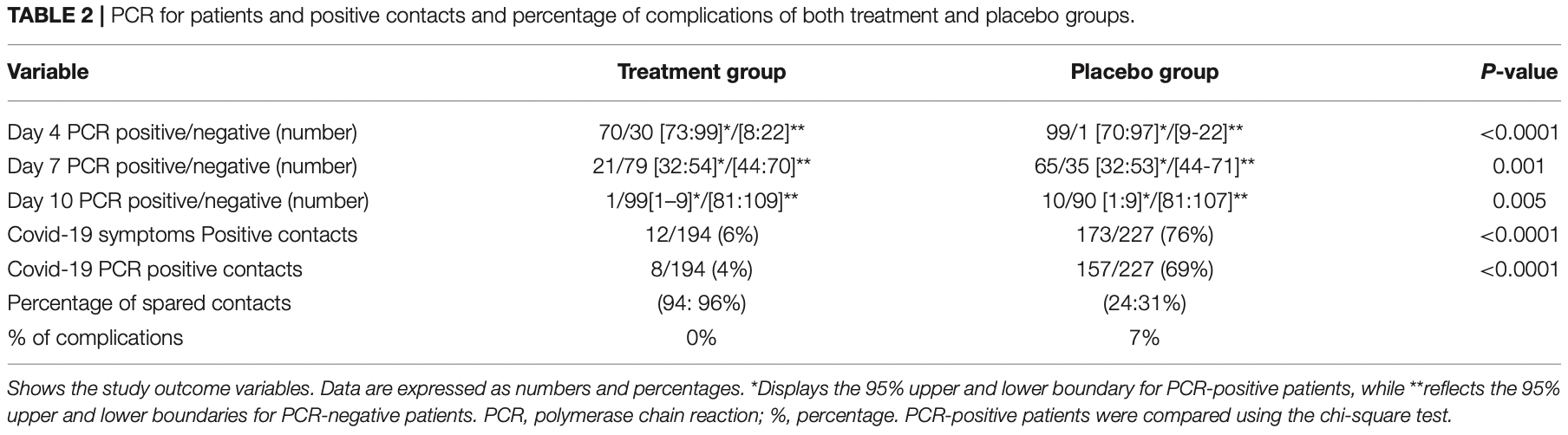
Combined Nasal, Oropharyngeal Povidone Iodine Plus Glycyrrhizic Acid Sprays, Accelerate Clinical and Laboratory Recovery and Reduces Household Transmission of SARS-CoV-2: A Randomized Placebo-Controlled Clinical Trial
et al., Frontiers in Medicine, doi:10.3389/fmed.2022.863917, PACTR202101875903773, Apr 2022
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT with 200 patients and 421 contacts, with 100 patients and their contacts treated with nasal and oropharyngeal sprays containing povidone-iodine and glycyrrhizic acid, showing significantly faster viral clearance and recovery, and significantly lower transmission.
SOC included vitamin C and zinc. The spray active ingredients included a compound of glycyrrhizic acid in the form of ammonium glycyrrhizate 2.5 mg/ml plus PVI 0.5% for oropharyngeal and dipotassium glycyrrhizinate 2.5 mg/ml plus PVI 0.5% for nasal spray. Patients were advised to concomitantly use oropharyngeal and nasal sprays 6 times per day. They were instructed to abstain from food, drink, and smoke for 20min, particularly after oropharyngeal spray. The oropharyngeal spray bottle contains an atomizer that ends with a long arm applicator to insert inside the mouth cavity and can be directed up, down, right, or left to cover the entire pharyngeal area.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
|
risk of hospitalization, 90.9% lower, RR 0.09, p = 0.06, treatment 0 of 100 (0.0%), control 5 of 100 (5.0%), NNT 20, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
recovery time, 14.6% lower, relative time 0.85, p = 0.008, treatment mean 7.6 (±2.0) n=100, control mean 8.9 (±2.0) n=100.
|
|
recovery time, 49.1% lower, relative time 0.51, p < 0.001, treatment mean 5.6 (±1.3) n=100, control mean 11.0 (±3.4) n=100, smell.
|
|
recovery time, 48.2% lower, relative time 0.52, p < 0.001, treatment mean 5.7 (±1.0) n=100, control mean 11.0 (±4.0) n=100, taste.
|
|
risk of no viral clearance, 67.7% lower, RR 0.32, p < 0.001, treatment 21 of 100 (21.0%), control 65 of 100 (65.0%), NNT 2.3, mid-recovery, day 7.
|
|
risk of no viral clearance, 90.0% lower, RR 0.10, p = 0.010, treatment 1 of 100 (1.0%), control 10 of 100 (10.0%), NNT 11, day 10.
|
|
risk of no viral clearance, 29.3% lower, RR 0.71, p < 0.001, treatment 70 of 100 (70.0%), control 99 of 100 (99.0%), NNT 3.4, day 4.
|
|
risk of transmission, 91.9% lower, RR 0.08, p < 0.001, treatment 12 of 194 (6.2%), control 173 of 227 (76.2%), NNT 1.4, symptomatic.
|
|
risk of transmission, 94.0% lower, RR 0.06, p < 0.001, treatment 8 of 194 (4.1%), control 157 of 227 (69.2%), NNT 1.5, PCR+.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Elsersy et al., 19 Apr 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Egypt, peer-reviewed, 8 authors, study period March 2021 - July 2021, this trial uses multiple treatments in the treatment arm (combined with glycyrrhizic acid) - results of individual treatments may vary, trial PACTR202101875903773.
Contact: hazelsersy@hotmail.com, hazem.anwar@med.menofia.edu.eg.
Combined Nasal, Oropharyngeal Povidone Iodine Plus Glycyrrhizic Acid Sprays, Accelerate Clinical and Laboratory Recovery and Reduces Household Transmission of SARS-CoV-2: A Randomized Placebo-Controlled Clinical Trial
Frontiers in Medicine, doi:10.3389/fmed.2022.863917
The COVID-19 pandemic is still posing challenging health and economic problems. Effective broad-spectrum antiviral therapy is urgently needed for the control of early SARS-CoV-2 infection to limit its spread and mutations. In this randomized placebocontrolled clinical study, we tested the effects of intranasal and oropharyngeal delivery of a compound of povidone-iodine 0.5% and glycyrrhizic acid 2.5 mg/ml on the laboratory (PCR) and clinical recovery from SARS-CoV-2 patients and their household contacts. 353 patients suspected of having COVID-19 infection were screened by chest CT and nasopharyngeal swab tests (PCR). 200 patients were randomly allocated to two equal groups: treatment and placebo groups. Treatment accelerated the recovery of PCR on days 4, 7, and 10, as evidenced by PCR-positive patients (70, vs. 99%, 20 vs. 65%, 1 vs. 10%) in both the treated and placebo groups, respectively. Treatment enhanced the early recovery of symptoms [day 7.6 ± 2 (CI 7:8.3) vs. 8.9 ± 2 (CI 8.3:9.6)]. Treatment promoted early recovery of anosmia and ageusia [5.6 ± 1 (CI, 4.8:6.4) vs. 11 ± 3 days, (CI, 10.8:12)] in both the treated and control groups (P < 0.0001). There was a notable reduction in transmission of the virus among the household close contacts in the treatment group (4%) vs. 76% in the placebo group. Combined PVI-GA nasal and oropharyngeal spray accelerates both laboratory and clinical recovery of SARS-CoV-2 infected patients in the early phases of the disease and reduces the household spread of the virus; thus, it may play an important role in controlling coronavirus outbreaks. Clinical Trial Registration: https://pactr.samrc.ac.za, PACTR202101875903773.
ETHICS STATEMENT The studies involving human participants were reviewed and approved by Fever and Liver Hospital Local Ethics Committee. Written informed consent to participate in this study was provided by the patients/participants or their legal guardian/next of kin.
AUTHOR CONTRIBUTIONS HE: Idea, intellectual property, protocol design, experimental design, data analysis, manuscript writing, pilot 1, and pilot 2 design and conduction. MZ: Data analysis, manuscript writing, extraction, and purification of Glycyrrhizic acid and its salts. A-EE: Experimental design, data analysis, manuscript writing, randomization, and allocation. MA-E: Experimental design, data analysis, Manuscript writing, design, and conduction of pilot 3. MA: Data analysis, Manuscript writing design, and conduction of pilot 4. ME: Data analysis, manuscript writing, and case report conduction. EM and NE: Data analysis, Manuscript writing, Glycyrrhizic acid, and its salts extraction. All authors contributed to the article and approved the submitted version.
FUNDING The present study was funded by donations from Elsersy Scientific Med. company.
SUPPLEMENTARY MATERIAL The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed. 2022.863917/full#supplementary-material Conflict of Interest: This project has been filed as a patent application for HE, international PCT PCT/EG/2021000030, national application number 2020/1599. The remaining authors..
References
Agyeman, Chin, Pharm, Liew, Ofori-Asenso, Smell and taste dysfunction in patients with COVID-19: a systemic review and meta-analysis, Mayo clinic proc, doi:10.1016/j.mayocp.2020.05.030
Bailly, Vergotenb, Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome, Pharmacology & Therapeutics, doi:10.1016/j.pharmthera.2020.107618
Bigliardi, Alsagoff, El-Kafrawi, Pyon, Wa et al., Povidone iodine in wound healing: a review of current concepts and practices, Int J Surg, doi:10.1016/j.ijsu.2017.06.073
Brann, Tsukahara, Weinreb, Lipovsek, Van Den Berge et al., non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia, Sci. Adv, doi:10.1126/sciadv.abc5801
Cazzolla, Lovero, Muzio, Testa, Schirinzi et al., Taste and smell disorders in COVID-19 patients. role of interleukin-6, ACS Chem Neurosci, doi:10.1021/acschemneuro.0c00447
Chen, Du, Potential natural compounds for preventing 2019-nCoV infection, Preprints, doi:10.20944/preprints202001.0358.v3
Del Rio, Omer, Malani, Winter of Omicron-the evolving COVID-19 pandemic, JAMA, doi:10.1001/jama.2021.24315
Frank, Brown, Capriotti, Westover, Pelletier et al., In Vitro efficacy of a povidone-iodine nasal antiseptic for rapid inactivation of SARS-CoV-2, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.3053
Guenezan, Garcia, Strasters, Jousselin, Lévêque et al., Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: a randomized clinical trial, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.5490
Harald, Symptomatic protective action of glycyrrhizin (Licorice) in COVID-19, Front Immunol, doi:10.3389/fimmu.2020.01239
He, Lau, Wu, Deng, Wang et al., Temporal dynamics in viral shedding and transmissibility of COVID-19, Nat Med, doi:10.1038/s41591-020-0869-5
Ibarra, Trocóniz, Fagiolino, Enteric reabsorption processes and their impact on drug pharmacokinetics, Sci Rep, doi:10.1038/s41598-021-85174-w
Ingredient, Panel, Final report on the safety assessment of glycyrrhetinic acid, potassium glycyrrhetinate, disodium succinoyl glycyrrhetinate, glyceryl glycyrrhetinate, glycyrrhetinyl stearate, stearyl glycyrrhetinate, glycyrrhizic acid, ammonium glycyrrhizate, dipotassium glycyrrhizate, disodium glycyrrhizate, trisodium glycyrrhizate, methyl glycyrrhizate, and potassium glycyrrhizinate, Int J Toxicol, doi:10.1080/10915810701351228
Letko, Marzi, Munster, Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B beta coronaviruses, Nat Microbiol, doi:10.1038/s41564-020-0688-y
Meinhardt, Radke, Dittmayer, Franz, Thomas et al., Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19, Nat Neurosci, doi:10.1101/2020.06.04.135012
Ming, Yin, Therapeutic effects of glycyrrhizic acid, Nat Prod Commun, doi:10.1177/1934578X1300800335
Naaber, Tserel, Kangro, Sepp, Jurjenson et al., Dynamics of antibody response to BNT162b2 vaccine after 6 months: a longitudinal prospective study, Lancet Reg Health Eur, doi:10.1016/j.lanepe.2021.100208
Ploeger, Mensinga, Sips, Seinen, Meulenbelt et al., The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling, Drug Metab Rev, doi:10.1081/DMR-100104400
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic treatments for Coronavirus disease. (COVID-19) a review, JAMA, doi:10.1001/jama.2020.6019
Sungnak, Huang, Bécavin, Berg, Queen et al., HCA lung biological network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med, doi:10.1038/s41591-020-0868-6
Van De Sand, Bormann, Alt, Schipper, Heilingloh et al., Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease, Viruses, doi:10.3390/v13040609
Van Gelderen, Bijlsma, Van Dokkum, Savelkoull, Glycyrrhizic acid: the assessment of a no effect level, Hum Exp Toxicol, doi:10.1191/096032700682694251
Wan, Shang, Graham, Baric, Li, Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS, J Virol, doi:10.1128/JVI.00127-20
Wang, Tian, Zhang, Zhang, Guo et al., Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China, BMJ Global Health, doi:10.1136/bmjgh-2020-002794
Wang, Yang, Yuan, Liu, Liu, The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb, Acta Pharm Sin B, doi:10.1016/j.apsb.2015.05.005
Wölfel, Corman, Guggemos, Seilmaier, Zange et al., Virological assessment of hospitalized patients with COVID, Nature, doi:10.1101/2020.03.05.20030502
Xu, Chen, Wang, Feng, Zhou et al., Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission, Sci China Life Sci, doi:10.1007/s11427-020-1637-5
Yuan, Wang, Chen, Shan, Di, Lactobacillus murinus improved the bioavailability of orally administered glycyrrhizic acid in rats, Front Microbiol, doi:10.3389/fmicb.2020.00597
Zou, Ruan, Huang, Liang, Huang et al., SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med, doi:10.1056/NEJMc2001737
DOI record:
{
"DOI": "10.3389/fmed.2022.863917",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2022.863917",
"abstract": "<jats:p>The COVID-19 pandemic is still posing challenging health and economic problems. Effective broad-spectrum antiviral therapy is urgently needed for the control of early SARS-CoV-2 infection to limit its spread and mutations. In this randomized placebo-controlled clinical study, we tested the effects of intranasal and oropharyngeal delivery of a compound of povidone-iodine 0.5% and glycyrrhizic acid 2.5 mg/ml on the laboratory (PCR) and clinical recovery from SARS-CoV-2 patients and their household contacts. 353 patients suspected of having COVID-19 infection were screened by chest CT and nasopharyngeal swab tests (PCR). 200 patients were randomly allocated to two equal groups: treatment and placebo groups. Treatment accelerated the recovery of PCR on days 4, 7, and 10, as evidenced by PCR-positive patients (70, vs. 99%, 20 vs. 65%, 1 vs. 10%) in both the treated and placebo groups, respectively. Treatment enhanced the early recovery of symptoms [day 7.6 ± 2 (CI 7:8.3) vs. 8.9 ± 2 (CI 8.3:9.6)]. Treatment promoted early recovery of anosmia and ageusia [5.6 ± 1 (CI, 4.8:6.4) vs. 11 ± 3 days, (CI, 10.8:12)] in both the treated and control groups (<jats:italic>P</jats:italic> &lt; 0.0001). There was a notable reduction in transmission of the virus among the household close contacts in the treatment group (4%) vs. 76% in the placebo group. Combined PVI-GA nasal and oropharyngeal spray accelerates both laboratory and clinical recovery of SARS-CoV-2 infected patients in the early phases of the disease and reduces the household spread of the virus; thus, it may play an important role in controlling coronavirus outbreaks.</jats:p><jats:sec><jats:title>Clinical Trial Registration</jats:title><jats:p><jats:ext-link>https://pactr.samrc.ac.za</jats:ext-link>, PACTR202101875903773.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fmed.2022.863917"
],
"author": [
{
"affiliation": [],
"family": "Elsersy",
"given": "Hazem E.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zahran",
"given": "Magdy A. H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elbakry",
"given": "Abd-Elazeem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abd-Elwahab",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Mohamed Milegy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elgandy",
"given": "Mohamed Salah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mohammed",
"given": "Eman H. M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elewa",
"given": "Nourhan M.",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T15:01:29Z",
"timestamp": 1650380489000
},
"deposited": {
"date-parts": [
[
2022,
4,
20
]
],
"date-time": "2022-04-20T02:10:12Z",
"timestamp": 1650420612000
},
"indexed": {
"date-parts": [
[
2022,
4,
20
]
],
"date-time": "2022-04-20T11:40:37Z",
"timestamp": 1650454837980
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
4,
19
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T00:00:00Z",
"timestamp": 1650326400000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2022.863917/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
4,
19
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
19
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1001/jama.2020.6019",
"article-title": "Pharmacologic treatments for Coronavirus disease. (COVID-19) a review.",
"author": "Sanders",
"doi-asserted-by": "publisher",
"first-page": "1824",
"journal-title": "JAMA",
"key": "B1",
"volume": "32",
"year": "2019"
},
{
"DOI": "10.1056/NEJMc2001737",
"article-title": "SARS-CoV-2 viral load in upper respiratory specimens of infected patients",
"author": "Zou",
"doi-asserted-by": "publisher",
"first-page": "1177",
"journal-title": "N Engl J Med.",
"key": "B2",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.24315",
"article-title": "Winter of Omicron—the evolving COVID-19 pandemic",
"author": "Del Rio",
"doi-asserted-by": "publisher",
"first-page": "319",
"journal-title": "JAMA.",
"key": "B3",
"volume": "327",
"year": "2021"
},
{
"DOI": "10.1016/j.lanepe.2021.100208",
"article-title": "Dynamics of antibody response to BNT162b2 vaccine after 6 months: a longitudinal prospective study",
"author": "Naaber",
"doi-asserted-by": "publisher",
"first-page": "100208",
"journal-title": "Lancet Reg Health Eur.",
"key": "B4",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1001/jamaoto.2020.3053",
"article-title": "In Vitro efficacy of a povidone-iodine nasal antiseptic for rapid inactivation of SARS-CoV-2",
"author": "Frank",
"doi-asserted-by": "publisher",
"first-page": "1054",
"journal-title": "JAMA Otolaryngol Head Neck Surg.",
"key": "B5",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1016/j.pharmthera.2020.107618",
"article-title": "Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome",
"author": "Bailly",
"doi-asserted-by": "crossref",
"key": "B6",
"volume-title": "Pharmacology & Therapeutics",
"year": "2020"
},
{
"DOI": "10.1177/1934578X1300800335",
"article-title": "Therapeutic effects of glycyrrhizic acid",
"author": "Ming",
"doi-asserted-by": "publisher",
"first-page": "415",
"journal-title": "Nat Prod Commun",
"key": "B7",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.3390/v13040609",
"article-title": "Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease",
"author": "van de Sand",
"doi-asserted-by": "publisher",
"first-page": "609",
"journal-title": "Viruses.",
"key": "B8",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1001/jamaoto.2020.5490",
"article-title": "Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: a randomized clinical trial",
"author": "Guenezan",
"doi-asserted-by": "publisher",
"first-page": "400",
"journal-title": "JAMA Otolaryngol Head Neck Surg.",
"key": "B9",
"volume": "147",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-85174-w",
"article-title": "Enteric reabsorption processes and their impact on drug pharmacokinetics",
"author": "Ibarra",
"doi-asserted-by": "publisher",
"first-page": "5794",
"journal-title": "Sci Rep.",
"key": "B10",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3389/fmicb.2020.00597",
"article-title": "Lactobacillus murinus improved the bioavailability of orally administered glycyrrhizic acid in rats",
"author": "Yuan",
"doi-asserted-by": "publisher",
"first-page": "579",
"journal-title": "Front Microbiol",
"key": "B11",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1081/DMR-100104400",
"article-title": "The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling",
"author": "Ploeger",
"doi-asserted-by": "publisher",
"first-page": "125",
"journal-title": "Drug Metab Rev.",
"key": "B12",
"volume": "33",
"year": "2001"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"article-title": "HCA lung biological network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes",
"author": "Sungnak",
"doi-asserted-by": "publisher",
"first-page": "681",
"journal-title": "Nat Med.",
"key": "B13",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1007/s11427-020-1637-5",
"article-title": "Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission",
"author": "Xu",
"doi-asserted-by": "publisher",
"first-page": "457",
"journal-title": "Sci China Life Sci.",
"key": "B14",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1128/JVI.00127-20",
"article-title": "Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS",
"author": "Wan",
"doi-asserted-by": "publisher",
"first-page": "e00127",
"journal-title": "J Virol",
"key": "B15",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1038/s41564-020-0688-y",
"article-title": "Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B beta coronaviruses",
"author": "Letko",
"doi-asserted-by": "publisher",
"first-page": "562",
"journal-title": "Nat Microbiol",
"key": "B16",
"volume": "5",
"year": "2020"
},
{
"article-title": "Potential natural compounds for preventing 2019-nCoV infection",
"author": "Chen",
"key": "B17",
"volume-title": "Preprints.",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.01239",
"article-title": "Symptomatic protective action of glycyrrhizin (Licorice) in COVID-19",
"author": "Harald",
"doi-asserted-by": "publisher",
"first-page": "1239",
"journal-title": "Front Immunol.",
"key": "B18",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.mayocp.2020.05.030",
"article-title": "Smell and taste dysfunction in patients with COVID-19: a systemic review and meta-analysis",
"author": "Agyeman",
"doi-asserted-by": "publisher",
"first-page": "1621",
"journal-title": "Mayo clinic proc",
"key": "B19",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1101/2020.06.04.135012",
"article-title": "Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19",
"author": "Meinhardt",
"doi-asserted-by": "publisher",
"first-page": "168",
"journal-title": "Nat Neurosci.",
"key": "B20",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1126/sciadv.abc5801",
"article-title": "non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19–associated anosmia",
"author": "Brann",
"doi-asserted-by": "crossref",
"key": "B21",
"volume-title": "Sci. Adv",
"year": "2020"
},
{
"DOI": "10.1021/acschemneuro.0c00447",
"article-title": "Taste and smell disorders in COVID-19 patients. role of interleukin-6",
"author": "Cazzolla",
"doi-asserted-by": "publisher",
"first-page": "2774",
"journal-title": "ACS Chem Neurosci.",
"key": "B22",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.apsb.2015.05.005",
"article-title": "The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "310",
"journal-title": "Acta Pharm Sin B.",
"key": "B23",
"volume": "5",
"year": "2015"
},
{
"DOI": "10.1016/j.ijsu.2017.06.073",
"article-title": "Povidone iodine in wound healing: a review of current concepts and practices",
"author": "Bigliardi",
"doi-asserted-by": "publisher",
"first-page": "260",
"journal-title": "Int J Surg",
"key": "B24",
"volume": "44",
"year": "2017"
},
{
"DOI": "10.1191/096032700682694251",
"article-title": "Glycyrrhizic acid: the assessment of a no effect level",
"author": "van Gelderen",
"doi-asserted-by": "publisher",
"first-page": "434",
"journal-title": "Hum Exp Toxicol.",
"key": "B25",
"volume": "19",
"year": "2000"
},
{
"article-title": "Final report on the safety assessment of glycyrrhetinic acid, potassium glycyrrhetinate, disodium succinoyl glycyrrhetinate, glyceryl glycyrrhetinate, glycyrrhetinyl stearate, stearyl glycyrrhetinate, glycyrrhizic acid, ammonium glycyrrhizate, dipotassium glycyrrhizate, disodium glycyrrhizate, trisodium glycyrrhizate, methyl glycyrrhizate, and potassium glycyrrhizinate",
"key": "B26",
"volume-title": "Int J Toxicol",
"year": "2007"
},
{
"DOI": "10.1136/bmjgh-2020-002794",
"article-title": "Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "e002794",
"journal-title": "BMJ Global Health.",
"key": "B27",
"volume": "5",
"year": "2020"
},
{
"article-title": "Virological assessment of hospitalized patients with COVID-2019",
"author": "Wölfel",
"key": "B28",
"volume-title": "Nature.",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0869-5",
"article-title": "Temporal dynamics in viral shedding and transmissibility of COVID-19",
"author": "He",
"doi-asserted-by": "publisher",
"first-page": "672",
"journal-title": "Nat Med.",
"key": "B29",
"volume": "26",
"year": "2020"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2022.863917/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Combined Nasal, Oropharyngeal Povidone Iodine Plus Glycyrrhizic Acid Sprays, Accelerate Clinical and Laboratory Recovery and Reduces Household Transmission of SARS-CoV-2: A Randomized Placebo-Controlled Clinical Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "9"
}
